Abstract
We report the first case of Mycobacterium bovis infection in a free-living fallow deer (Dama dama) in northwest Italy, the epidemiological analysis (i.e., tracing source and dissemination during outbreaks), and the potential source of infection in a historically animal tuberculosis (aTB)-free area. Gross lung and lymph node lesions were histologically consistent with a severe parasitic bronchopneumonia due to lungworms associated with severe mycobacterial infection. The lesions contained numerous densely packaged, acid-fast bacilli, raising suspicion of an active, open form of aTB. Acid-fast organisms were characterized as M. bovis SB0120-ETR 45533, one of the most common profiles in Italy. Epidemiological investigation into the most recent outbreaks caused by M. bovis SB0120-ETR 45533 within a 50-km radius of where the fallow deer was retrieved disclosed two different situations: one case involving a water buffalo (Bubalus bubalis) in 2008 and one involving a donkey in 2016. Analysis of spoligotype and VNTR-type pattern circulating and recorded in northwest Italy from 1999 to 2014 suggested that in the cases of the donkey and the fallow deer, the source of infection was most likely attributable to spillover from outbreaks in domestic species: cattle and water buffalo, respectively. According to the European Commission, aTB status of livestock does not depend on aTB cases in wildlife (Council Directive 64/432/33C of 26 June 1964); nevertheless, the primary aim of aTB eradication should include global monitoring of all susceptible species if the re-emergence of M. bovis from established wildlife reservoir is to be prevented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal tuberculosis (aTB) is a chronic infectious disease caused by Mycobacterium bovis (M. bovis). Cattle are the main host, but spillover to livestock, wildlife, and humans can easily occur when proactive ecological and environmental factors are present (Gortázar et al. 2011a; Fitzgerald and Kaneene 2012; Amato et al. 2016). Haydon et al. (2002) proposed one of the most complete definitions of “reservoir” and extended it to include one or more epidemiologically connected populations or an environment where the pathogen can be permanently maintained and from which the infection is transmitted to a susceptible population. This definition should also be applied to M. bovis because it often spreads in multi-host contexts in which several species and environmental factors interact in an epidemiological context (Gortázar et al. 2007; Nugent 2011; Fitzgerald and Kaneene 2012). Indeed, to be a competent reservoir, a host species must be susceptible, able to transmit the disease, and abundant enough (Corner 2006). The role of reservoir can change, however, depending on ecological factors. For example, the wild boar (Sus scrofa) is a well-recognized reservoir host in Spain (Naranjo et al. 2008) but a spillover (or dead-end) host in northwest Italy since it does not contribute to the spread of infection (Serraino et al. 1999; Dondo et al. 2005).
Among wild hoofed ruminants, the family Cervidae may play a major role in the epidemiology of aTB (Martín-Hernando et al. 2010) mainly because of their ethology (spatial aggregation, high number of intra- and interspecific contacts), and longevity (Gortázar et al. 2011a). Some species are considered to be a reservoir in North America (Fitzgerald and Kaneene 2012; Miller and Sweeney 2013), France (Richomme et al. 2013), and Spain (Aranaz et al. 2004; Gortázar et al. 2011b). In several countries, however, aTB has also been observed as a spillover or a spillback event between livestock and wildlife (Gortázar et al. 2011a; Nugent 2011) or spreading within farmed deer herds (Griffin and Buchan 1994; Busch et al. 2017). In Europe, M. bovis infection has been reported in red deer (Cervus elaphus) (Richomme et al. 2013) and fallow deer (Dama dama) (Martín-Hernando et al. 2010; Gortázar et al. 2011b; Amato et al. 2016; Kohl et al. 2018), and less frequently in roe deer (Capreolus capreolus) (Balseiro et al. 2009).
Though measures against aTB in cattle in Italy were first enforced in 1954, only since 1995 has stricter regulation in compliance with European Community requirements strengthened the eradication scheme, leading to an aTB herd prevalence of 0.46% in 2011 (Chiavacci et al. 2014). Piedmont, an aTB official free region, has applied an additional regional plan to reduce the aTB herd prevalence to less than 0.1%. According to European (EC Reg. No. 853/20) and national (Ministerial Decree No. 592 of 15/12/1995) laws, active surveillance (test and slaughter method) on species other than bovids is limited to cases of epidemiological correlation with aTB outbreaks and to production of raw milk from animals cohabiting with cattle. Since 1997, the regional wildlife health status monitoring plan has included aTB surveillance on hunted and found dead animals as wild boar, roe deer, red deer, fallow deer, and mouflon (Ovis orientalis), while for carnivores, it is planned only if gross suspected lesions were observed at necropsy.
