Abstract
Purpose
To explore the efficacy and safety of pegaptanib sodium as maintenance therapy in Japanese patients with neovascular, age-related macular degeneration (AMD) after induction therapy (LEVEL-J study).
Methods
A multi-center, prospective study was conducted at 21 medical institutions between 2009 and 2011. Of Japanese neovascular AMD patients with choroidal neovascularization who showed improvement in visual acuity (VA) with induction therapy, those who were scheduled for intravitreal injections of pegaptanib as maintenance therapy were recruited. LogMAR VA was assessed. Booster treatment (unscheduled treatment with other agents) was allowed during the study period if symptoms were judged to have worsened. Safety was assessed by monitoring adverse events and intraocular pressure (IOP).
Results
Of 75 patients included in the analysis, 80 % completed the 54-week study period. Their mean age was 74.7 ± 6.9 years, and 54 patients (72.0 %) were men. The mean number of pegaptanib injections was 5.7 ± 2.6. Booster treatment was not required in 40 eyes (53.3 %). Mean logMAR VA was 0.61 ± 0.31 before induction therapy, 0.26 ± 0.24 before maintenance therapy, and 0.29 ± 0.28 at 54 weeks. No notable change in VA was observed during maintenance therapy. Adverse events were reported in 4 patients (5.3 %), including increased intraocular pressure, cancer, gallstones and recurrence of breast cancer, but mean IOP remained stable during maintenance therapy.
Conclusions
The results of this exploratory study suggest that maintenance therapy with pegaptanib is potentially an effective and well-tolerated option in Japanese patients with neovascular AMD in whom induction therapy has been successful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neovascular age-related macular degeneration (AMD) with choroidal neovascularization (CNV) is characterized by central scotoma, image distortion, and decreased visual acuity (VA) [1, 2]. In particular, when CNV involves the central fovea, social blindness often occurs. Neovascular AMD is a major cause of blindness in the elderly in developed countries throughout the world, including Japan [2–5]. The likelihood of onset increases with age, and the medical prominence of neovascular AMD is increasing each year [6, 7]. Although treatment was previously limited to photodynamic therapy (PDT), since the development of drugs for intravitreal (IVT) injection that specifically target vascular endothelial growth factor (VEGF), the treatment of neovascular AMD has changed dramatically. In Japan, pegaptanib sodium (Macugen, Pfizer, New York, NY, USA) was approved in 2008, and ranibizumab (Lucentis, Novartis, East Hanover, NJ, USA) was approved in 2009. Currently, both of these drugs are used in clinical practice. Pegaptanib is an RNA aptamer that targets VEGF165 [8], and ranibizumab is a monoclonal antibody fragment that binds to all VEGF isoforms [9, 10]. Because ranibizumab inhibits all VEGF, unlike pegaptanib, which does not inhibit other VEGF-A activity such as VEGF121, its antiangiogenic effects are potent. However, normal VEGF-A activity may be blocked, so systemic safety, such as an increased risk of cardiovascular disease, is a concern [11]. On the other hand, because pegaptanib has a lower risk of systemic adverse effects than ranibizumab, it is considered suitable when long-term efficacy must be safely maintained.
Treatment with VEGF inhibitors requires an induction phase to inhibit CNV activity and improve VA, and a maintenance phase to preserve VA after improvement, in which multiple drug injections are required. A small-scale clinical trial in the United States reports promising results after induction therapy with a non-selective VEGF inhibitor, followed by maintenance therapy with pegaptanib and, when necessary, at the physician’s discretion, unscheduled therapy (booster treatment) with other drugs [12, 13]. Therefore, an exploratory study (Evaluation of efficacy and safety in maintaining visual acuity with sequential treatment of neovascular AMD: LEVEL) was conducted to apply this method of treatment on a larger scale [14]. In order to discuss the efficacy of maintenance therapy with pegaptanib after improvement of VA with induction therapy in Japanese patients, a similar study in Japan was necessary.
Here, a prospective, exploratory study was conducted in Japanese AMD patients in whom VA had improved with induction therapy and who were to receive pegaptanib as maintenance therapy. The purpose of this study was to explore the efficacy and safety of pegaptanib as a maintenance therapy in Japanese patients.
