Abstract
During the past century, anthropogenic activities have altered the distribution of mercury (Hg) on the earth’s surface. The impacts of such alterations to the natural cycle of Hg can be minimized through coordinated management, policy decisions, and legislative regulations. An ability to quantitatively measure environmental Hg loadings and spatiotemporal trends of their fate in the environment is critical for science-based decision making. Here, we outline a Hg monitoring program for temperate estuarine and marine ecosystems on the Atlantic Coast of North America. This framework follows a similar, previously developed plan for freshwater and terrestrial ecosystems in the U.S. Methylmercury (MeHg) is the toxicologically relevant form of Hg, and its ability to bioaccumulate in organisms and biomagnify in food webs depends on numerous biological and physicochemical factors that affect its production, transport, and fate. Therefore, multiple indicators are needed to fully characterize potential changes of Hg loadings in the environment and MeHg bioaccumulation through the different marine food webs. In addition to a description of how to monitor environmental Hg loads for air, sediment, and water, we outline a species-specific matrix of biotic indicators that include shellfish and other invertebrates, fish, birds and mammals. Such a Hg monitoring template is applicable to coastal areas across the Northern Hemisphere and is transferable to arctic and tropical marine ecosystems. We believe that a comprehensive approach provides an ability to best detect spatiotemporal Hg trends for both human and ecological health, and concurrently identify food webs and species at greatest risk to MeHg toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic releases of mercury (Hg) to the environment have impacted the biosphere substantially, and the ocean has received a major environmental insult as a result of increased Hg releases (Mason and Sheu 2002; Sunderland and Mason 2007). Overall, it appears that Hg in the open ocean has increased substantially during the past 200 years, primarily as a result of greater atmospheric Hg deposition (Mason et al. 1994; Selin et al. 2008). Additional local- and regional-scale contamination of the coastal zone has resulted from human activities and associated runoff from the terrestrial environment (USEPA 1997; Balcom et al. 2004). Therefore, while the impact of anthropogenic emissions in North America on the open ocean may be difficult to quantify (Sunderland and Mason 2007), there is growing evidence demonstrating increased levels of Hg in U.S. estuarine and coastal waters and sediments (Mason and Lawrence 1999; Varekamp et al. 2003; Conaway et al. 2007; Fitzgerald et al. 2007). Elevated inputs of Hg are a health concern because inorganic Hg can be converted to methylmercury (MeHg) (Clarkson and Magos 2006), a neurotoxin that bioaccumulates and biomagnifies through aquatic food webs, and in some cases adversely impacting high trophic-level aquatic organisms (Wolfe et al. 1998; Scheuhammer et al. 2007; Wolfe et al. 2007). Humans are exposed to MeHg principally by the consumption of contaminated fish (Fitzgerald and Clarkson 1991; Mahaffey 1998), and elevated MeHg levels in fish have resulted in fish-consumption advisories at state, provincial, and federal levels (Rice et al. 2003; Mahaffey et al. 2004; Environment Canada 2008) because of their proven negative effects to humans (Anderson 2008). Aquatic sediments and low-oxygen waters are the main sites for the conversion of inorganic Hg to MeHg (methylation), which is mediated primarily by sulfate-reducing bacteria (Compeau and Bartha 1985; Gilmour et al. 1992). Of the many potential stressors and contaminants in the coastal environment, the issue of MeHg contamination is one of the most pervasive concerns, as evidenced by the extensive fish-consumption advisories that exist for much of the North American coastline.
The coastal zone plays a crucial role in Hg and MeHg cycles, acting as a site of inorganic Hg entrapment, MeHg formation, and high biological productivity. Sunderland and Mason (2007) estimate that about 8.2 Mmol of Hg yr−1 (range, 5.2–11.4 Mmol yr−1) is trapped in the estuarine and coastal zone, which is comparable to the estimated point source Hg inputs to the atmosphere of 11.3–16.9 Mmol yr−1. While the coastal zone is a sink for inorganic Hg, mass balance considerations suggest that estuaries and near-shore marine systems are a net source of MeHg to the coastal ocean (Cossa et al. 1996; Hammerschmidt and Fitzgerald 2004, 2006b).
While a number of field studies have focused on Hg speciation in estuarine and coastal ecosystems (e.g., Baeyens et al. 1998; Benoit et al. 1998; Kannan et al. 1998; Bloom et al. 1999; Hammerschmidt et al. 2004; Heyes et al. 2004; Sunderland et al. 2004; Hollweg et al. 2009), there has been little coordinated monitoring of Hg and MeHg changes in the coastal zone, the effect of these changes on fish MeHg levels, and on humans and wildlife. This is surprising, both because the majority of fish consumed by humans are from coastal and open-ocean marine environments (FAO 2004; Sunderland 2007), and because there are indications that Hg trends have increased over the past decade in wildlife (Rigét et al. 2007).
Structure of Monitoring Program
There is a substantial effort underway to develop a Hg monitoring program across the United States (Mason et al. 2005; Harris et al. 2007), which, while not specifically excluding the coastal zone, has a strong focus on terrestrial and freshwater environs. To augment this effort, the design of a marine monitoring program that quantifies inputs of Hg to coastal and estuarine systems, and associated exposure and impact of MeHg on organisms, is needed. In 2006, the workshop Fate and Bioavailability of Mercury in Aquatic Ecosystems and Effects on Human Exposure was convened by the Dartmouth Toxic Metals Research Program, where marine Hg scientists and human health experts were gathered to articulate research and monitoring needs (Chen et al. 2008). From that workshop, we determined that a coordinated and expanded monitoring program is needed to evaluate: (1) spatial and temporal patterns of Hg deposition and transport, (2) MeHg formation and bioaccumulation, (3) wildlife populations at risk from MeHg exposure, and (4) human exposure. These priorities are also embraced by the proposed National Mercury Monitoring Program (Harris et al. 2007). That program identified several monitoring design elements including a national distribution of 10–20 monitoring stations that would include intensive sites bounded by multiple cluster or extensive sites. Intensive sites would establish cause and effect relationships between Hg deposition and environmental change based on a comprehensive range of measurements, while cluster sites would use fewer measurements to better characterize environmental responses in varying ecosystem types. We suggest following this monitoring design for estuarine and marine ecosystems.
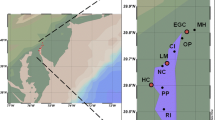
Our region of interest includes the estuarine and coastal ecosystems bounded by Chesapeake Bay north to the Gulf of Maine (Fig. 1). We organized our Hg monitoring program into four major habitat types: estuarine, coastal, semi-pelagic, and pelagic. We define these habitat types using the following criteria: (1) estuarine areas are semi-closed coastal waters that have free access to the ocean and variable, but generally shallow, water depths; (2) coastal areas extend out to a depth of 100 m (and are often within 3 nautical miles [5.6 km] from shore); (3) semi-pelagic areas represent the middle to outer continental shelf and range in water depth between 100 and 200 m (and are often located from 3 to 200 nautical miles [5.6–370.0 km] from shore but occasionally extend further); and (4) pelagic areas are open ocean waters that are >200 m in depth (and are usually >200 nautical miles [>370 km] from shore). Within these habitat types, we recognize different and relatively independent marine food webs exist that should be monitored separately for their ability to transfer MeHg to upper trophic levels. For example, benthic and demersal food webs at the bottom of the ocean need to be monitored separately from pelagic food webs higher in the water column.
Area for consideration of a standardized Hg monitoring network, including sites of potential Hg monitoring stations and their association with existing Hg deposition network stations. Using the National Atmospheric Deposition Program’s (NADP) wet deposition of chloride pattern as a proxy for marine influence on precipitation chemistry, a sharp change in influence is clearly present [David Gay, personal communication]. Marine air greatly influences precipitation chemistry within the first 400–500 km along the Gulf Coast, and less than 250 km along the mid-Atlantic and Northeast coasts of the United States and Canada. See the NADP chloride deposition measurements (http://nadp.sws.uiuc.edu/isopleths/maps2006/cldep.gif).
We have identified five categories of indicators: abiotic measurements, invertebrates, fish, birds, and mammals. Within the biotic categories, we based taxonomic selection on multiple criteria (Table 1). A description of the target taxa within each of the four major habitat types is followed by sampling strategies that identify best tissues for use and how selenium (Se) interacts with MeHg. The best indicators for evaluating toxicity and spatiotemporal trends of MeHg for protection of human health can differ from those for ecological health. In order to encompass both for fish, we created low and high trophic-level categories that reflect Hg levels for ecological and human health concerns, respectively.
