Abstract
Several hypotheses have been examined as potential causes of global amphibian declines, including emerging infectious diseases and environmental contaminants. Although these factors are typically studied separately, animals are generally exposed to both stressors simultaneously. We examined the effects of the herbicide atrazine and the insecticide chlorpyrifos on the susceptibility of tiger salamander larvae, Ambystoma tigrinum, to a viral pathogen, Ambystoma tigrinum virus (ATV). Environmentally relevant concentrations of atrazine (0, 20, 200 μg/L) and chlorpyrifos (0, 2, 20, 200 μg/L) were used along with ATV in a fully factorial experimental design whereby individually housed, 4-week-old larvae were exposed for 2 weeks. Atrazine alone was not lethal to larvae, and chlorpyrifos alone was lethal only at the highest concentration. When combined with ATV, chlorpyrifos increased susceptibility to viral infection and resulted in increased larval mortality. A significant interactive effect between atrazine and ATV was detected. Atrazine treatments slightly decreased survival in virus-exposed treatments, yet slightly increased survival in the virus-free treatments. These findings corroborate earlier research on the impacts of atrazine, in particular, on disease susceptibility, but exhibit greater effects (i.e., reduced survival) when younger larvae were examined. This study is the first of its kind to demonstrate decreases in amphibian survival with the combination of pesticide and a viral disease. Further examination of these multiple stressors can provide key insights into potential significance of environmental cofactors, such as pesticides, in disease dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphibian declines are occurring worldwide due to a variety of anthropogenic impacts (Blaustein and Kiesecker, 2002). Whereas clear mechanisms likely underlie many of these declines (e.g., habitat loss and invasive species), other causes are far less understood and can involve subtle and complex interactions (Collins and Storfer, 2003). These “enigmatic” declines account for a large proportion of worldwide population losses and require further empirical and field-based investigation (Stuart et al., 2004). Two primary suspects for these enigmatic declines are emerging infectious diseases and contaminant exposure.
Disease is thought to play a key role in amphibian declines worldwide (Daszak et al., 2003; Stuart et al., 2004). Several diseases have been implicated in these declines: ranaviral diseases, chytridiomycosis, saprolegniosis, and Ribeiroia infection (Carey, 1993; Berger et al., 1998; Daszak et al., 1999; Kiesecker, 2002; Johnson et al., 2007). In particular, Ranaviruses and the chytrid fungus, Batrachochytrium dendrobatidis, are well-documented for causing population die-offs throughout the world (Daszak et al., 1999, 2003). What is far less clear about both of these pathogens is the reason(s) for their apparent sudden emergence (Chinchar, 2002; Daszak et al., 2003; Collins and Halliday, 2005). One proposed mechanism for this emergence is that exposure to contaminants can weaken host immune responses and increase susceptibility to infection and disease (Carey, 2000; Christin et al., 2003; Forson and Storfer, 2006a; Rohr et al., 2008).
Several pesticides have been shown to exhibit strong negative impacts on amphibians. Chlorpyrifos is the most widely used insecticide in the United States and, although targeted to act on insect nervous systems (Kiely et al., 2004), it also is known to alter nervous function in amphibians (Colombo et al., 2005). These effects result in both behavioral modifications and a reduction in growth (Widder and Bidwell, 2006). Chlorpyrifos also has a large range of lethal concentration (LC50) values ranging from 1 to 3,000 μg/L for differing amphibian species (Barron and Woodburn, 1995). It is estimated that concentrations up to 64 μg/L occur in natural settings (van den Brink et al., 1996), with residues in natural ponds found at >20 μg/L (Hurlbert et al., 1970).
Atrazine is one of the most widely used herbicides across the United States and is frequently detected in a wide variety of water bodies at concentrations up to 250 μg/L (Solomon et al., 1996). Atrazine has been shown to disrupt endocrine function in two species of frogs (Hayes et al., 2002, 2003). Atrazine has other large negative effects in several amphibian species in terms of growth inhibition, developmental suppression, and decreased survival (e.g., Larson et al., 1998; Diana et al., 2000; Storrs and Kiesecker, 2004; Rohr et al., 2006). In addition, atrazine has been found to elicit negative effects indirectly via depleting amphibian food resources (Boone and James, 2003) and by increasing amphibian susceptibility to trematode infection (Kiesecker, 2002; Rohr et al., 2008).