Since 1998, data on M. bovis strains collected from livestock and wildlife in Piedmont have been systematically recorded in the database of our Institute. Through analysis of these records, we can trace back all aTB cases that have occurred in Piedmont. This study reports the first case of generalized aTB in a naturally infected free-living adult fallow deer (Dama dama) in Piedmont, the epidemiological analysis, and the identification of a potential source of infection in a historically aTB-free area (Decision 2007/174/EC of 20/03/2007).
Material and methods
Case index and setting
Piedmont is located in the northwest corner of Italy. It is bordered by France to the west and by Liguria to the south, Aosta Valley to the north, Lombardy to the east, and Emilia-Romagna to the southeast. Two-thirds of the topography is alpine and hilly. The Alps stretch in an arch that sweeps from south to west to north and then east, enclosing much of the Po Valley. The region is subdivided into 8 administrative provinces: Torino (TO), Cuneo (CN), Asti (AT), Alessandria (AL), Vercelli (VC), Biella (BI), Novara (NO), and Verbano-Cusio-Ossola (VCO).
A small population (estimated 1000 animals) of fallow deer inhabits the region in small colonies distributed in flat and hilly areas (Carnevali et al. 2009); hunting management is aimed to “freeze” the current situation to avoid any further expansion of this non-native species. In 2014, a free-living adult (about 8–10 years old) female leucistic fallow deer (Dama dama) was humanely euthanatized by the veterinary service because of its extremely poor condition. The animal belonged to a group of about 20 animals reared on a woodland located in the province of NO very close to a safari park; the park is a private area of about 40 ha, where the forest opened into glades inhabited by deer, foxes, weasels, reptiles, hawks, and other bird species. The park held about 600 animals of different species and both wild and domestic ruminants, including cattle and water buffaloes.
Diagnostic investigations
The carcass was delivered to our Institute and submitted to necropsy and diagnostic procedures to determine the cause of the poor condition and for aTB surveillance.
Histopathology
Tissue samples from the heart, lungs, and mediastinal and bronchial lymph nodes were fixed in 10% neutral buffered formalin and processed by standard paraffin wax techniques. Samples were cut in 4 ± 2 μ sections and stained with hematoxylin-eosin (HE) and Ziehl-Neelsen (ZN) histochemical acid-fast stain. Slides were evaluated microscopically at increasing magnification (× 10, × 20, × 40).
Immunohistochemical (IHC) analysis using a polyclonal antibody raised against M. bovis (Bioss Inc., Woburn, MA, USA) was performed. The sections were submitted to heat-induced antigen retrieval using citrate buffer (pH 6.0) and then incubated for 1 h at room temperature with the antibody diluted 1:100. Immunodetection was performed using the ChemMate Dako Envision Detection kit, Peroxidase/DAB, Rabbit/Mouse (DakoCytomation, Glostrup, Denmark) with an incubation of 20 min and the visualization of the antibody binding was obtained via 3,3′-diaminobenzidine (DAB). All slides were evaluated microscopically at increasing magnification (× 10, × 20, × 40,); diagnosis of mycobacteriosis was based on the observation by ZN and IHC, respectively, of multiple granulomas associated with acid-fast and brown-staining rods.
Ancillary exams (bacteriology, virology)
Portions of lung, liver, gut, spleen, and kidney tissue were submitted to bacteriology. The specimens were streaked directly on 5% sheep blood agar-based plates (Columbia agar, Liofilchem, Roseto degli Abruzzi, Italy), and Gassner Agar (Microbiol, Uta, Italy), then incubated aerobically for 24–48 h at 37 °C. A Columbia agar plate containing 5% sheep blood was incubated under microaerophilic conditions (5% CO2 added) for 72 h at 37 °C. After incubation the most prevalent and suggestive colonies were subsequently identified using API galleries (bioMérieux Italia, Bagno a Ripoli, Italy). Isolation and identification of Salmonella spp. from the gut and liver was performed according to the Terrestrial Manual of Diagnostic Tests (OIE 2016a). Spleen samples were submitted to direct isolation of Brucella spp. (OIE 2016b) and to detection of Pestiviruses (Peletto et al. 2016). In order to exclude infection by thermotolerant Campylobacter spp., Mycobacterium avium subsp. paratuberculosis, and Verotoxigenic E. coli, feces were analyzed according to previously published methods (Biagini et al. 2013; Bellio et al. 2014; OIE 2008; OIE 2014).