There are several existing, long-term marine Hg monitoring programs. They include the National Atmospheric Deposition Program’s Mercury Deposition Network for air (Lamb and Bowersox 2000), the National Oceanographic and Atmospheric Administration’s (NOAA) Mussel Watch Program for shellfish (Chase et al. 2001), the U.S. Environmental Protection Agency’s (USEPA) National Coastal Assessment Program for shellfish and marine fish (Cunningham et al. 2003), the U.S. Geological Survey’s Contaminant Exposure and Effects—Terrestrial Vertebrates program for terrestrial wildlife (Cohen et al. 2003), and the Atlantic, Arctic and Pacific Seabird Egg Contaminant Monitoring Programs of Environment Canada (Pearce et al. 1979; Braune 2007). We use these existing programs as a basis for identifying indicators and recommending Hg monitoring locations. Broad justifications and specific methodologies not detailed in our plan appear in Harris et al. (2007). Associated abiotic measurements and scientific names for the program’s indicator organisms are provided in Tables 2 and 3.
Indicator Compartments
Abiotic Indicators
Major sources of inorganic Hg to the coastal zone include direct atmospheric deposition, rivers (including runoff of atmospherically deposited Hg), and water pollution control facilities, with most of the MeHg derived from in situ transformation of inorganic Hg (Mason et al. 1999; Balcom et al. 2004, 2008). Given these sources, it is important to measure both direct atmospheric inputs and to quantify watershed contributions of inorganic Hg to the coastal zone. Collection of sediment cores and determination of total Hg and MeHg in both surficial sediment and water at intensive sites is recommended (Table 2).
At intensive sites, event-based wet deposition collection (Driscoll et al. 2007) and Hg atmospheric speciation and deposition should be measured. While the atmospheric concentration of Hg0 is reflective of the global atmospheric Hg pool and is not a sensitive local indicator of short-term regional change (Slemr et al. 2003), levels of ionic gaseous and particulate Hg show a higher regional variability that may reflect irregular regional emissions (Schroeder and Munthe 1998; Ryaboshapko et al. 2007a, b). Ionic gaseous and particulate Hg species are, however, relatively difficult to measure (Landis et al. 2002), especially in the coastal zone (Laurier and Mason 2007), and, although there is large uncertainty in the current estimates of Hg dry deposition, new approaches are being developed (Skov et al. 2006). Atmospheric Hg speciation measurements at intensive sites should be coupled with flux estimates from other sources into the coastal zone. Specifically, measurements of groundwater and surface river water fluxes and Hg speciation should provide an ability to examine the role of Hg and MeHg transport from associated watersheds to the coastal zone.
Precipitation and other climatic variables also influence export to coastal ecosystems, especially with respect to sporadic and extreme events. Measurements of Hg in wet deposition are accomplished relatively easily and are monitored currently at the national level by the Mercury Deposition Network (MDN), which includes 11 coastal sites within our study area (MDN 2008; Fig. 1).
Changes in atmospheric Hg deposition also are recorded by sediments, and historical records of input have been inferred from the analysis of estuarine and wetland sediment cores (Varekamp et al. 2003; Conaway et al. 2007). There is a large body of experimental and observational evidence for their reliability, and well-established protocols for the collection, processing, and interpretation of these records (Porcella 1996). However, there are confounding effects of watershed input, sediment mixing (physical and bioturbation), and other factors that impact the level of temporal resolution.
While the relationship between biota and sediment Hg and MeHg levels is difficult to construct (Mason 2000), measurements of sediment MeHg provide an integrative measure of the impact of changes in Hg input and other factors on net production of MeHg (Benoit et al. 2003). MeHg in sediment and interstitial waters is available for uptake by benthic organisms and may be an important source to overlying water, either by upward diffusion or bioadvection. It has been shown, for numerous freshwater (Benoit et al. 2003) and some marine (Heyes et al. 2006; Kim et al. 2006) ecosystems, that there is a relationship between short-term Hg methylation rate measured using assays and in situ MeHg concentration or %MeHg in sediments; however, these correlations do not necessarily extend to all coastal marine deposits (Hammerschmidt and Fitzgerald 2004, 2006b; Ogrinc et al. 2007; Hammerschmidt et al. 2008). Assays of Hg methylation are not recommended for the extensive cluster sites. Nonetheless, sediment MeHg concentration and %MeHg may be useful proxies of the rate of change in bulk MeHg concentration and relative production. Measurements of %MeHg in sediment will allow assessment of whether changes of sedimentary MeHg content is related directly or indirectly to changes in atmospheric Hg input, as well as provide an abiotic indicator of potential biological exposure.
Total Hg and MeHg measurements in water, both in bulk and in the dissolved and particulate fractions, have been made in many coastal ecosystems (Kannan et al. 1998; Mason et al. 1999; Whalin et al. 2007; Balcom et al. 2008). Concentrations in water can be influenced by factors unrelated to Hg inputs, such as the variation in particulate matter and dissolved (DOC) and particulate organic carbon (POC), which are needed to improve interpretation (Table 2). Despite spatial variation in these factors, studies have shown a reasonable correlation between MeHg in water and MeHg in freshwater fish, reflecting the influences of bioaccumulation at the base of the pelagic food chain (Brumbaugh 2001). As levels of Hg species in water vary seasonally and with depth within a particular water body, these measurements must be assessed with consideration of anticipated spatial variability. Generally, the relationship between Hg and MeHg concentrations in water and estuarine and marine organisms has not been well documented and is poorly described in the literature.
Marine Invertebrate Indicators
Selection of indicator species at the base of pelagic and benthic food webs in marine systems should include invertebrate species representing different functional feeding groups, such as benthic infauna, benthic epifauna, and epi- and meso-pelagic biota (Tables 1, 3). Inclusion of both benthic and pelagic food webs facilitates an understanding of differing pathways of MeHg transfer into higher trophic levels. Studies in freshwater ecosystems suggest that pelagic feeding organisms have the ability to bioaccumulate greater concentrations of MeHg than benthic feeding fauna (Gorski et al. 2002; Power et al. 2002). However, little is known for marine systems; specifically, some areas may differ from freshwater systems in the manner MeHg is transferred to higher trophic positions.
Benthic infauna that live in the sediments of estuarine and coastal areas are useful taxa for monitoring MeHg bioaccumulation directly from ingestion or absorption from sediment. Benthic amphipods are inhabitants of many sediments and are used frequently in estuarine toxicity testing (e.g., Leptocheirus, Corophium), and which readily accumulate MeHg (Lawrence and Mason 2001). They obtain their food, and likely their MeHg, from ingesting detrital material and sediments. The most abundant sediment infauna are polychaetes.
In estuarine and coastal waters, several nonnative, epifaunal species are common. The periwinkle is a primary consumer that grazes on biofilms and periphyton on sediment and rock surfaces. The green crab also is nonnative to the U.S., but now inhabits coastal waters across the study area. It is a secondary consumer that feeds on small fish, benthic invertebrates, and detrital material. MeHg concentrations in these two species range greatly across sites and appear to be related modestly with sediment Hg levels [Chen et al., unpublished data]. Lawrence and Mason (2001) showed that the bioaccumulation factor decreased with increasing sediment organic content. Importantly, the fraction of total Hg as MeHg can vary widely in invertebrates (Tremblay et al. 1996a, b; Gorski et al. 2002; Mason and Benoit 2003).
Benthic invertebrate indicators for human health risk that inhabit estuary, coastal, and semipelagic areas include a wide variety of shellfish such as crabs, mussels, clams, oysters, and lobsters. Being important commercially harvested species, the monitoring of these taxa for certain contaminants is conducted by governmental regulatory agencies. For example, the NOAA Mussel Watch and Gulfwatch Programs have historically collected contaminant data for blue mussels, eastern oyster, and other bivalves across broad spatial and temporal scales (Chase et al. 2001), although they do not measure MeHg. Several studies have documented elevated muscle Hg concentrations in Atlantic lobster (Greig et al. 1975; Vassiliev et al. 2005; Hammerschmidt and Fitzgerald 2006a), including monitoring efforts by the USEPA (Cunningham et al. 2003). Differences in total Hg concentrations vary less than other metals for synoptically collected, co-located species (Chase et al. 2001), yet there is evidence to suggest that concentrations of MeHg vary in bivalves according to feeding strategy.
Lastly, cephalopods, such as squid are important ecologically and for human health. In the northeastern Atlantic Ocean, benthic cephalopods had significantly higher Hg levels than pelagic species (Bustamante et al. 2006). In the Mediterranean Sea, cephalopods Hg levels can be substantially higher than small fishes of similar trophic position, and appear to account for the highly elevated Hg liver body burdens in marine mammals (Frodello et al. 2000). Euphausiids are also typically relevant epi- and meso-pelagic organisms for monitoring MeHg availability (Braune 1987b; Monteiro et al. 1996).
Fish Indicators
There are numerous fish species that, when routinely sampled for Hg, are useful indicators of human and ecosystem health (Tables 1, 3). For assessing and monitoring trends in ecological health risk, we also emphasize species that are target prey for piscivorous fish and wildlife, and species that maintain sustainable and robust populations. Because there is a growing body of evidence of Hg-related effects to freshwater (Drevnick et al. 2008; LaRose et al. 2008) and estuarine fish species (del Carmen Alvarez et al. 2006), the sustainability of healthy populations of high trophic-level fish species with elevated Hg levels needs to be considered in context with negative impacts from Hg. For assessing and monitoring trends in human health risk, we emphasize commercially and/or recreationally valuable fish that have documented patterns of Hg bioaccumulation.