Tiger salamanders (Ambystoma tigrinum), like many other amphibian species, have undergone widespread epizootics throughout the western United States (Jancovich et al., 1997, 2005). A major cause for these die-offs has been the emergence of a single host ranaviral pathogen, Ambystoma tigrinum virus (ATV) (Jancovich et al., 1997, 2005; Storfer et al., 2007). It is hypothesized that environmental cofactors might have triggered some of these recent outbreaks (Jancovich et al., 2005; Forson and Storfer, 2006a, b).
There is some evidence that pesticides could be important cofactors for amphibian declines in general. Pesticide contamination occurs throughout the United States, from agricultural ponds to relatively undisturbed sites in the Sierra Nevada mountains of California (McConnell et al., 1998). The concentrations detected at most of these sites are typically too low to cause mortality directly, even among amphibian larvae. The sublethal effects of these low concentrations, and in particular, their interaction with biotic stressors are not well-understood. One study performed on 12-week-old A. tigrinum larvae showed increased infection rates with ATV when also exposed to atrazine at 16 μg/L (Forson and Storfer, 2006a). Only until recently, have the potential interactive effects of pesticides and pathogens in amphibians been explored (Kiesecker, 2002; Christin et al., 2003).
In addition to interactions with pathogens, recent studies have found interactive effects of sublethal concentrations of atrazine with other pesticides. Belden and Lydy (2000) found that atrazine potentiates the toxicity of chlorpyrifos in chironomid midges. When sublethal concentrations of each pesticide are combined, midge survival is significantly reduced. This potentiating effect has shown mixed results among amphibian taxa. The pesticide combination results in lethality in the African clawed frog, Xenopus laevis, but not in the wood frog, Rana sylvatica, or the green frog, Rana clamitans (Wacksman et al., 2006). Combined effects of atrazine and chlorpyrifos have not been yet examined in salamander taxa, but may have important implications for disease dynamics because the two pesticides often are applied at the same sites (Wacksman et al., 2006).
In this study, we examine the effects of the multiple stressors of a viral pathogen (ATV) and the pesticides atrazine and chlorpyrifos on 4-week-old tiger salamander larvae. We also examine interactions between the two pesticides, at several ecologically relevant concentrations, with one another and ATV in a fully factorial experimental design. We hypothesize that the presence of pesticides increases susceptibility to ATV and results in reduced survival of larval salamanders.
Methods
Salamander Larvae
We used laboratory-bred larvae to ensure no previous exposure to the pathogen or pesticides. Eggs were obtained from five full sibship families at Arizona State University (Tempe, AZ, USA). Families originated from animals collected along the Mogollon Rim (Coconino County, AZ, USA). Larvae were reared individually in round polyethylene containers (12.7-cm × 7.6-cm) filled with 500 ml of artesian spring water treated with Reptisafe (Zoo Med Laboratories, San Luis Obispo, CA, USA), which was aerated for at least 24 hours. Complete water changes were performed weekly both during the rearing period and the experiment. Upon hatching, larvae were fed daily 0.015 g of egg dry mass of hatched brine shrimp. Larvae were maintained on a 12:12 hour light:dark cycle in an environmental chamber kept at 20 ± 1°C. The experiment began once larvae reached 4 weeks of age (mass = 0.10 ± 0.0018 g; SVL = 0.88 ± 0.21 cm).
Experimental Design
A fully factorial 3 (0, 20, 200 μg/L atrazine) × 4 (0, 2, 20, 200 μg/L chlorpyrifos) × 2 (virus/no virus) design was used, replicating each treatment 10 times with individual animals as replicates, for a total of 240 animals. Individuals from each of the five families were distributed equally to all treatments. Larvae were individually housed in plastic cups filled with 500 ml of well water. Pesticide aliquots were administered first, with virus aliquots immediately following. Using a previous protocol (Forson and Storfer, 2006a), we administered to each individual cup an aliquot of control cell media or media containing the viral strain CAP, which originates from the same population that our experimental salamanders were drawn from (Kaibab region, Arizona). The viral strain used was passed through cell culture only twice, and therefore was suitable for use. Larvae in the viral treatment were exposed to 1 × 104 plaque forming units of Ambystoma tigrinum virus via water bath for 7 days (estimated LC50; Brunner et al., 2005). Pesticides were reapplied after the water change on day 7, whereas virus exposure occurred only during the initial week. The experiment was concluded after 2 weeks, when death had subsided in virus treatments for 3 consecutive days. Surviving larvae were killed at the end of the experiment with a water bath overdose of MS-222.