aTB diagnostic protocol
Tissue samples (about 25 g) from lymph nodes (retropharyngeal, thoracic, and mesenteric) and the lung were processed for mycobacterial culture as previously described (Balseiro et al. 2009). A liquid culture system VersaTREK/ESP (©TREK Diagnostic Systems, Cleveland, OH, USA) was applied on the same tissues for isolation of Mycobacterium spp. All suspected colonies were submitted to ZN staining and then typed by molecular methods (Balseiro et al. 2009).
Epidemiological investigations
Following the occurrence of a aTB case in a fallow deer in a historically officially TB free (OTF) area, the local veterinary services ordered and supervised enforced surveillance of targeted species (wild boar and wild ruminants) in the province of NO. From 2014 to 2017, 468 animals (227 roe deer, 153 fallow deer, 78 wild boar, 9 red deer, and 1 mouflon) were hunted and the lungs and lymph nodes were submitted to the aTB diagnostic protocol previously described. Moreover, as required by regional plan, 109 red foxes (Vulpes vulpes) and 15 Eurasian badger (Meles meles) found dead in the province of NO were necropsied between 2014 and 2017.
Molecular profiles of the strain isolated in the fallow deer were compared with those recorded in our Institute’s M. bovis strains database. The data were reported in a dataset (Microsoft Office Excel 2010), and descriptive analysis was performed using SAS® version 9.4 statistical software. The absolute and relative frequencies of the case index’s spoligo and VNTR type were calculated at the species and year level; 95% confidence interval (95% CI) with the exact binomial method was calculated for each relative frequency. Geographical distribution of the typing patterns was depicted using an open-source geographic information system (QGIS version 2.14 “Essen,” Free Software Foundation, Inc., Boston, MA, USA,) applying WGS 84/UTM zone 32 (EPSG:32632) as coordinate systems.
Results
Case index
At necropsy, the fallow deer showed cachexia, serious dehydration, diffuse nodular dermatitis, and cutaneous ulcerations, associated with severe tick and lice infestation. Gross lesions in the thoracic cavity showed a few greenish, necrotizing nodules along the dorsal and caudal layers of the lung, together with small calcified granulomas in the bronchial lymph node referable to a chronic parasitic bronchopneumonia overlapping a pulmonary form of aTB. A few calcified nodules on the surface of the liver and peritoneum suggested chronic parasitic polyserositis due to Cisticercus tenuicollis.
Histopathological examination of the lungs revealed severe non-encapsulated granulomatous and necrotizing multifocal to coalescing inflammation with rare Langhans giant cells (Fig. 1a). Multifocal areas of necrosis with mineralization surrounded by epithelioid macrophages, lymphocytes, plasma cells, neutrophils, and rare Langhans giant cells were observed in the lymph nodes (Fig. 1b); these areas were partly or completely enclosed by a thin capsule. Numerous densely packaged, acid-fast bacilli (Fig. 1c) with strong immunopositivity (Fig. 1d) was detected in the tissues. The pattern and the involvement of the deep airways suggested an open, active form of aTB.
Acid-fast organisms (AFOs) were isolated by both liquid culture system VersaTREK/ESP and traditional cultures on solid media after 4 and 12 days of incubation, respectively. AFOs were identified as M. bovis and characterized by spoligo and ETR typing as SB0120-ETR 45533.
Ancillary exams revealed the presence of Salmonella enterica serovar Napoli in fecal excretion, Corynebacterium jeikeium in lung abscesses, and a focal degenerated parasitic cyst on the epicardium suggestive of cysticercosis.
History of aTB in the outbreak area (province of Novara)
Wildlife surveillance 2014–2017
Necropsy performed on all hunted and found dead animals did not reveal gross lesions referable to aTB. Further microbiology and molecular tests were conducted on hunting species isolating only Mycobacterium avium complex mycobacteria (n = 4) and Mycobacterium Other Than Tuberculosis (n = 1) in 5 wild boars.
aTB outbreaks 2014–2017
No other aTB cases were detected in the area, except for an adult, female donkey found dead in a mountain pasture in 2016 (1500 m above sea level) about 50 km from the site where the fallow deer was found, but in the province of BI, which is also an OTF area (Decision 204/2012/EU). At necropsy, severe, multifocal to coalescing granulomatous lesions with many multinucleated Langhans giant cells and moderate fibrosis in the lungs, liver, large intestines, and associated lymph nodes were detected. In all organs, the lesions contained numerous acid-fast bacilli. Bacterial culture of tissue samples and molecular typing identified M. bovis SB0120-ETR 45533.