In estuaries, mummichogs are ubiquitous because they can withstand broad environmental conditions. They are often used for examining environmental Hg loadings (Khan and Weis 1993) and their potential ecological effects (Zhou et al. 1998). Some studies indicate they can build a tolerance to contaminants, including MeHg (Weis 2002), although individuals with elevated MeHg levels are more prone to being preyed on because of impairment to predator-avoidance behaviors (Smith and Weis 1997). While the mummichog inhabits quiet backwaters, the sand lance prefers shallow, sandy brackish waters and is an important prey item for estuarine, coastal, and even semi-pelagic birds. The striped bass is a common, recreationally fished species of estuaries and has been shown to accumulate >0.5 μg/g, ww of Hg (Davis et al. 2002; Mason et al. 2006). However, because it has multiple feeding strategies, migrates among areas of differing Hg sensitivity, and exhibits poor growth–Hg relationships, its use as an indicator species is limited. A species of less importance to the sport fishery industry, but one that has commercial and subsistence interests is the scup or porgy. It is found in estuaries and nearshore areas, and is a primary indicator species used by the USEPA National Contaminant Assessment Program (NCAP) and rarely exceeds 0.10 μg/g (ww) (Cunningham et al. 2003).
In coastal areas, the striped anchovy and butterfish are common and widespread forage fish for piscivorous wildlife. Bluefish are a recreationally important, coastal piscivore that have: (1) well-defined age–growth and growth–Hg relationships, (2) are widely distributed, and (3) been characterized for MeHg contamination in a variety of coastal waters (Ashley and Horwitz 2000; Burger et al. 2005; Hammerschmidt and Fitzgerald 2006a). The winter flounder is a highly valued species for Hg monitoring because of its recreational and commercial interests, extensive use by the NCAP, and prevalence of tumors related to contaminant exposure (Moore et al. 2004)—making it a more sensitive indicator species than the closely related summer flounder (Paralichthys dentatus). Both flounder species are routinely sampled by the NCAP and generally have fillets < 0.10 μg/g (ww) of Hg.
The source of MeHg in semipelagic and pelagic habitats is unknown. MeHg bioaccumulation in these regions may result from (1) deep ocean waters, (2) shelf, slope, and/or deeper ocean sediments, and (3) hydrothermal vents (Kraepiel et al. 2003; Lamborg et al. 2006; Hollweg et al. 2009; Liu et al. 2009), hydrologic advection (Hammerschmidt and Fitzgerald 2006b) and bioadvection from the coastal zone (Fitzgerald et al. 2007), or methylation in waters of high-biological productivity (Topping and Davies 1981; Mason and Sullivan 1999; Chen et al. 2008). Different fish species emerge as suitable indicators of MeHg availability in these offshore habitats. Ecological risk in semipelagic waters can be well documented using Atlantic herring (Braune 1987b). Measurements of Hg in sedentary, demersal species such as the Atlantic cod provide an opportunity to monitor MeHg bioaccumulation in specific locations over time (e.g., Staveland et al. 2005). Other high trophic-level species with commercial value and known Hg concentrations include the yellowfin tuna (Kraepiel et al. 2003) and related species.
For pelagic waters, the North Atlantic saury is one of the most abundant epipelagic species (<200 m depths) in our study area and serves as prey for many high trophic-level species. A more novel fish group to monitor are lanternfish that are found in mesopelagic environments (>300 m in depth) and migrate diurnally over hundreds of meters in depth. They regularly form the prey base for pelagic seabirds such as the Leach’s storm-petrel, which forage nocturnally on lanternfish at the ocean surface (Montevecchi et al. 1992). Concentrations of Hg in lanternfish have been used to support the current understanding of deepwater MeHg production in the open ocean, and to identify temporal and spatial patterns in oceanic Hg bioavailability (Monteiro et al. 1996; Martins et al. 2006).
While high trophic-level species such as the blue shark and swordfish are of commercial and recreational interest, because they can bioaccumulate concentrations of Hg that are harmful for human consumption (often >1.0 μg/g, ww; unpublished data from the U.S. Food and Drug Administration [FDA] and USEPA; Branco et al. 2007), low densities and wide-ranging abilities make their use as indicators of specific areas challenging. Although average shark Hg levels have been generically described by the FDA, some species can attain exceedingly high muscle Hg levels depending on their size, prey base, and geographic origin. For example, Garica-Hernandez et al. (2007) found smooth hammerhead sharks (Sphyrna zygaena) in the Gulf of California surpassing 21.0 μg/g (ww) in their muscle tissue. Based on δ15 N values, less variable prey bases in some shark species, such as the blue shark, make it a more preferable species to monitor Hg levels over spatiotemporal scales of interest than species with highly variable prey bases, such as the shortfin mako shark (Isurus oxyrinchus) (Estrada et al. 2003).
Sampling strategies can vary widely and depend on the target habitat and species. Fish are typically analyzed on a whole body basis for ecological health monitoring, while muscle tissue is removed and the fillet is analyzed for assessment of human exposure. Both approaches tend to analyze total Hg on a wet weight (ww) basis. Most of the Hg in fish muscle is in the methyl form (Bloom 1992). Lower trophic-level fish also provide an ability to predict MeHg transfer rates to higher trophic levels. For fish species of commercial and recreational interest, acquisition of tissue samples for Hg analysis using biopsy plugs of muscle directly at the dock is a viable and cost-effective strategy (Bank et al. 2007a). Biopsies and other nonlethal sampling methods are recommended for sharks, which have experienced tremendous declines because of overfishing (Myers et al. 2007).
Since variation in fish Hg concentrations is commonly influenced by the growth characteristics of length, age, or weight, those fish species with well-defined, growth–Hg relationships are the best candidates as indicator species. Monitoring Hg programs using fish should always acquire total length, weight, and ideally other important metrics such as age. There are other associated data that increase interpretation powers, such as Se levels and stable isotope information. The role of bioavailable Se is of growing interest to risks imparted by MeHg from fish to humans (Ralston et al. 2007).
Bird Indicators
Suggested bird species for monitoring Hg trends vary according to foraging guild (i.e., piscivore vs. invertivore) and habitat type (Tables 1, 3). Our selection criteria for birds emphasizes breeding individuals because they are generally territorial (or have small home ranges), are likely consuming prey items that have higher MeHg concentrations than in the winter (Ramlal et al. 1993; Leermakers et al. 1995), and are more reflective of local conditions compared to migrants. Summertime Hg concentrations can also be better linked to meaningful endpoints of adverse effects, such as reproductive success (Burgess and Meyer 2008; Evers et al. 2008). Use of multiple bird species for monitoring Hg provides the most comprehensive coverage for detecting changes in various habitat types and food webs that may not be predictive from one another (Pearce et al. 1989).
In estuaries, there are often species of high conservation concern (Ackerman et al. 2007) with apparently high sensitivity to Hg input (Heinz et al. 2009), and recently documented adverse reproductive effects from Hg (Schwarzbach et al. 2006). Ammodramus sparrows are recommended as indicators for estuarine invertivore food webs. One species, the saltmarsh sharp-tailed sparrow has Hg body burdens that tend to exceed those in associated songbirds (Shriver et al. 2006), and in some estuaries, lowered reproductive success is related to elevated blood Hg levels (Lane et al. 2008). Indicators of the estuarine piscivore food web include a choice of over 10 species of wading birds in our study area. Unfledged wading birds, particularly the black-crowned night-heron, is often used as the indicator age group (Rattner et al. 2000; Henny et al. 2002). Areas without wading bird sampling opportunities can be evaluated for MeHg availability in piscivores through sampling of the belted kingfisher. Kingfishers are a relatively unique indicator, as they are one of the few birds that can be used to compare MeHg availability across marine and freshwater habitats (Evers et al. 2005).
In coastal waters of the Gulf of Maine, the common eider regularly forages on the blue mussel (Wayland et al. 2001), while the piscivorous black guillemot depends on benthic fish (Butler and Buckley 2002). Mean Hg concentrations in eggs of the black guillemot are significantly greater than those in associated seabirds (Goodale et al. this issue), and because their prey occupy relatively small home ranges, guillemots are valuable indicators for characterizing distinct areas of interest. The osprey breeds along our entire study area and is an important indicator species since it is an obligate piscivore, is found across the northern hemisphere, and is commonly monitored for Hg in both freshwater (Hughes et al. 1997) and estuarine and marine ecosystems (Golden and Rattner 2003; Henny et al. 2008; Rattner et al. 2008).
The common tern is another ubiquitous piscivore that regularly forages in coastal and offshore areas and has well-described Hg body burdens (Braune 1987a; Burger et al. 1994; Nisbet et al. 2002). The common tern has Hg body burdens that are comparable to the much larger double-crested cormorant (Goodale et al. this issue). Mercury monitoring efforts with cormorant eggs are an efficient approach because of the cormorant’s common status and colonial nesting tendencies.