Pesticide Preparation
For initial exposure and each subsequent water change, a new 100-mg/L stock solution was created for each pesticide. Technical grade atrazine and chlorpyrifos were dissolved in 100% methanol and then subsequently diluted 10x into distilled water to create working solutions. These solutions were vigorously shaken for 10 minutes to ensure homogenization. From this, 100 and 1,000 μl of solutions were placed in their respective treatments of 500 ml of water to obtain 20 and 200 μg/L concentrations of each pesticide. For chlorpyrifos, a 2 μg/L concentration also was added by using 10 μl of the stock solution. Control treatments were administered: 1000 μl of a 10% methanol solvent control. Stock solution concentrations of both pesticides were verified via gas chromatography (University of Idaho Analytical Science Laboratory, Moscow, ID, USA). Previous research shows negligible degradation of both pesticides during a 7-day period in laboratory setups (Manzanti et al., 2003).
Variables Measured
Survival of individuals was monitored daily, with deceased organisms immediately preserved in 95% ethanol. To quantify virus loads and infection status of individuals, we used quantitative real-time PCR. Methodology of DNA extraction and qPCR followed Forson and Storfer (2006a). Tail tissue from larvae were extracted via DNeasy kits (Qiagen) and quantified via a Nanodrop spectrophotometer. Samples were diluted to 20-ng/L concentrations and examined in triplicate. Reactions contained 100-ng template DNA, 300-nmol forward primer, 900-nmol reverse primer, 240-nmol probe, and Taqman 2X Universal PCR master mix (no AmpErase UNG; Applied Biosystems, Foster City, CA, USA). Reactions were run for 40 cycles of 95°C denaturing (20 s), 54°C annealing (20 s), and 72°C extension (30 s) on an ABI 7300 Real-time PCR System using Real-time PCR System Sequence Detection Software version 1.2.3 (Applied Biosystems). All virus-treated animals were examined along with a random sampling of 20% of the no-virus controls to verify lack of contamination.
Statistical Analyses
We used logistic regressions to detect significant interactions between treatments of virus, atrazine, and chlorpyrifos using the response variables of both total dead and infected (PROC LOGISTIC, SAS 9.0). The total number of viral copies data were log transformed (Log (x + 1)) to meet normality assumptions. We analyzed these transformed data using two-way ANOVA. Due to the bimodal nature of the data, we also statistically examined differences in viral load between non-zero values (i.e., only infected individuals).
Results
Mortality
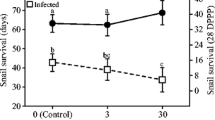
The logistic regression analysis listed three key components to the overall model: virus, chlorpyrifos, and a virus × atrazine interaction. Virus exposed treatments resulted in significantly higher mortality across all treatments relative to nonvirus treatments (χ2 = 40.59, degrees of freedom (df) = 1, p = 0.0001). Significant increase in mortality due to pesticide exposure was detected with chlorpyrifos (χ2 = 16.88, df = 3, p = 0.0007), with increasing concentrations resulting in higher mortality regardless of virus presence (Figure 1). The combination of virus and chlorpyrifos resulted in an additive increase in mortality relative to the pesticide alone, although the interaction term was not significant (χ2 = 1.88, df = 3, p = 0.6). We found no significant main effect of atrazine on mortality (χ2 = 2.46, df = 2, p = 0.29) and no significant interactive effects of atrazine by chlorpyrifos on mortality (χ2 = 7.59, df = 6, p = 0.27). There was a significant interactive effect of atrazine by virus (χ2 = 6.68, df = 2, p = 0.03) with the combination of atrazine slightly reducing mortality in no-virus treatments and slightly increasing mortality in virus treatments (Figure 2). The three-way interaction effect between virus, chlorpyrifos, and atrazine was not significant (χ2 = 8.36, df = 6, p = 0.21), although combined pesticide treatments resulted in the greatest mortality in virus-exposed animals (Figure 3).