Epidemiological investigations and analysis of the Institute’s M. bovis strains database
Analysis of the spoligotyping pattern revealed a total of 2019 strains with 77 different spoligotypes isolated from livestock and wildlife between 1998 and 2017 in Piedmont (Online Resource 1). The most frequently recorded spoligotype was SB0120 (65.43%, n = 1321) followed by SB0134 and SB0121 (9.56%, n = 193 and 8.57%, n = 173, respectively). The frequency of the other spoligotypes was less than 3% (Online Resource 1). Online Resource 2 presents the data on VNTR typing of SB0120 isolated strains; the most frequent profiles were 45533 (21.57%, n = 285/1321) followed by 54534 (17.64%, n = 233/1321), 54533 (16.12%, n = 213/1321), and other profiles with a prevalence of less than 7%.
M. bovis SB0120 45533 and SB0120 54534 were isolated from animals from 66 and 64 different farms, respectively; the one showed the widest host range with spreading among cattle, goats, buffalo, donkey, fallow deer, and wild boar (Table 1) other than humans (data not shown). The high variability of the M. bovis strains seems to be a feature of the infection in cattle because only one or two patterns were recorded for the other species (Table 1). An exception was wild boars, in which 7 different patterns were observed, 3 of which were identified only in this species (Table 1); however, these 3 patterns were detected also in cattle from farms outside the region during the same period (data not shown).
The temporal distribution of the aTB outbreaks indicated a progressive decline in the top five spoligotype-VNTR patterns (SB0120-ETR 45533, SB0120-ETR 54534, SB0120-ETR 54533, SB0134-ETR 54534, SB0120-ETR 54563) in Piedmont (Fig. 2). Between 12 and 4 outbreaks of SB0120-ETR 45533 and SB0120-ETR 54534 were recorded per year in livestock until 2009, when the frequency dropped to less than 4 outbreaks per year. Other patterns showed a lower frequency that fluctuated between 3 and 4 outbreaks per year with sporadic peaks (Fig. 2).
Epidemiological investigations highlighted that before 2014, only one aTB outbreak of SB0120-ETR 45533 was reported in the same area where the infected fallow deer came from; this outbreak was caused by M. bovis SB0120-VNTR 45533 and occurred in 2008 in water buffalo (Bubalus bubalis) in a safari park less than 7 km away from the place where the fallow deer was found. As reported by Rossi et al. (2008), specific surveillance of cohabiting animals (6 water buffaloes) or belonging to a closer fence (n = 2 red deer, n = 2 cows, n = 10 goats, n = 2 Barbary sheep) conducted in vivo (gamma IFN) and post mortem (bacterial culture) showed microbiological positivity only in another water buffalo that resulted positive to M. bovis with the same spoligotype profile but with a different VNTR type (54534 instead of 45533). The lack of data on previous movements of infected animals precluded determination of the source of infection; however, introduction via trade could be probable.
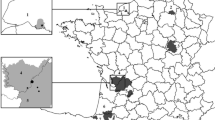
The geographical distribution of SB0120-45533 between 2002 and 2016 confirms the absence of an overlap between the outbreaks in cattle and the case of the fallow deer and the buffaloes (Fig. 3). The available data indicate the buffaloes in the safari park as the only possible source of aTB for the fallow deer; restriction of contact due to the fenced area makes direct contact less likely. Otherwise, as emerged from the epidemiological survey, lax biosecurity might have led to environmental contamination of the external area, resulting in aTB infection in the fallow deer grazing there.
In the case of the donkey, epidemiological investigation disclosed that the animal was previously reared on a farm located in an area with a consistent history of aTB (province of Cuneo, CN) and where spoligotype SB0120-45533 was frequent in the past (Fig. 3). For these reasons, the investigations ended, with cattle claimed as the source of the infection in the donkey.
Discussion and conclusions
This is the first report of aTB infection in a wild fallow deer in Italy, different from the cases that occurred in Sicily (Amato et al. 2016) or Germany (Kohl et al. 2018) where the animals were kept in a herd and a zoo, respectively.