Describing the availability of MeHg in pelagic waters of our study area using birds is challenging. The Leach’s storm-petrel is best. Although this seabird nests on outer coastal islands in the Gulf of Maine, it forages along the continental shelf on mesopelagic organisms such as myctophids, amphipods, and euphausiids (Montevecchi et al. 1992). Atmospheric deposition of Hg on the ocean surface increasingly reflects global airshed Hg concentrations as the distance from mainland increases (Gill and Fitzgerald 1987). Blood Hg levels in the storm-petrel may provide a relatively accessible technique for monitoring changes in food web MeHg availability that is related to either global Hg pools or MeHg sources more distant from the continent.
Bird tissues regularly used for monitoring environmental Hg loads for short-term exposure are blood and eggs (usually as ww) and, for longer-term exposure, feathers (as fresh weight) (Evers et al. 2005). Feathers provide a good measure for examining long-term Hg trends (Thompson et al. 1992; Monteiro and Furness 1997). These three tissues are generally analyzed for total Hg because they are mostly representative of MeHg (Wolfe et al. 2007). Similar to fish, other measurements are typically required to best describe Hg body burdens. While bird age past the first 4–5 years is generally unknown (unless uniquely marked), the size, molt status, sex, and age class (juvenile vs. adult) are important for interpreting Hg levels (Evers et al. 2005). An understanding of Se levels and stable isotopes is also useful. The potential protective role of bioavailable Se in birds appears to be complex (Scheuhammer et al. 2008).
Mammal Indicators
Our suite of mammalian indicators includes a broad mix of terrestrial and marine taxa (Tables 1, 3). Although mustelids are used widely as indicators of MeHg availability in freshwater ecosystems (Strom 2008; Klenavic et al. 2008), two species, the American mink and North American river otter, also forage regularly in estuarine and marine ecosystems where fish are a dominant prey (Yates et al. 2005; Lake et al. 2007).
Pinnipeds are the most accessible marine mammal to be utilized as indicators for coastal habitats. Seals depend on ledges or beaches for pupping and resting, which provides a feasible method for tracking individual MeHg body burdens. Harbor seals are the most common pinnipeds in our study area, have the smallest home range compared to associated seal species, and are most accessible for sampling purposes. Their importance as a global sentinel species is also well recognized (Ross et al. 1996). Harbor seals selectively forage on small, schooling fish and squid, and their diet changes seasonally (Payne and Selzer 1989).
In semi-pelagic waters, toothed cetaceans forage on prey items such as squid and fish that are higher in the food web than krill and other invertebrates. Their Hg body burdens therefore average higher than baleen cetaceans (Hansen et al. 1990). The harbor porpoise is one of the more common and ubiquitous toothed cetacean in coastal and semi-pelagic waters, feeding on cephalopods and Atlantic herring (Fontaine et al. 1994). In more southern waters of our study area, the bottlenosed dolphin is often used as an indicator of contaminants, including Hg (Kuehl and Haebler 1995; Frodello et al. 2000).
In pelagic and more coastal waters of the northern Atlantic Ocean, pilot whales are useful indicator species because of their dietary importance to many native cultures (Andersen et al. 1987), where consumption has been regulated due to elevated Hg concentrations in muscle tissue (up to 3.3 μg/g, ww) (Weihe et al. 1996). The diet of pilot whales includes fish and cephalopods (Katona et al. 1993). Beaked whales are rarely accessible for sampling purposes unless they are stranded. Their novel value as indicators of MeHg availability in open ocean habitats is their longevity and high trophic-level position, which can result in highly elevated liver Hg concentrations (Bustamante et al. 2003).
Marine mammals are known to bioaccumulate varying levels of MeHg depending on species, diet, age, sex, reproductive status, geographic distribution, and range of ocean habitat (Nagakura et al. 1974; Gaskin et al. 1979; Dietz et al. 1996; Wagemann et al. 1998; Das et al. 2003). Multiple tissues are regularly used to characterize Hg body burdens, including skin, blubber, muscle, kidney, and liver. Muscle is more likely to contain a higher proportion of MeHg (50–100%), while liver contains a lower percentage (O’Hara et al. 2003). While sampling live marine mammals is challenging, samples taken from individuals stranded or by-catch from fish nets does provide a routinely available approach for acquiring tissues. As in fish and birds, the protective or toxic role of associated Se levels should be determined as well. Marine mammals have adapted to elevated levels of dietary MeHg by sequestering it as a nontoxic, inorganic form in the liver, often with a 1:1 ratio with Se (Koeman et al. 1973; Itano et al. 1984; Ikemoto et al. 2004).
Conclusions
A high resolution and comprehensive program for monitoring environmental Hg loads in air, sediment, and water of estuarine and marine environments, and the subsequent ecological response in invertebrates, fish, birds, and mammals, in terms of both human and ecological health concerns is described above. Our intention for developing such a detailed list of indicator compartments is to provide multiple selection options to accommodate existing monitoring efforts, and different site-specific objectives, expertise, and funding. To best detect temporal changes in environmental loading, we recommend a minimum effort to include measurements of wet Hg deposition, MeHg and percent MeHg in estuarine/marine sediment, total Hg and MeHg in young fish occupying small home ranges, high trophic-level fish of greatest local interest for human consumption, and relative breeding birds (Fig. 2).
Simplified universal food web components recommended for mercury sampling. Recommended sampling design for intensive sites includes all components. For cluster sites, include at least primary components (p) and secondary components (s) when possible. The influence of squid and other cephalopods as a transfer mechanism for MeHg in the food web may be important for higher trophic levels.
Mercury data have been amassed for several coastal areas and represent multiple compartments measured in parallel with process-level investigations, including the Chesapeake Bay (Mason et al. 1999; Lawson et al. 2001; Hollweg et al. 2009); Long Island Sound (Rolfhus and Fitzgerald 2001; Balcom et al. 2004; Hammerschmidt et al. 2004; Hammerschmidt and Fitzgerald 2006a); Acadia National Park (Bank et al. 2007b; Kahl et al. 2007); and outer Bay of Fundy (Gaskin et al. 1979; Pearce et al. 1979; Braune 1987a,b; Elliot et al. 1992; Sunderland et al. 2004, 2008; Harding et al. 2005; Ritchie et al. 2006) (Fig. 1). These locations are strong candidates for the basis of an organized, standardized monitoring program that tracks estuarine and marine environmental Hg loadings as well as watershed contributions. Within these and other areas, site considerations should encompass protected areas, which commonly serve as important shellfish beds, nursery areas for fish, and areas of wildlife conservation. Examples of such areas include National Wildlife Refuges, National Parks, National Estuarine Reserves, and The Nature Conservancy preserves.
Establishing a standardized, long-term Hg monitoring network in coastal areas is essential for monitoring and demonstrating the impact of domestically produced Hg emissions, as well as the increasing global Hg loads stemming from rapidly growing sources in Asia that, based on current models and estimates, contribute an estimated 54% of the global anthropogenic Hg emissions in 2000 (Pacyna et al. 2006). Today, those sources are projected to contribute at even higher rates, potentially offsetting emission declines in other regions of the northern hemisphere (e.g., eastern United States; Monson et al. 2009). The subsequent uncertainty for increases in the global Hg pool from Asia and elsewhere, combined with effects from climate change (e.g., Faroe Islands; Booth and Zeller 2005) and ocean acidification (Caldeira and Wickett 2003), both of which may exacerbate MeHg availability, are especially serious threats to marine-based human and ecological health. While our template for monitoring environmental Hg loading is most applicable to marine ecoregions identified by Spalding et al. (2007) in the temperate North Atlantic realm, it can be modified for other biogeographic areas by using a simplified universal food web approach (Fig. 2). While arctic realms are of increasing concern for the magnitude of Hg loadings (Lindberg et al. 2002) and related adverse implications to wildlife (Braune et al. 2006) and human health (Jewett et al. 2003), there is also evidence that marine ecoregions encompassing equatorial realms support high trophic marine inhabitants with elevated Hg levels that may reflect habitat sensitivity to environmental Hg loading (Evers et al. 2009). The threats posed by anthropogenically redistributed Hg on marine habitats require strong scientific underpinnings to understand the considerable complexities in Hg biogeochemistry and MeHg production. This understanding is critical to properly regulating and managing Hg at local, continental, and global scales. Standardized Hg monitoring programs can provide the necessary linkages between those science and policy needs.
References
Ackerman JT, Eagles-Smith CA, Takekawa JY, Demers SC, Adelsbach TL, Bluso JD, et al. (2007) Mercury concentrations and space use of pre-breeding American avocets and black-necked stilts in San Francisco Bay. Science of the Total Environment 384:452–466.