Proportion of salamander larvae that survived viral and chlorpyrifos exposure. Pesticide levels span ecologically relevant concentrations and viral exposure level typically results in 50% survival. Logistic regression analyses are performed on total proportions, and therefore no standard error bars are present. Each bar represents all larvae in a particular treatment, and therefore combines larvae across all atrazine treatments.
Proportion of salamander larvae that survived viral and atrazine exposure. Pesticide levels span ecologically relevant concentrations and viral exposure level typically results in 50% survival. Logistic regression analyses are performed on total proportions, and therefore no standard error bars are present. Each bar represents all larvae in a particular treatment, and therefore combines larvae across all chlorpyrifos treatments.
Infection
The logistic regression model showed no interactive effect between the two pesticides on infection rate was detected (χ2 = 6.33, p = 0.85). When examined separately, increased chlorpyrifos concentration resulted in higher infection rates (F3,6 = 10.25, p = 0.01; Figure 4), whereas atrazine had no significant effect (F2,6 = 2.69, p = 0.14).
Quantitative PCR estimates of viral loads among individuals show that death corresponds strongly to infection in virus exposed treatments. Dead individuals in the virus treatment exhibited significantly higher viral loads (F1,57 = 7.12, p = 0.01) than surviving individuals, with 86% of dead exhibiting greater than 1 × 106 viral genome copies. Analysis comparing viral loads did not signify differences with exposure to atrazine (F2,104 = 0.24, p = 0.79) or chlorpyrifos (F3,104 = 0.34, p = 0.8), or to an interactive effect of both pesticides (F6,104 = 0.29, p = 0.94). Examination of infected only animals also did not signify difference in viral loads with exposure to atrazine (F2,47 = 0.05, p = 0.95), chlorpyrifos (F3,47 = 0.88, p = 0.46), or their interaction (F6,47 = 0.85, p = 0.54).
Discussion
Both contaminants and disease are thought to be critical components contributing to amphibian declines (Collins and Storfer, 2003). Several studies have examined the impacts of pesticides on amphibians (for review see Relyea and Hoverman, 2006), but few have investigated their importance in contributing to disease susceptibility and emergence. Our examination of pesticide exposure on a salamander/virus system exhibits key effects otherwise overlooked in single-stressor experiments. Although we did not find any significant interactions between the two pesticides, there were interactions between each pesticide and Ambystoma tigrinum virus treatments. Specifically, this study showed three key results concerning the effects of chlorpyrifos: (1) a direct lethal impact on 4-week-old salamander larvae at 200 μg/L; (2) increased susceptibility to viral infection; and (3) an increased additive mortality effect when combined with virus. Atrazine exhibited mixed results by increasing survival slightly in no-virus treatments, and yet slightly decreasing survival in virus exposed treatments. Following, we discuss each of these results in detail.
Impacts of Chlorpyrifos
The widely used insecticide chlorpyrifos has significant impacts on the survival of young tiger salamander larvae, both in the absence and presence of ATV exposure. On its own, chlorpyrifos results in a 20% reduced survival rate at the highest concentration (Figure 1). When this concentration is combined with virus, larval survival decreases >60% compared with the control. We believe this dramatic decrease is primarily the result of increased susceptibility of salamander larvae to viral infection, as suggested by an increased infection rate among exposed larvae (Figure 4), as well as high viral loads among those that died. Although no statistical interactive effect on mortality between chlorpyrifos and viral exposure was detected, >80% of dead individuals exhibited viral loads typical of a diseased individual (>1 × 106 viral copies/genome), suggesting that the combination of virus and pesticide exposure interact with one another. One would anticipate decreased viral loads with increasing pesticide concentrations if pesticides were acting separately to kill the salamanders more quickly. Instead, we see an increase in infection rate with increasing concentrations (Figure 4). Further study incorporating increased sample sizes would likely provide increased power to statistically detect an interactive effect. Nonetheless, combined ATV and chlorpyrifos exposure results in decreased survival and increased infection rate, even at the lowest tested concentration (2 μg/L) of chlorpyrifos (Figure 1).