Moreover, this case of aTB in wildlife occurred in a historically officially aTB-free (OTF) area. Detailed epidemiological investigation has become mandatory to define the source of infection and the role of the species involved. No other aTB cases were identified in the study area, except for the water buffaloes in a nearby safari park in 2008 and the donkey in 2016. Unlike the water buffalo, which belong to the Bovidae family known to be susceptible to aTB (Iannaccone et al. 2018), donkey has is highly resistant to mycobacterial infection (O’Reilly and Daborn 1995; Pavlik et al. 2004), and no eradication program can be applied because the intradermal tuberculin test is unreliable (Pavlik et al. 2004).
Epidemiological investigation showed different sources of infection for the aTB outbreaks reported in 2014 and 2016: both were spillover (or dead-host) events (from the buffalo to the fallow deer and from the cattle to the donkey) particularly in the donkey, in which sporadic cases are reported in horses only in areas where aTB is present in livestock (Cousins 2001), as occurred in southwest Piedmont in the past.
In Piedmont, maintenance host or spillback events in wildlife have never been reported, except for aTB infection in a few wild boar (Chiavacci et al. 2014), which is usually considered a “spillover host” because M. bovis is unable to persist in this population without interspecific contacts with a primary reservoir, usually cattle. As a family, Cervidae are very susceptible to aTB infection (Gortázar et al. 2012) and their large home range and longevity make them ideal spatial vectors with a risk of spillback in new locations (Nugent 2011), as occurred in Spain (Parra et al. 2006) and England (Delahay et al. 2007).
In the present study, the necropsied fallow deer was considerably debilitated, underscoring the important role of concurrent parasitic and bacterial diseases in the exacerbation and generalization of aTB. As reported by Johnson et al. (2008) and by Martín-Hernando et al. (2010), the occurrence of generalized aTB in fallow deer, which could result from a synergy between stressors and the high susceptibility of this species to M. bovis, turn the infected animals into dangerous spatio-temporal vectors and “super spreaders” (Nugent 2011). However, our investigation revealed no secondary spillovers or spillback or intraspecific transmission, although the gross lesions and microscopic patterns suggested active M. bovis shedding. Moreover, being female and sharing the habitat within a social group increased the chances of transmission of infection.
The finding of a group of fallow deer with only 1 infected animal was also reported by Gortázar et al. (2011a), in which the lower prevalence in this species than the deer or the wild boar was probably due to a lower susceptibility to M. bovis and a lower intraspecific transmission rate. It can be concluded that the co-infections might have led to a breakdown in the immune system, as demonstrated by the non-encapsulated lung lesions typical of a weakened immune system, making the contagious period relatively short. Furthermore, the good condition of the cohort may have made the infection through direct or indirect contact less likely to occur.
In our opinion, the absence of interspecific transmission (secondary spillover) could be due to the fragmentation of fallow deer populations in Italy, their ethology, and variable but restricted home range; these features, besides a highly anthropic rural ecosystem that limits interactions with other species, could have excluded or made the spread of infection unlikely just as it would have lessened the role of fallow deer in aTB epidemiology in Piedmont.
Amato and collaborators (Amato et al. 2016) reported observing specific aTB cutaneous lesions. In our case, we did not investigate whether mycobacteria were established in the skin lesions and overlapping with the parasite infestation. We believe that such lesions should always be submitted to diagnostic investigation of open pulmonary aTB because scratching and licking can promote the establishment of M. bovis in the derma through pre-existing breaches in the skin surface. Moreover, this event can increase the risk of exposure to aTB in other animals and environmental contamination, when males mark their territories by rubbing against trees and these olfactory “infected” traces are caught by the nose and Jacobson’s organ of other susceptible species.
SB0120 spoligotype associated with the 45533 VNTR profile is one of the most common strains in Piedmont; its isolation from both wildlife and livestock supports the hypothesis proposed by Hauer et al. (2015) that there is no host-specific barrier for different M. bovis genotypes. The occurrence of strains with the same spoligotype and VNTR-type profile in different areas and in multiple species probably overlaps with a progressive reduction in the variability of circulating strains after aTB control in Piedmont was improved with use of the gamma-interferon test as an ancillary to the tuberculin skin test. In agreement with previous studies (Smith 2012; Hauer et al. 2015; Amato et al. 2017), we support the hypothesis for a “modeling” of some dominant clonal complexes in which their advantage is the fact that they are “lucky clones in the right place at the right time”, which makes them very widespread and therefore more likely to resist neutral forces (population bottleneck) active in an animal population where aTB control measures are starting. Alternatively, a selective advantage (management risk factors) for some strains could have occurred in wildlife (e.g., wild boars) because of the absence of selective culling.