Andersen A, Julshamn K, Ringdal O, Msrkore J (1987) Trace elements intake in the Faroe Islands. II. Intake of mercury and other elements by consumption of pilot whales (Globjcephalus meleanus). Science of the Total Environment 65:63–68.
Anderson HA (2008) Eighth International Conference on Mercury as a Global Pollutant (ICMGP): human health and exposure to methylmercury. Environmental Research 107:1–3.
Ashley J, Horwitz RJ (2000) Assessment of PCBs, Selected Organic Pesticides and Mercury in Fishes from New Jersey: 1998–1999 Monitoring Program. Patrick Center for Environmental Research, The Academy of Natural Sciences, Philadelphia.
Baeyens W, Meuleman C, Muhaya B, Leermakers M (1998) Behaviour and speciation of mercury in the Scheldt estuary (water, sediments and benthic organisms). Hydrobiologia 366:63–97.
Balcom PH, Fitzgerald WF, Vandal GM, Lamborg CH, Rolfhus KR, Langer CS, et al. (2004) Mercury sources and cycling in the Connecticut River and Long Island Sound. Marine Chemistry 90:53–74.
Balcom PH, Hammerschmidt CR, Fitzgerald WF, Lamborg CH, O’Connor JS (2008) Seasonal distributions and cycling of mercury and methylmercury in the waters of New York/New Jersey Harbor estuary. Marine Chemistry 109:1–17.
Bank MS, Chesney E, Shine JP, Maage A, Senn DB (2007a) Mercury bioaccumulation and trophic transfer in sympatric snapper species from the Gulf of Mexico. Ecological Applications 17:2100–2110.
Bank MS, Burgess DS, Evers DC, Loftin CF (2007b) Mercury contamination of biota from Acadia National Park, Maine: a review. Establishing paired gauged watersheds at Acadia National Park for long-term research on acidic deposition, nitrogen saturation, forest health, and mercury biogeochemistry (1998–2002), Kahl S (editor). Environmental Monitoring and Assessment 126:105–115.
Benoit JM, Gilmour CC, Mason RP, Riedel GS, Riedel GF (1998) Behavior of mercury in the Patuxent River estuary. Biogeochemistry 40:249–265.
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In: Biogeochemistry of Environmentally Important Trace Elements. ACS Symposium Series, American Chemical Society, pp 262–297.
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Canadian Journal of Fisheries and Aquatic Sciences 49:1010–1017.
Bloom NS, Gill GA, Cappellino S, Dobbs C, McShea L, Driscoll C, et al. (1999) Speciation and cycling of mercury in Lavaca Bay, Texas, sediments. Environmental Science and Technology 33:7–13.
Booth S, Zeller D (2005) Mercury, food webs, and marine mammals: implications of diet and climate change for human health. Environmental Health Perspectives 113:521–526.
Branco V, Vale C, Canario J, Neves dos Santos M (2007) Mercury and selenium in blue shark (Prionace glauca, L. 1758) and swordfish (Xiphias gladius, L. 1758) from two areas of the Atlantic Ocean. Environmental Pollution 150:373–380.
Braune BM (1987a) Comparison of total mercury levels in relation to diet and molt for nine species of marine birds. Archives of Environmental Contaminants and Toxicology 16:217–224.
Braune BM (1987b) Mercury accumulation in relation to size and age of Atlantic Hering (Clupea harengus harengus) from the Southwestern Bay of Fundy, Canada. Archives of Environmental Contaminants and Toxicology 16:311–320.
Braune BM (2007) Temporal trends of organochlorines and mercury in seabird eggs from the Canadian Arctic, 1975–2003. Environmental Pollution 148:599–613.
Braune BM, Mallory ML, Gilchrist HG (2006) Elevated mercury levels in a declining population of ivory gulls in the Canadian Arctic. Marine Pollution Bulletin 52:978–982.
Brumbaugh WG (2001) A national pilot study of mercury contamination of aquatic ecosystems along multiple gradients: bioaccumulation in fish. Columbia, MO: U.S. Geological Survey
Burger J, Nisbet IC, Gochfeld M (1994) Heavy metal and selenium levels in feathers of known-aged common terns (Sterna hirundo). Archives of Environmental Contaminants and Toxicology 26:351–355.
Burger J, Stern AH, Gochfield M (2005) Mercury in commercial fish: optimizing individual choices to reduce risk. Environmental Health Perspectives 113:266–271.
Burgess NM, Meyer MW (2008) Methylmercury exposure associated with reduced productivity in common loons. Ecotoxicology 17:83–91.
Bustamante P, Garrigue C, Breau L, Caurant F, Dabin W, Greaves J, et al. (2003) Trace elements in two odontocete species (Kogia breviceps and Globicephala macrorhynchus) stranded in New Caledonia (South Pacific). Environmental Pollution 24:263–271.
Bustamante P, Lahaye V, Durnez C, Churlaud C, Caurant F (2006) Total and organic Hg concentrations in cephalopods from the north eastern Atlantic waters: influence of geographical origin and feeding ecology. Science of the Total Environment 368:585–596.
Butler RG, Buckley DE (2002) Black guillemot (Cepphus grylle). In: The Birds of North America Online, Poole A (editor), Ithaca, NY: Cornell Laboratory of Ornithology.
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365.
Chase ME, Jones SH, Hennigar P, Sowles J, Harding GCH, Freeman K, et al. (2001) Gulfwatch: monitoring spatial and temporal patterns of trace metal and organic contaminants in the Gulf of Maine (1991–1997) with the blue mussel, Mytilus edulis L. Marine Pollution Bulletin 42:491–505.
Chen CY, Serrell N, Evers DC, Fleishman BJ, Lambert KF, Weiss J, et al. (2008) Methylmercury in marine ecosystems: from sources to seafood consumers—a work group report. Environmental Health Perspectives. doi:10.1289/ehp.11211
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Critical Reviews in Toxicology 36:609–662.
Cohen JB, Rattner BA, Golden NH (2003) Use of retrospective data to assess ecotoxicological monitoring needs for terrestrial vertebrates residing in Atlantic coast estuaries. Ecotoxicology 12:365–375.
Compeau G, Bartha R (1985) Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Applied and Environmental Microbiology 50:498–502.
Conaway CH, Ross JRM, Looker R, Mason RP, Flegal AR (2007) Decadal mercury trends in San Francisco estuary sediments. Environmental Research 105:53–66.
Cossa D, Coquery M, Martin JM, Gobell C (1996) Mercury fluxes at the ocean margins. In: Global and Regional Mercury Cycles: Sources, Fluxes and Mass Balances, Baeyens RE, Vasiliev O (editors), Dordrecht, The Netherlands: Kluwer Academic Publishers, pp 229–248.
Cunningham P, Cooter W, Sullivan E (2003) Mercury in Marine Life Database. Washington, DC: U.S. Environmental Protection Agency, Office of Wetlands, Oceans, and Watersheds.
Das K, Debacker V, Fillet S, Bouquegneau JM (2003) Heavy metals in marine mammals. In: Toxicology of Marine Mammals, Vos J, Bossart GD, Fournier M, O’Shea T (editors), Boca Raton, FL: CRC Press, pp 135–167.
Davis JA, May MD, Greenfield BK, Fairey R, Roberts C, Ichikawa G, et al. (2002) Contaminant concentrations in sport fish from San Francisco Bay, 1997. Marine Pollution Bulletin 44:1117–1129.
del Carmen-Alvarez M, Murphy CA, Rose KA, McCarthy ID, Fuiman LA (2006) Maternal body burdens of methylmercury impair survival skills of offspring in Atlantic croaker (Micropogonias undulates). Aquatic Toxicology 80:329–337.
Dietz R, Riget F, Johansen P (1996) Lead, cadmium, mercury and selenium in Greenland marine animals. Science of the Total Environment 186:67–93.
Drevnick PA, Roberts AP, Otter RR, Hammerschmidt CR, Klaper R, Oris J (2008) Mercury toxicity in livers of northern pike (Esox luscius) from Isle Royale, U.S.A. Comparative Biochemistry and Physiology Part C 147:331–338.
Driscoll CT, Abbott M, Bullock R, Jansen J, Leonard D, Lindberg S, et al. (2007) Airsheds and watersheds. In: Harris R, Krabbenhoft D, Mason R, Murray MW, Reash R, Saltman T (eds), Ecosystem Response to Mercury Contamination: Indicators of Change. Boca Raton, FL: CRC Press, pp 13–46.
Elliott JE, Scheuhammer AM, Leighton FA, Pearce PA (1992) Heavy metal and metallothionein concentrations in Atlantic Canadian seabirds. Archives of Environmental Contamination and Toxicology 22:63–73.
Environment Canada (2008) Mercury and the Environment: Fish Consumption. Available: http://www.ec.gc.ca/MERCURY/EN/fc.cfm.
Estrada JA, Rice AN, Lutcavage ME, Skomal GB (2003) Predicting trophic position in sharks of the north-west Atlantic Ocean using stable isotope analysis. Journal of the Marine Biological Association 83:1347–1350.