Impacts of Atrazine
Several studies have exhibited impacts of atrazine on amphibians, including alterations of development, behavior, and survival (Hayes et al., 2002; Storrs and Kiesecker, 2004; Rohr et al., 2006). Previous work has shown that atrazine increases disease susceptibility in tiger salamanders (Forson and Storfer, 2006a). Relative to this work, our study was performed on younger larvae (4 vs. 12 weeks), thus we expected to find an even greater susceptibility to atrazine due to both reduced overall size and incompletely developed immune systems (Charlemagne, 1979). Interestingly, we found an interactive effect occurring where survival slightly decreased when atrazine was combined with virus, but actually increased when no virus was present (Figure 2). Admittedly, these slight changes (~10%) on their own may not amount to meaningful impacts to natural population dynamics. Increased mortality in the presence of combined virus and atrazine corroborates early findings on the potential negative impacts on larval susceptibility (Forson and Storfer, 2006a). The increased survival in atrazine treatments has been found in other amphibian studies (Forson and Storfer, 2006b; Storrs and Kiesecker, 2004), although the mechanism for this increased survival is not known.
Atrazine Interaction with Chlorpyrifos
Unlike previous studies (Belden and Lydy, 2000; Wacksman et al., 2006), we did not detect any enhancement of toxicity of chlorpyrifos by atrazine in control or virus-exposed treatments. This corroborates findings on two amphibian species (Rana clamitans), which also did not exhibit enhanced toxicity effects of the two pesticides when combined (Wacksman et al., 2006). The atrazine concentrations used in this study were at least five times lower than the concentration used in the study by Wacksman et al., (1,000 μg/L), and therefore enhanced toxicity on A. tigrinum might still occur at higher levels of exposure. However, our high level (200 μg/L) represents the ecologically relevant high level found in nature (Hayes et al., 2003).
Despite the lack of a synergistic effect, treatments containing virus and increasing concentrations of the two pesticides exhibited lower survival (Figure 3). A compelling result is the 50% decrease in survival between the virus exposed animals with no pesticides and those with the highest combined concentration treatments (200 μg/L of chlorpyrifos, 20 and 200 μg/L of atrazine). Areas where pulses of multiple pesticides are simultaneously introduced should have significant impacts on viral disease dynamics. In particular, ATV generally follows density-dependent dynamics (Brunner et al., 2004), and an increased proportion of the susceptible population could lead to increased severity of epizootics (Forson and Storfer, 2006a). Future examination of pesticide impacts on disease dynamics should continue to involve multiple pesticides because pesticide use is ubiquitous and pervasive, even in relatively undisturbed habitats.
Conclusions
Changes in parasite densities, as well as host susceptibility and survival, can have dramatic impacts on host-pathogen population dynamics. Slight shifts in these variables can alter the balance often maintained in host-pathogen interactions, driving one to local extinction (McCallum and Dobson, 1995; De Castro and Bolker, 2005). Introduction of pesticides into these systems might provide a key advantage to pathogens by weakening hosts (e.g., immune suppression). Indeed, emerging diseases are increasingly appreciated as a cause of host extinctions (Daszak et al., 2000; De Castro and Bolker, 2005), and the role of environmental cofactors could be important in determining how new epizootics occur.
Typically, disease susceptibility and contaminant impact on amphibians are studied separately. Understanding how these two interact is of crucial interest to deciphering a potential cause of amphibian decline. This study shows that low concentrations of commonly used pesticides can increase tiger salamander susceptibility to an emerging virus. Whereas previous studies have shown nonlethal impacts of combining nutrients and/or pesticides with disease (Johnson et al., 2007; Forson and Storfer, 2006a, b), this is the first to demonstrate lethal impact on amphibians. More field and laboratory-based studies should follow up these results to determine impacts not only in a more natural setting, but also to determine the indirect impacts on the surrounding aquatic communities. It could be that contaminant exposure plays an important, but largely overlooked, role in amphibian disease dynamics.