Moreover, adaptation to the environment and/or to the host of some clonal complexes cannot be excluded (Hauer et al. 2015; Ghavidel et al. 2012). The higher virulence of certain persistent strains could be enhanced using a WGS approach (Hauer et al. 2015) sustained by experimental in vitro and in vivo models for the study of the pathogenicity and infection of more frequent M. bovis clones in different animal species.
aTB infection in wildlife is a huge concern owing to the risk of the reintroduction of M. bovis in OTF areas with potential transmission to livestock. Wildlife aTB management is globally challenging due to biases in epidemiological studies: uncertainty about its ecology in different animal species, difficulty in obtaining samples and population data, lack of validated tests, and overlapping of jurisdictions and authority (Woebeser 2009). Furthermore, estimating aTB prevalence can be highly inaccurate, mainly in areas where it is thought to be less than 5% (Clifton-Hadley and Wilesmith 1991; Abernethy et al. 2006; Delahay et al. 2007; Fitzgerald and Kaneene 2012). Because aTB prevalence in cattle is very low in some European countries (annual report of the Standing Committee on Plants Animal Food and Feed, 2018), optimal surveillance and possible control strategies should be set up according to the peculiarities of an area and animal species to prevent spillback and the re-emergence of aTB (Hardstaff et al. 2014). According to the European Commission, the aTB status of livestock does not depend on aTB cases in wildlife (Council Directive 64/432/33C of 26 June 1964); nonetheless, global monitoring of all susceptible species ought to be the primary aim of aTB eradication efforts to prevent the re-emergence of M. bovis from established wildlife reservoirs.
References
Abernethy DA, Denny GO, Menzies FD, McGuckian P, Honhold N, Roberts AR (2006) The Northern Ireland programme for the control and eradication of Mycobacterium bovis. Vet Microbiol 112(2–4):231–237
Amato B, Mignacca SA, Pacciarini ML, Vitale M, Antoci S, Cucinotta S, Puleio R, Biasibetti E, Fiasconaro M, Capucchio MT, Di Marco Lo Presti V (2016) An outbreak of bovine tuberculosis in a fallow deer herd (Dama dama) in Sicily. Res Vet Sci 106:116–120
Amato B, Di Marco Lo Presti V, Gerace E, Capucchio MT, Vitale M, Zanghì P, Pacciarini ML, Marianelli C, Boniotti MB (2017) Molecular epidemiology of Mycobacterium tuberculosis complex strains isolated from livestock and wild animals in Italy suggests the need for a different eradication strategy for bovine tuberculosis. Transbound Emerg Dis 1–9
Aranaz A, De Juan L, Montero N, Sánchez C, Galka M, Delso C, Alvarez J, Romero B, Bezos J, Vela AI, Briones V, Mateos A, Domínguez L (2004) Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. J Clin Microbiol 42(6):2602–2608
Balseiro A, Oleaga A, Orusa R, Robetto S, Zoppi S, Dondo A, Goria M, Gortázar C, Marin JFG, Domenis L (2009) Tuberculosis in roe deer from Spain and Italy. Vet Rec 164:468–470
Bellio A, Traversa A, Adriano D, Bianchi MD, Colzani A, Gili S, Dondo A, Gallina S, Grattarola C, Maurella C, Zoppi S, Zuccon F, Decastelli L (2014) Occurrence of thermotolerant Campylobacter in raw poultry meat, environment and pigeon stools collected in open-air markets. Ital J Food Saf 3:1706
Biagini D, Lazzaroni C, Zoppi S (2013) Microbial inoculum for litter conditioning in calves rearing: preliminary results on litter quality and health. Italian J Anim Sci 12:93–94
Busch F, Bannerman F, Liggett S, Griffin F, Clarke J, Lyashchenko KP, Rhodes S (2017) Control of bovine tuberculosis in a farmed red deer herd in England. Vet Rec 180(3):8
Carnevali L, Pedrotti L, Riga F, Toso S (2009) Banca Dati Ungulati. Status, distribuzione, consistenza, gestione e prelievo venatorio delle popolazioni di Ungulati. in Italia. Rapporto 2001–2005. [117], 1–168. 2009. ISPRA (Istituto Superiore per la Protezione e la Ricerca Ambientale). Biologia e Conservazione della Fauna. http://www.isprambiente.gov.it/it/pubblicazioni/documenti-tecnici/banca-dati-ungulati-status-distribuzione. Accessed 17 October 2018