Patients and methods
Patients
Selected from AMD patients ≥50 years of age with subfoveal CNV, this study included patients scheduled for IVT injections of pegaptanib every 6 weeks for 48 weeks. The main inclusion criteria were patients with AMD, 30–120 days before enrollment in the study, who, as a result of induction therapy with (1) ranibizumab 1–3 times, (2) PDT 1–2 times, (3) pegaptanib 1–3 times, or a suitable combination of (1) to (3), had VA improvement ≥0.2 logMAR. The main exclusion criteria were: subfoveal scarring or atrophy; subfoveal hemorrhage of the treated eye; extending to hemorrhage ≥50 % of the entire lesion or ≥1 disc area; opacification of the optic media; other causes leading to CNV; presence or complications of diabetic retinopathy; severe cardiac disease (New York Heart Association class III or IV); clinically significant peripheral vascular disease; or a stroke within 12 months before enrollment.
Study design
This was a multi-center, prospective study conducted at 21 institutions in Japan from April 2009 to December 2011. Approval was obtained from the ethics committee at each participating institution, and written informed consent was obtained from all patients who participated in the study.
Pegaptanib was administered according to the package insert, generally at a dose of 0.3 mg/eye by IVT injection, every 6 weeks, from day 0 until 48 weeks. If symptoms were judged to have worsened, then unscheduled treatment with other agents (booster treatment) was allowed at the physician’s discretion. Because this study was conducted under real clinical conditions, the dosing interval could also be changed within 7 days before and after the scheduled date of administration at the physician’s discretion.
Visual acuity and central point thickness (CPT) measured by optical coherence tomography (OCT) were assessed on day 0 (before the start of maintenance therapy) as the baseline and then every 6 weeks for 54 weeks. Before and ≥30 min after injection, intraocular pressure (IOP) and adverse events were assessed.
Statistical analysis
Patients who met the inclusion and exclusion criteria and who received the study drug at least once during the study were included for analysis. When using decimal VA, it was converted to logMAR values, and basic (descriptive) statistics were calculated at each evaluation. The proportions of subjects were calculated based on changes in VA before starting induction therapy to week 54 of maintenance therapy at 3 levels: gain of ≥15 letters, gain of ≥0, and loss of <15 letters. For CPT, basic statistics were also calculated.
In addition to calculating the number of injections, basic statistics for the number of induction treatments, VA before induction treatment, baseline VA, and CPT were also calculated according to booster treatment status (boosted or non-boosted), in order to evaluate factors related to whether or not booster treatment was required. To analyze safety, the frequency of adverse events and basic statistics for IOP were calculated.
As the nature of this study was exploratory rather than confirmatory, statistical analyses were mainly performed in a descriptive manner. No statistical power approach for determining sample size was applied.
Results
A total of 77 patients (77 eyes) were enrolled from 16 medical institutions. Excluding one patient (one eye) who did not meet the inclusion criteria, and one patient (one eye) who asked to withdraw from the study before starting maintenance therapy, 75 patients (75 eyes) were analyzed. Of these, 60 patients (80 %) completed the 54-week study period; 15 patients (20 %) could not be followed up until the 54th week because of patient request (nine eyes, 60.0 %), treatment for adverse events (three eyes, 20.0 %), missed follow-up visits (two eyes, 13.3 %), and missing data (one eye, 6.7 %).
Patients’ characteristics
Table 1 shows the patients’ characteristics. The patients’ ages ranged from 58 to 92 years, with a mean age of 74.7 years; 54 (72.0 %) patients were men. There were 43 patients (57.3 %) with a medical history, most commonly hypertension (27 patients, 62.8 %) and diabetes (4 patients, 9.3 %). The AMD diagnosis was: typical AMD in 50 eyes; polypoidal choroidal vasculopathy (PCV) in 21 eyes; and retinal angiomatous proliferation (RAP) in four eyes. The most common results of fluorescein angiography of typical AMD were “occult with no classic features” (32 eyes), followed by “predominantly classic” (10 eyes) and “minimally classic” (8 eyes).
For induction therapy, 56 eyes (74.7 %) received ranibizumab, 2 eyes (2.7 %) received pegaptanib, 3 eyes (4.0 %) received PDT, and 14 eyes (18.7 %) received a combination of ranibizumab and PDT. Induction therapy was performed over 14 weeks (median), and during this time, patients received a mean of 2.8 treatments. After induction therapy, CPT was 86–817 μm (mean ± SD 228.5 ± 101.4 μm). CPT was ≤200 μm in 31 eyes (41.9 %) and >225 μm in 28 eyes (37.8 %).
Maintenance therapy
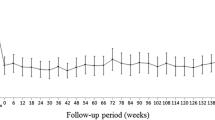
With induction therapy, VA improved ≥0.3 logMAR. LogMAR VA (mean ± SD) was 0.61 ± 0.31 before induction therapy and 0.26 ± 0.24 before maintenance therapy. During maintenance therapy, logMAR did not change greatly and was 0.29 ± 0.28 at 54 weeks. The improvement in mean logMAR VA obtained during induction was well sustained (Fig. 1). For VA before induction therapy and at week 54 of maintenance therapy, 33 eyes (55.0 %) had ≥15-letter improvement, and 59 eyes (98.3 %) had ≥0-letter improvement. Only one eye had lost <15 letters in VA (logMAR VA: before induction therapy 0.30; 54 weeks 0.40). During maintenance therapy, mean retinal thickness did not change substantially (Fig. 2).