References
Barron MG, Woodburn KB (1995) Ecotoxicology of chlorpyrifos. Reviews of Environmental Contamination and Toxicology 144:1–93
Belden JB, Lydy MJ (2000) Impact of atrazine on organophoshate insecticide Toxicity. Environmental Toxicology and Chemistry 19:2266–2274
Berger L, Speare R, Daszak P, Green DE, Cunningham AA Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences USA 95:9031–9036
Blaustein AR, Kiesecker JM (2002) Complexity in conservation: lessons from the global decline of amphibian populations. Ecology Letters 5:597–608
Boone MD, James SM (2003) Interactions of an insecticide, herbicide and natural stressors in amphibian community mesocosms. Ecological Applications 13:829–841
Brunner JL, Schock DM, Davidson EW, Collins JP (2004) Intraspecific reservoirs: complex life history and the persistence of a lethal ranavirus. Ecology 85:560–566
Brunner JL, Richards K, Collins JP (2005) Dose and host characteristics influence virulence of ranavirus infections. Oecologia 44: 399-406
Carey C (2000) Infectious disease and worldwide declines of amphibian populations, with comments on emerging diseases in coral reef organisms and in humans. Environmental Health Perspectives 108:143–150
Carey C (1993) Hypothesis concerning the causes of the disappearance of boreal toads from the mountains of Colorado. Conservation Biology 7:355–362
Charlemagne J (1979) Thymus independent anti-horse erythrocyte antibody response and suppressor T cells in the Mexican axolotl (Amphibia, Urodela, Ambystoma mexicanum). Immunology 36:643–648
Chinchar VG (2002) Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Archives of Virology 147:447–470
Christin MS, Gendron AD, Brousseau P, Me’Nard L, Marcogliese DJ, Cyr D, Ruby S, Fournier M (2003) Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environmental Toxicology and Chemistry 22:1127–1133
Collins JP, Storfer AS (2003) Global amphibian declines: sorting the hypotheses. Diversity and Distributions 9:89–98
Collins JP, Halliday T (2005) Forecasting changes in amphibian biodiversity: aiming at a moving target. Philosophical Transactions of the British Royal Society: B 360:309–314
Colombo A, Federica O, Bonfanti P (2005) Exposure to the organophosphorus pesticide chlorpyrifos inhibits acetylcholinesterase activity and affects muscular integrity in Xenopus laevis larvae. Chemosphere 61:1665–1671
Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R (1999) Emerging infectious diseases and amphibian population declines. Emerging Infectious Disease 5:735–748
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife- threats to biodiversity and human health. Science 287:443–449
Daszak P, Cunningham AA, Hyatt AD (2003) Infectious disease and amphibian population declines. Diversity and Distributions 9:141–150
De Castro F, Bolker B (2005) Mechanisms of disease-induced extinction. Ecology Letters 8:117–126
Diana SG, Resetarits WJ, Schaeffer DJ, Beckmen KB, Beasley VR (2000) Effects of atrazine on amphibian growth and survival in artificial aquatic communities. Environmental Toxicology and Chemistry 19:2961–2967
Forson D, Storfer AS (2006a) Atrazine increases ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum. Ecological Applications 16:2325–2332
Forson D, Storfer AS (2006b) Effects of atrazine and iridovirus infection on survival and life history characteristics in long-toed salamanders, Ambystoma macrodactylum. Environmental Toxicology and Chemistry 25:168–173
Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A (2002) Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proceedings of the National Academy of Sciences USA 99:5476–5480
Hayes TB, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A (2003) Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environmental Health Perspectives 111:568–575
Hurlbert SH, Mulla MS, Keith JO, Westlake WE, Düsch ME (1970) Biological effects and persistence of Dursban in freshwater ponds. Journal of Economic Entomology 1:43–52
Jancovich JK, Davidson EW, Parameswaran N, Mao J, Chinchar VG, Collins JP, Jacobs BL, Storfer AS (2005) Evidence for emergence of an amphibian iridoviral disease because of human-enhanced spread. Molecular Ecology 14:213–224