Chiavacci L, Dondo A, Goria M, Moda G, Ruocco L, Vignetta P, Zoppi S (2014) Tuberculosis eradication in Italy. In: Thoen CO, Steele JH, Kaneene JB (eds) zoonotic tuberculosis: Mycobacterium bovis and other pathogenic mycobacteria, 3rd edn. Wiley & Sons, Inc., Hoboken, New Jersey, pp 357–369
Clifton-Hadley RS, Wilesmith JW (1991) Tuberculosis in deer: a review. Vet Rec 129(1):5–12
Corner LA (2006) The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: how to assess the risk. Vet Microbiol 112:303–312
Cousins DV (2001) Mycobacterium bovis infection and control in domestic livestock. Rev Sci Tech 20:71–85
Delahay RJ, Smith GC, Barlow AM, Walker N, Harris A, Clifton-Hadley RS, Cheeseman CL (2007) Bovine tuberculosis infection in wild mammals in the south-west region of England: a survey of prevalence and a semi-quantitative assessment of the relative risks to cattle. Vet J 173(2):287–301
Dondo A, Zoppi S, Angelillo M, Buonincontro G, Garrone A, Squadrone S, Benedetto A, Goria M (2005) Diagnosi post mortem di tubercolosi nei bovini: esiti e considerazioni sul protocollo diagnostico applicato nel periodo 2001-2004 in Piemonte. Il Progresso Veterinario 8(LX):351–358
Fitzgerald SD, Kaneene JB (2012) Wildlife reservoirs of bovine tuberculosis worldwide: hosts, pathology, surveillance, and control. Vet Pathol 50(3):488–499
Ghavidel M, Mansury D, Nourian K, Ghazvini K (2012) The most common spoligotype of Mycobacterium bovis isolated in the world and the recommended loci for VNTR typing; a systematic review. Microb Pathog 118:310–315
Gortázar C, Ferroglio E, Höfle U, Frölich K, Vicente J (2007) Diseases shared between wildlife and livestock: a European perspective. Eur J Wildl Res 53:241–256
Gortázar C, Torres MJ, Acevedo P, Aznar J, Negro JJ, de la Fuente J, Vicente J (2011a) Fine-tuning the space, time, and host distribution of mycobacteria in wildlife. BMC Microbiol 11(1):27
Gortázar C, Vicente J, Boadella M, Ballesteros C, Galindo RC, Garrido J, Aranaz A, de la Fuente J (2011b) Progress in the control of bovine tuberculosis in Spanish wildlife. Vet Microbiol 151:170–178
Gortázar C, Delahay EJ, Mcdonald RA, Boadella M, Wilson GJ, Gavier-Widen D, Acevedo P (2012) The status of tuberculosis in European wild mammals. Mammal Rev 42:193–206
Griffin JF, Buchan GS (1994) Aetiology, pathogenesis and diagnosis of Mycobacterium bovis in deer. Vet Microbiol 40(1–2):193–205
Hardstaff JL, Marion G, Hutchings MR, White PC (2014) Evaluating the tuberculosis hazard posed to cattle from wildlife across Europe. Res Vet Sci 97:S86–S93
Hauer A, De Cruz K, Cochard T, Godreuil S, Karoui C, Henault S, Bulach T, Bañuls AL, Biet F, Boschiroli ML (2015) Genetic evolution of Mycobacterium bovis causing tuberculosis in livestock and wildlife in France since 1978. PLoS One 10(2):1–17
Haydon DT, Cleaveland S, Taylor LH, Laurenson MK (2002) Identifying reservoirs of infection: a conceptual and practical challenge. Emerg Infect Dis 8(12):1468–1473
Iannaccone M, Cosenza G, Pauciullo A, Martino G, Capparelli R (2018) The SNP g.4667G>A at 3′UTR of IFNG gene is associated with susceptibility to bovine tuberculosis in Mediterranean water buffalo (Bubalus bubalis). Anim Genet 49(5):496–497
Johnson LK, Liebana E, Nunez A, Spencer Y, Clifton-Hadley R, Jahans K, Ward A, Barlow A, Delahay R (2008) Histological observations of bovine tuberculosis in lung and lymph node tissues from British deer. Vet J 175(3):409–412
Kohl TA, Utpatel C, Niemann S, Moser I (2018) Mycobacterium bovis persistence in two different captive wild animal populations in Germany: a longitudinal molecular epidemiological study revealing pathogen transmission by whole-genome sequencing. J Clin Microbiol 56(9):1–9
Martín-Hernando MP, Torres MJ, Aznar J, Negro JJ, Gandía A, Gortázar C (2010) Distribution of lesions in red and fallow deer naturally infected with Mycobacterium bovis. J Comp Pathol 142(1):43–50