Evers DC, Burgess N, Champoux L, Hoskins B, Major A, Goodale W, et al. (2005) Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 14:193–222.
Evers DC, Savoy L, DeSorbo CR, Yates D, HansonW, Taylor KM, et al. (2008) Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 17:69–81.
Evers DC, Graham RT, Perkins P, Michener R, Divoll T (2009) Mercury concentrations in the goliath grouper of Belize: an anthropogenic stressor of concern. Endangered Species Research. doi:10.3354/esr00158
[FAO] Food and Agriculture Organization (2004) The State of World Fisheries and Aquaculture, Rome: Food and Agriculture Organization of the United Nations.
Fitzgerald WF, Clarkson TW (1991) Mercury and monomethylmercury: present and future concerns. Environmental Health Perspectives 96:159–166.
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chemical Reviews 107:641–662.
Fontaine PM, Hammill MO, Barrette C, Kingsley MC (1994) Summer diet of the harbour porpoise (Phocoena phocoena) in the estuary and the northern Gulf of St. Lawrence. Canadian Journal of Fisheries and Aquatic Sciences 51:172–178.
Frodello JP, Roméo M, Viale D (2000) Distribution of mercury in the organs and tissues of five toothed-whale species of the Mediterranean. Environmental Pollution 108:447–452.
Garcia-Hernandez J, Cadena-Cardenas L, Betancourt-Lozano M, Garcia-De-La-Parra LM, Garcia-Rico LG, Marquez-Farias F (2007) Total mercury content found in edible tissues of top predator fish from the Gulf of California, Mexico. Toxicological and Environmental Chemistry 89:507–522.
Gaskin DE, Stonefield KI, Suda P, Frank R (1979) Changes in mercury levels in harbor porpoises from the Bay of Fundy, Canada, and adjacent waters during 1969–1977. Archives of Environmental Contamination and Toxicology 8:733–762.
Gill GA, Fitzgerald WF (1987) Mercury in surface waters of the open ocean. Global Biogeochemical Cycles 1:199–212.
Gilmour CC, Henry EA, Mitchell R (1992) Sulfate stimulation of mercury methylation in fresh-water sediments. Environmental Science and Technology 26:2281–2287.
Golden NH, Rattner BA (2003) Ranking terrestrial vertebrate species for utility in biomonitoring and vulnerability to environmental contaminants. Reviews of Environmental Contamination and Toxicology 176:67–136.
Goodale MW, Evers DC, Mierzykowski SE, Bond AL, Burgess NM, Otorowski CI, et al. (this issue) Marine foraging birds as bioindicators of mercury in the Gulf of Maine. EcoHealth. doi:10.1007/s10393-009-0211-7
Gorski PR, Cleckner LB, Hurley JP, Sierszen ME, Armstrong DE (2002) Factors affecting enhanced mercury bioaccumulation in inland lakes of Isle Royale National Park, USA. The Science of the Total Environment 304:327–348.
Greig RA, Wenzloff D, Shelpuk C (1975) Mercury concentrations in fish, North Atlantic offshore waters—1971. Pesticide Monitoring Journal 9:15–20.
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environmental Science and Technology 38:1487–1495.
Hammerschmidt CR, Fitzgerald WF (2006a) Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Archives of Environmental Contamination and Toxicology 51:416–424.
Hammerschmidt CR, Fitzgerald WF (2006b) Methylmercury cycling in sediments on the continental shelf of southern New England. Geochimica et Cosmochimica Acta 70:918–930.
Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Visscher PT (2004) Biogeochemistry of methylmercury in sediments of Long Island Sound. Marine Chemistry 90:31–52.
Hammerschmidt CR, Fitzgerald WF, Balcom PH, Visscher PT (2008) Organic matter and sulfide inhibit methylmercury production in sediments of New York/New Jersey Harbor. Marine Chemistry 109:165–182.
Hansen CT, Nielsen CO, Dietz R, Hansen MM (1990) Zinc, cadmium, mercury and selenium in minke whales, belugas and narwhals from West Greenland. Polar Biology 10:529–539.
Harding G, Dalziel J, Vass P (2005) Prevalence and bioaccumulation of methyl mercury in the food web of the Bay of Fundy, Gulf of Maine. In: The Changing Bay of Fundy: Beyond 400 Years, Percy JA, Evans AJ, Wells G, Rolston SJ (editors), Dartmouth, Nova Scotia and Sackville, New Brunswick, Canada: Environment Canada—Atlantic Region, Occasional Report No. 23, pp. 76–77
Harris R, Krabbenhoft DP, Mason R, Murray MW, Reash R, Saltman T (2007) Ecosystem Response to Mercury Contamination: Indicators of Change. Boca Raton, FL: CRC Press, 216 pp
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2009) Species differences in the sensitivity of avian embryos to methylmercury. Archives of Environmental Contamination and Toxicology 56:129–138
Henny CJ, Hill EF, Hoffman DJ, Spalding MG, Grove RA (2002) Nineteenth century mercury: hazard to wading birds and cormorants of the Carson River, Nevada. Ecotoxicology 11:213–231.
Henny CJ, Grove RA, Kaiser JL (2008) Osprey distribution, abundance, reproductive success and contaminant burdens along lower Columbia River, 1997/1998 versus 2004. Archives of Environmental Contamination and Toxicology 54:525–534.
Heyes A, Miller C, Mason RP (2004) Mercury and methylmercury in Hudson River sediment: impact of tidal resuspension on partitioning and methylation. Marine Chemistry 90:75–89.
Heyes A, Mason RP, Kim EH, Sunderland E (2006) Mercury methylation in estuaries: insights from using measuring rates using stable mercury isotopes. Marine Chemistry 102:134–147.
Hollweg TA, Gilmour CC, Mason RP (2009) Methylmercury production in Chesapeake Bay and mid-Atlantic continental margin sediments. Marine chemistry (in press)
Hughes KD, Ewins PJ, Clark KE (1997) A comparison of mercury levels in feathers and eggs of osprey (Pandion haliaetus) in the North American Great Lakes. Archives of Environmental Contamination and Toxicology 33:441–452.
Ikemoto T, Kunito T, Tanaka H, Baba N, Miyazaki N, Tanabe S (2004) Detoxification mechanism of heavy metals in marine mammals and seabirds: interaction of selenium with mercury, silver, copper, zinc, and cadmium in liver. Archives of Environmental Contaminants and Toxicology 47:402–413.
Itano K, Kawai S, Miyazaki N, Tatsukawa R, Fujiyama T (1984) Mercury and selenium levels in striped dolphins caught off the Pacific Coast of Japan. Agricultural and Biological Chemistry 48:1109–1116.
Jewett SC, Zhang X, Naidu AS, Kelley JJ, Dasher D, Duffy LK (2003) Comparison of mercury and methylmercury in northern pike and Arctic grayling from western Alaska rivers. Chemosphere 50:383–392.
Kahl JS, Nelson SJ, Fernandez I, Haines T, Norton S, Wiersma GB, et al. (2007) Watershed nitrogen and mercury geochemical fluxes integrate landscape factors in long-term research watersheds at Acadia National Park, Maine, USA. Environmental Monitoring and Assessment 126:9–25.
Kannan K, Smith RG, Lee RF, Windom HL, Heitmuller PT, Macauley JM, et al. (1998) Distribution of total mercury and methyl mercury in water, sediment, and fish from south Florida estuaries. Archives of Environmental Contamination and Toxicology 34:109–118.
Katona SK, Rough V, Richardson DT (1993) A Field Guide to Whales, Porpoises, and Seals from Cape Cod to Newfoundland, Washington, DC: Smithsonian Institution Press.
Khan AT, Weis JS (1993) Bioaccumulation of heavy metals in two populations of mummichog (Fundulus heteroclitus). Bulletin of Environmental Contamination and Toxicology 51:1– 5.
Kim EH, Mason RP, Porter ET, Soulen HL (2006) The impact of resuspension on sediment mercury dynamics, and methylmercury production and fate: a mesocosm study. Marine Chemistry 102:300–315.
Klenavic K, Champoux L, O’Brien M, Daoust PY, Evans RD, Evans HE (2008) Mercury concentrations in wild mink (Mustela vison) and river otters (Lontra canadensis) collected from eastern and Atlantic Canada: relationship to age and parasitism. Environmental Pollution 156:359–366
Koeman JH, Peeters WHM, Koudstaal-Hol CHM, Tjloe PS, deGoeij JJM (1973) Mercury-selenium correlations in marine mammals. Nature 245:385–386.
Kraepiel AM, Keller K, Chin HB, Malcolm E.G, Morel FM (2003) Sources and variations of mercury in tuna. Environmental Science and Technology 37:5551–5558.
Kuehl DW, Haebler R (1995) Organochlorine, organobromine, metal, and selenium residues in bottlenose dolphins (Tursiops truncatus) collected during an unusual mortality event in the Gulf of Mexico, 1990. Archives of Environmental Contamination and Toxicology 28:494– 499.