Jancovich JK, Davidson EW, Morado JF, Jacobs BL, Collins JP (1997) Isolation of a lethal virus from the endangered tiger salamander Ambystoma tigrinum stebbinsi. Diseases of Aquatic Organisms 31:161–167
Johnson TJ, Chase JM, Dosch KL, Hartson RB, Gross JA, Larson DJ, Sutherland DR, Carpenter SR (2007) Aquatic eutrophication promotes pathogenic infection in amphibians. Proceedings of the National Academy of Sciences USA 104:15781–15786
Kiely T, Donaldson D, Grude A (2004) Pesticide industry sales and usage: 2000 and 2001 market estimates. EPA(7503C)-733-R-04-001. Final/Technical Report. U.S. Environmental Protection Agency, Washington, DC
Kiesecker JM (2002) Synergism between trematode infection and pesticide exposure: a link to amphibian limb deformities in nature? Proceedings of the National Academy of Sciences USA 99:9900–9904
Larson DL, McDonald S, Fivizzani AJ, Newton WE, Hamilton SJ (1998) Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: Duration, larval growth, and hormonal response. Physiological Zoology 71:671 – 679
Manzanti L, Rice C, Bialek K, Sparling D, Stevenson C, Johnson WE, Kangas P, Rheinstein J (2003) Aqueous-phase disappearance of atrazine, metolachlor, and chlorpyrifos in laboratory aquaria and outdoor macrocosms. Archives of Environmental Contamination and Toxicology 44:67–76
McConnell LL, LeNoir JS, Datta S, Seiber JN (1998) Wet deposition of current-use pesticides in the Sierra Nevada mountain range, California, USA. Environmental Toxicology and Chemistry 17:1908–1916
Hamish McCallum H, Dobson A (1995) Detecting disease and parasite threats to endangered species and ecosystems. Trends in Ecology and Evolution 10:190–194
Relyea R, Hoverman J (2006) Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecology Letters 9:1157–1171
Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD, Schoff PK, Beasley VR (2008) Agrochemicals increase trematode infections in a declining amphibian species. Nature doi:10.1038/nature07281
Rohr JR, Sager T, Sesterhenn TM, Palmer BD (2006) Exposure, postexposure, and density-mediated effects of atrazine on amphibians: Breaking down net effects into their parts. Environmental Health Perspectives 114:46–50
Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, La Point TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW Jr, Williams WM (1996) Ecological risk assessment of atrazine in North American surface waters. Environmental Toxicology and Chemistry 15:31–76
Storfer A, Alfaro ME, Ridenhour BJ, Jancovich JK, Mech SG, Parris MJ, Collins JP (2007) Phylogenetic concordance analysis shows an emerging pathogen is novel and endemic. Ecology Letters 10:1075–1083
Storrs SI, Kiesecker JM (2004) Survivorship patterns of larval amphibians exposed to low concentrations of atrazine. Environmental Health Perspectives 112:1054–1057
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
van den Brink PJ, van Wijngaarden RPA, Lucassen WGH, Brock TCM., Leeuwangh P (1996) Effects of the insecticide Dursban 4E (active ingredient chlorpyrifos) in outdoor experimental ditches: II. Invertebrate community responses and recovery. Environmental Toxicology and Chemistry 15:1133–1142
Wacksman MN, Maul JD, Lydy MJ (2006) Impact of atrazine on chlorpyrifos toxicity in four aquatic vertebrates. Archives of Environmental Contamination and Toxicology 51:681–689
Widder PD, Bidwell JR (2006) Cholinesterase activity and behavior in chlorpyrifos-exposed Rana sphenocephala tadpoles. Environmental Toxicology and Chemistry 25:2446–2454
Acknowledgments
The authors thank J. Baumsteiger, J. Eastman, J. Stewart, K. Benyo, and S. Spear for their help in setting up and taking down the experiment. J. Roth and J. Collins provided the salamander larvae used in this experiment. Additional support was provided by A. Hart, D. Fisher, and B. Han. Funding for this project was provided by a grant from the National Science Foundation. The authors also thank the anonymous reviewers for comments to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kerby, J.L., Storfer, A. Combined Effects of Atrazine and Chlorpyrifos on Susceptibility of the Tiger Salamander to Ambystoma tigrinum Virus. EcoHealth 6, 91–98 (2009). https://doi.org/10.1007/s10393-009-0234-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-009-0234-0