Miller RS, Sweeney SJ (2013) Mycobacterium bovis (bovine tuberculosis) infection in north American wildlife: current status and opportunities for mitigation of risks of further infection in wildlife populations. Epidemiol Infect 141(7):1357–1370
Naranjo V, Gortázar C, Vicente J, de la Fuente J (2008) Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet Microbiol 127(1–2):1–9
Nugent G (2011) Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: a New Zealand case study. Vet Microbiol 151:34–42
O’Reilly LM, Daborn CJ (1995) The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis 76(1):1–46
OIE (2008) Office International des Epizooties, Chapter 2.9.10. Verocytotoxygenic Escherichia coli in: manual of diagnostic tests and vaccines for terrestrial animals 2018, web format http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/ accessed: 04/08/2018
OIE (2014) Office International des Epizooties, Chapter 2.1.15. Paratuberculosis (Johne’s disease) in manual of diagnostic tests and vaccines for terrestrial animals 2018, web format http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/ accessed: 04/08/2018
OIE (2016a) Office International des Epizooties, Chapter 2.9.8. Salmonellosis in manual of diagnostic tests and vaccines for terrestrial animals 2018, web format http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/ accessed: 04/08/2018
OIE (2016b) Office International des Epizooties, Chapter 2.1.4. Brucellosis (infection with B. abortus, B. melitensis and B. suis) in manual of diagnostic tests and vaccines for terrestrial animals 2018, web format http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/ accessed: 04/08/2018
Parra A, Garcia A, Inglis NF, Tato A, Alonso JM, Hermoso de Mendoza M, Hermoso de Mendoza J, Larrasa J (2006) An epidemiological evaluation of Mycobacterium bovis infections in wild game animals of the Spanish Mediterranean ecosystem. Res Vet Sci 80:140–146
Pavlik I, Jahn P, Dvorska L, Bartos M, Novotny L, Halouzka R (2004) Mycobacterial infections in horses: a review of the literature. Veterinarni Medicina – UZPI 49:427–440
Peletto S, Caruso C, Cerutti F, Modesto P, Zoppi S, Dondo A, Acutis PL, Masoero L (2016) A new genotype of border disease virus with implications for molecular diagnostics. Arch Virol 161(2):471–477
Richomme C, Boadella M, Courcoul A, Durand B, Drapeau A, Corde Y, Hars J, Payne A, Fediaevsky A, Boschiroli ML (2013) Exposure of wild boar to Mycobacterium tuberculosis complex in France since 2000 is consistent with the distribution of bovine tuberculosis outbreaks in cattle. PLoS One 8(10):e77842
Rossi F, Zoppi S, Bergagna S, Tinelli F, Borella A, Bollo E, Suma G, Goria M, Dondo A (2008) Focolaio di tubercolosi da Mycobacterium bovis in un parco safari in Piemonte. Atti del X Convegno Nazionale SIDILV, pp 282–283
Serraino A, Marchetti G, Sanguinetti V, Rossi MC, Zanoni RG, Catozzi L, Bandera A, Dini W, Mignone W, Franzetti F, Gori A (1999) Monitoring of transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. J Clin Microbiol 37(9):2766–2771
Smith NH (2012) The global distribution and phylogeography of Mycobacterium bovis. Infect Genet Evol 12:857–865
Woebeser G (2009) Bovine tuberculosis in Canadian wildlife: an updated history. Can Vet J 50(11):1169–1176
Acknowledgments
We thank the staff of the General Diagnostic and Biotechnology laboratories of our Institute for their technical assistance.
Funding
This study was in part funded by the Italian Ministry of Health (Project n. IZS PLV 06/16 RC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Blasio, A., Varello, K., Vitale, N. et al. Animal tuberculosis in a free-ranging fallow deer in northwest Italy: a case of “lucky strain survival” or multi-host epidemiological system complexity?. Eur J Wildl Res 65, 74 (2019). https://doi.org/10.1007/s10344-019-1316-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-019-1316-0