Lake JL, Ryba SA, Serbst J, Brown CF, Gibson L (2007) Mercury and stable isotopes of carbon and nitrogen in mink. Environmental Toxicology and Chemistry 26:2611–2619.
Lamb D, Bowersox V (2000) The national atmospheric deposition program: an overview. Atmospheric Environment 34:1661–1663.
Lamborg CH, Von Damm KL, Fitzgerald WF, Hammerschmidt CR, Zierenberg R (2006) Mercury and monomethylmercury in fluids from Sea Cliff submarine hydrothermal field, Gorda Ridge. Geophysical Research Letters 33:L17606..
Landis MS, Stevens RK, Schaedlich F, Prestbo EM (2002) Development and characterization of an annular denuder methodology for the measurement of divalent inorganic reactive gaseous mercury in ambient air. Environmental Science & Technology 36:3000–3009.
Lane OP, Major A, O’Brien K, Pau N, Evers DC (2008) Methylmercury Availability in New England Estuaries As Indicated by Saltmarsh Sharp-tailed Sparrow, 2004 – 2007. Report BRI 2008-11, Gorham, ME: BioDiversity Research Institute.
LaRose C, Canuel R, Lucotte M, Di Giulio R (2008) Toxicological effects of methylmercury on walleye (Sander vitreus) and perch (Perca flavescens) from lakes of the boreal forest. Comparative Biochemistry and Physiology Part C 147:139–149.
Laurier F, Mason RP (2007) Mercury concentration and speciation in the coastal and open ocean boundary layer. Journal of Geophysical Research 112:D6
Lawrence AL, Mason RP (2001) Factors controlling the bioaccumulation of mercury and methylmercury by the estuarine amphipod Leptocheirus plumulosus. Environmental Pollution 111:217–231
Lawson NM, Mason RP, Laporte JM (2001) The fate and transport of mercury, methylmercury, and other trace metals in Chesapeake Bay tributaries. Water Research 35:501–515.
Leermakers M, Meuleman C, Baeyens W (1995) Mercury speciation in the Scheldt estuary. Environmental Science and Technology 36:1245–1256.
Lindberg SE, Brooks S, Lin CJ, Scott KJ, Landis MS, Stevens RK, et al. (2002) Dynamic oxidation of gaseous mercury in the Arctic troposphere at polar sunrise. Water Air and Soil Pollution 80:641–652.
Liu B, Schaider LA, Mason RP, Bank MS, Rabalais NN, Swarzenski PW, et al. (2009) Disturbance impacts on mercury dynamics in northern Gulf of Mexico sediments. Journal of Geophysical Research. doi:10.1029/2008JG000752
Mahaffey K (1998) Methylmercury exposure and neurotoxicity. JAMA 280:737–738.
Mahaffey KR, Clickner RP, Bodurow CC (2004) Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environmental Health Perspectives 112:562–570.
Martins I, Costa V, Porteiro FM, Santos RS (2006) Temporal and spatial changes in mercury concentrations in the North Atlantic as indicated by museum specimens of glacier lanternfish Benthosemal glaciale (Pisces: Myctophidae). Environmental Toxicology 21:528–532
Mason RP (2000) The bioaccumulation of mercury, methylmercury and other toxic trace metals into pelagic and benthic organisms. In: Coastal and Estuarine Risk Assessment, Newman MC, Hale RC (editors), Boca Raton, FL: CRC Press, pp 127–149.
Mason RP, Benoit JM (2003) Organomercury compounds in the environment. In: Organometallic Compounds in the Environment, Craig PJ (editor), Chichester, UK: John Wiley & Sons.
Mason RP, Lawrence Al (1999) Concentration, distribution, and bioavailability of mercury and methylmercury in sediments of Baltimore Harbor and Chesapeake Bay, Maryland, USA. Environmental Toxicology and Chemistry 18:2438–2447.
Mason RP, Sheu GR (2002) Role of the ocean in the global mercury cycle. Global Biogeochemical Cycles 16:40–41.
Mason RP, Sullivan KA (1999) The distribution and speciation of mercury in the South and equatorial Atlantic. Deep Sea Research 46:937–956.
Mason RP, Fitzgerald WF, Morel FMM (1994) The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochimica et Cosmochimica Acta 58:3191–3198.
Mason RP, Lawson NM, Lawrence AL, Leaner JJ, Lee JG, Sheu GR (1999) Mercury in the Chesapeake Bay. Marine Chemistry 65:77–96.
Mason RR, Abbott ML, Bodaly RA, Bullock OR, Evers DC, Lindberg SE, et al. (2005) Monitoring the response to changing mercury deposition. Environmental Science and Technology 39:14A–22A.
Mason RP, Heyes D, Sveinsdottir A (2006) Methylmercury concentrations in fish from tidal waters of the Chesapeake bay. Archives of Environmental Contamination and Toxicology 51:425–437.
[MDN] Mercury Deposition Network (2008) The Mercury Deposition Network webpage. Available: http://nadp.sws.uiuc.edu/mdn/
Monson BA (2009) Trend reversal of mercury concentrations in piscivorous fish from Minnesota lakes: 1982–2006. Environmental Science and Technology. doi:10.1021/es8027378
Monteiro LR, Furness RW (1997) Accelerated increase in mercury contamination in North Atlantic mesopelagic food chains as indicated by time series of seabird feathers. Environmental Toxicology and Chemistry 16:2489–2493.
Monteiro LR, Costa V, Furness RW, Santos RS (1996) Mercury concentrations in prey fish indicate enhanced bioaccumulation in mesopelagic environments. Marine Ecology Progress Series 141:21–25.
Montevecchi WA, Birt-Friesen VL, Cairns DK (1992) Reproductive energetics and prey harvest of Leach’s storm-petrels in the Northwest Atlantic. Ecology 73:823–832.
Moore M, Lefkovitz L, Hall M, Hillman R, Mitchell D, Burnett J (2004) Reduction in organic contaminant exposure and resultant hepatic hydropic vacuolation in winter flounder (Pseudopleuronectes americanus) following improved effluent quality and relocation of the Boston sewage outfall into Massachusetts Bay, USA: 1987–2003. Marine Pollution Bulletin 50:156–166.
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315:1846–1850.
Nagakura K, Amira S, Kurihara M, Koga T, Fujita T (1974) Mercury content of whales. Bulletin of the Tokai Regional Fisheries Research Laboratory 78:41–46.
Nisbet ICT, Montoya JP, Burger J, Hatch JJ (2002) Use of stable isotopes to investigate individual differences in diets and mercury exposures among common terns Sterna hirundo in breeding and wintering grounds. Marine Ecology Progress Series 242:267–274.
Ogrinc N, Monperrus M, Kotnik J, Fajon V, Vidimova K, Amouroux D, et al. (2007) Distribution of mercury and methylmercury in deep-sea surficial sediments of the Mediterranean Sea. Marine Chemistry 107:31–48.
O’Hara TM, Woshner V, Bratton G (2003) Inorganic pollutants in Arctic marine mammals. In: Toxicology of Marine Mammals, Vos J, Bossart GD, Fournier M, O’Shea TJ (editors), London: Taylor and Francis.
Pacyna E.G, Pacyna JM, Steenhuisen F, Wilson S (2006) Global anthropogenic mercury emission inventory for 2000. Atmospheric Environment 40:4048–4063.
Payne PM, Selzer LA (1989) The distribution, abundance and selected prey of the harbor seal, Phoca vitulina concolor, in southern New England. Marine Mammal Science 5:173–192.
Pearce PA, Peakall DB, Reynolds LM (1979) Shell thinning and residues of organochlorines and mercury in seabird eggs, eastern Canada, 1970–76. Pesticide Monitoring Journal 13:61–68.
Pearce PA, Elliott JE, Peakall DB, Norstrom RJ (1989) Organochlorine contaminants in eggs of seabirds in the northwest Atlantic, 1968–1984. Environmental Pollution 56:217–235.
Porcella D (1996) Protocol for Estimating Historic Atmospheric Mercury Deposition. Electric Power Research Institute Technical Report-TR-106768, Palo Alto, CA
Power M, Klein GM, Guiguer KR, Kwan MKH (2002) Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. Journal of Applied Ecology 39:819–830.
Ralston NVC, Blackwell JL, Raymond LJ (2007) Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biological Trace Element Research 119:255–268.
Ramlal PS, Kelly CA, Rudd JWM, Furutani A (1993) Sites of methyl mercury production in remote Canadian Shield lakes. Canadian Journal of Fisheries and Aquatic Sciences 50:972–979.
Rattner BA, Hoffman DJ, Melancon MJ, Olsen GH, Schmidt SR, Parsons KC (2000) Organochlorine and metal contaminant exposure and effects in hatching black-crowned night herons (Nycticorax nycticorax) in Delaware Bay. Archives of Environmental Contamination and Toxicology 39:38–45.
Rattner BA, Golden NH, Toschik PC, McGowan PC, Custer TW (2008) Concentrations of metals in blood and feathers of nestling ospreys (Pandion haliaetus) in Chesapeake and Delaware Bays. Archives of Environmental Contamination and Toxicology 54:114–122.
Rice DC, Schoeny R, Mahaffey K (2003) Methods and rationale for derivation of a reference dose for methylmercury by the US EPA. Risk Analysis 23:107–115.
Rigét F, Dietz R, Born EW, Sonne C, Hobson KA (2007) Temporal trends of mercury in marine biota of west and northwest Greenland. Marine Pollution Bulletin 54:72–80.
Ritchie CD, Richards W, Arp PA (2006) Mercury in fog on the Bay of Fundy (Canada). Atmospheric Environment 40:6321–6328.
Rolfhus KR, Fitzgerald WF (2001) The evasion and spatial/temporal distribution of mercury species in Long Island Sound, CT–NY. Geochimica et Cosmochimica Acta 65:407–418.
Ross P, DeSwart R, Addison R, Van Loveren H, Vos J, Osterhaus A (1996) Contaminant-induced immunotoxicology in harbour seals: wildlife at risk. Toxicology 112:157–169
Ryaboshapko A, Bullock OR, Christensen J, Cohen M, Dastoor A, Ilyin I, et al. (2007a) Intercomparison study of atmospheric mercury models: 1. Comparison of models with short-term measurements. Science of the Total Environment 376:228–240.
Ryaboshapko A, Bullock OR, Christensen J, Cohen M, Dastoor A, Ilyin I, et al. (2007b) Intercomparison study of atmospheric mercury models: 2. Modelling results vs. long-term observations and comparison of country deposition budgets. Science of the Total Environment 377:319–333.
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO: A Journal of the Human Environment 36:12–19.
Scheuhammer AM, Basu N, Burgess NM, Elliott JE, Campbell GD, Wayland M, et al. (2008) Relationships among mercury, selenium, and neurochemical parameters in common loons (Gavia immer) and bald eagles (Haliaeetus leucocephalus). Ecotoxicology 17:93–101.
Schroeder WH, Munthe J (1998) Atmospheric mercury—an overview. Atmospheric Environment 32:809–822.
Schwarzbach SE, Albertson JD, Thomas CM (2006) Effects of predation, flooding, and contamination on reproductive success of California clapper rails (Rallus longirostris obsoletus) in San Francisco Bay. Auk 123:45–60.
Selin NE, Jacob DJ, Yantosca RM, Strode S, Jaeglé L, Sunderland EM (2008) Global 3-D land-ocean-atmosphere model for mercury: present-day vs. preindustrial cycles and anthropogenic enhancement factors for deposition. Global Biogeochemical Cycles 22; doi: 10.1029/2007GB003040
Shriver G, Evers DC, Hodgman TP, MacCulloch BJ, Taylor RJ (2006) Mercury in sharp-tailed sparrows breeding in coastal wetlands. Environmental Bioindicators 1:129–135.
Skov H, Brooks SB, Goodsite ME, Lindberg SE, Meyers TP, Landis MS, et al. (2006) Fluxes of reactive gaseous mercury measured with a newly developed method using relaxed eddy accumulation. Atmospheric Environment 40:5452–5463.
Slemr F, Brunk E.G, Ebinghaus R, Temme C, Munthe J, Wangberg I, et al. (2003) Worldwide trend of atmospheric mercury since 1977. Geophysical Research Letters 30:23.1–23.4
Smith GM, Weis JS (1997) Predator-prey relationships in mummichogs (Fundulus heteroclitus (L.)): Effects of living in a polluted environment. Journal of Experimental Marine Biology and Ecology 209:75–87.
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, et al. (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57:573–583.
Staveland G, Marthinsen I, Norheim G, Julshamn K (2005) Levels of environmental pollutants in flounder (Platichthys flesus L.) and cod (Gadus morhua L.) caught in the waterway of Glomma, Norway. II. Mercury and arsenic. Archives of Environmental Contamination and Toxicology 24:187–193.
Strom SM (2008) Total mercury and methylmercury residues in river otters (Lutra canadensis) from Wisconsin. Archives of Environmental Contamination and Toxicology 54:546–554.
Sunderland EM (2007) Mercury exposure from domestic and imported estuarine and marine fish in the US seafood market. Environmental Health Perspectives 115:235–242.
Sunderland EM, Mason RP (2007) Human impacts on open ocean mercury concentrations. Global Biogeochemical Cycles 21. doi:10.1029/2006GB002876
Sunderland EM, Gobas FAPC, Heyes A, Branfireun BA, Bayer AK, Cranston RE, et al. (2004) Speciation and bioavailability of mercury in well-mixed estuarine sediments. Marine Chemistry 90:91–105.
Sunderland EM, Cohen MD, Selin NE, Chmura GL (2008) Reconciling models and measurements to assess trends in atmospheric mercury deposition. Environmental Pollution 156:526–535
Thompson DR, Furness RW, Walsh PM (1992) Historical changes in mercury concentrations in the marine ecosystem of the north and north-east Atlantic Ocean as indicated by seabird feathers. The Journal of Applied Ecology 29:79–84.
Topping G, Davies IM (1981) Methylmercury production in the marine water column. Nature 290:243–244.
Tremblay A, Lucotte M, Meili M, Cloutier L, Pichet P (1996a) Total mercury and methylmercury contents of insects from boreal lakes: ecological, spatial and temporal patterns. Water Quality Research Journal of Canada 31:851–873.
Tremblay A, Lucotte M, Rheault I (1996b) Methylmercury in a benthic food web of two hydroelectric reservoirs and a natural lake of northern Quebec (Canada). Water Air and Soil Pollution 91:255–26.
United State Environmental Protection Agency [USEPA] (1997) The Incidence and Severity of Sediment Contamination in Surface Water of the United States, EPA 823-R–97-006, -007, -008, Washington, DC: Office of Science and Technology.
Varekamp JC, Kreulen B, ten Brink MRB, Mecray EL (2003) Mercury contamination chronologies from Connecticut wetlands and Long Island Sound sediments. Environmental Geology 43:268–282.
Vassiliev T, Bayer R, Congelton W, Bushway R, Vetlino J (2005) Heavy metal concentrations in lobster (Homarus americanus). Journal of Shellfish Research 24:680–681.
Wagemann R, Trebacz E, Boila G, Lockhart WL (1998) Methylmercury and total mercury in tissues of arctic marine mammals. The Science of the Total Environment 218:19–31.
Wayland M, Garcia-Fernandez AJ, Neugebauer E, Gilchrist HG (2001) Concentrations of cadmium, mercury and selenium in blood, liver and kidney of common eider ducks from the Canadian Arctic. Environmental Monitoring and Assessment 71:255–267.
Weihe P, Grandjean P, Debes F, White R (1996) Health implications for Faroe Islanders of heavy metals and PCBs from pilot whales. The Science of the Total Environment 186:141–148.
Weis J (2002) Tolerance to environmental contaminants in the mummichog, Fundulus heteroclitus. Human and Ecological Risk Assessment 8:933–953.
Whalin L, Kim EH, Mason R (2007) Factors influencing the oxidation, reduction, methylation and demethylation of mercury in coastal waters. Marine Chemistry 107:278–294.
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environmental Toxicology and Chemistry 17:146–160.
Wolfe MF, Atkeson T, Bowerman W, Burger K, Evers DC, Murray MW, et al. (2007) Wildlife indicators. In: Ecosystem Response to Mercury Contamination: Indicators of Change, Harris R, Krabbenhoft DP, Mason R, Murray MW, Reash R, Saltman T (editors), SETAC, Webster, NY: CRC Press, pp 123–189.
Yates D, Mayack D, Munney K, Evers DC, Taylor RJ, Kaur T, et al. (2005) Mercury levels in mink and river otter in northeastern North America. Ecotoxicology 14:263–274.
Zhou T, Weis P, Weis JS (1998) Mercury burden in two populations of Fundulus heteroclitus after sublethal methylmercury exposure. Aquatic Toxicology 42:37–47.
Acknowledgments
Through a grant from the National Institute of Environmental Health Sciences, Dartmouth College organized a Hg workshop in November 2006 that provided the opportunity to discuss and describe a standardized marine mercury monitoring network based on the consensus of a group of interdisciplinary mercury scientists. We thank Wing Goodale of BioDiversity Research Institute for expertly generating the study area map, and David Gay, Coordinator for the National Atmospheric Deposition Program, which includes the Mercury Deposition Network. Effort toward manuscript preparation was partially supported by NIH Grant Number P42 ESO7373 from the NIEHS and the RI-INBRE Grant Number P20RR016457 from NCRR, NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evers, D.C., Mason, R.P., Kamman, N.C. et al. Integrated Mercury Monitoring Program for Temperate Estuarine and Marine Ecosystems on the North American Atlantic Coast. EcoHealth 5, 426–441 (2008). https://doi.org/10.1007/s10393-008-0205-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-008-0205-x