Mean logMAR visual acuity from before starting induction therapy to week 54 of maintenance therapy. Closed circles indicate mean logMAR visual acuity; error bars indicate standard error of the mean; and a vertical broken line indicates the baseline (evaluation before maintenance therapy). Comparison before induction therapy vs. before maintenance therapy: P < 0.0001 (paired t-test, two-sided). Comparison before induction therapy vs week 54 of maintenance therapy: P < 0.0001 (paired t-test, two-sided)
During maintenance therapy, a mean of 7.1 ± 2.6 injections was given. The number of pegaptanib injections was 5.7 ± 2.6 (median 6, range 1–9), and 13 eyes (17.3 %) received the maximum number (9) of pegaptanib injections. In 40 eyes (53.3 %), booster treatment was not required. In patients who received booster treatment (which was coincidentally ranibizumab including one patient who received a combination of ranibizumab and PDT), the number of days from before the start of maintenance therapy until the first booster was given was 135.3 ± 94.6 days. In these patients, the mean number of booster treatments was 3.0 ± 1.9 times (median 2, range 1–7), and the most frequent number of booster treatments was one (10 eyes, 28.6 %), followed by two (8 eyes, 22.9 %). The number of induction treatments, VA before induction treatment, baseline VA, and CPT were compared according to boosted vs non-boosted, but none of these was related to whether booster treatment was given (Fig. 3).
Factors before starting maintenance therapy and relationship to whether booster treatment was given. a Mean number of induction treatments, P = 0.3643 for between-group comparison. b Mean logMAR visual acuity before induction treatment, P = 0.5558 for between-group comparison. c Mean logMAR visual acuity at baseline, P = 0.9952 for between-group comparison. d Mean central point thickness at baseline, P = 0.8753 for between-group comparison. For comparison between groups, an unpaired t-test (two-sided) was used. Error bars indicate the standard error of the mean
Safety
Adverse events occurred in 4 of 75 patients (5.3 %), including one event each of increased IOP, cancer, gallstones, and breast cancer recurrence. As an adverse reaction, increased IOP occurred in one patient (1.3 %). Slightly increased IOP was sustained ≥30 min after injection, but mean IOP remained stable during maintenance therapy (Fig. 4).
Discussion
The present study was conducted using a similar design to the LEVEL study performed in the USA [14]. In the LEVEL study, in neovascular AMD patients who had one to three induction treatments 30–120 days before study enrollment and who had clinical/anatomical improvement, the efficacy and safety of maintenance therapy with pegaptanib were investigated. As maintenance therapy, pegaptanib 0.3 mg was administered by IVT injection every 6 weeks for 48 weeks, with follow-up to 54 weeks. Booster treatment was allowed for clinical deterioration at the physician’s discretion. Of 568 patients in the LEVEL study, 86 % completed 1 year of treatment with pegaptanib. Mean logMAR VA improvement during induction (improvement of 49.6–65.5 letters) was well preserved (54-week mean, 61.8 letters). From before induction until week 54, 41 % of patients had ≥15-letter improvement on an ETDRS VA chart. During maintenance, mean CPT was relatively stable. About half of the patients did not receive booster treatment up to week 54. Pegaptanib was well tolerated, and there were almost no ocular or systemic adverse events. IOP remained stable from the start to completion of treatment. Although direct comparison of the present study’s results with the LEVEL study results may not be appropriate, the efficacy of pegaptanib as maintenance therapy in Japanese patients in the present study using a similar treatment method is noteworthy.
Problems of safety with VEGF inhibitors include ocular and systemic adverse reactions. In this study, only 1 of 75 eyes (1.3 %) had increased IOP. Currently, post-marketing surveillance of pegaptanib in all patients is being conducted in Japan (target sample size: 3,500 patients, 3,500 eyes), and based on interim results, of the 1,759 patients included in the safety analysis, 43 (2.4 %) have had adverse reactions. Most of these have been ocular events such as increased IOP, with almost no systemic events [15]. In a phase II trial in Japan, the adverse reactions that occurred during a 1-year treatment period were almost all ocular events judged to be attributed to the administration technique [16]. Subsequently, in a study where treatment was continued for another year, most adverse reactions were also related to the administration technique [17].
Systemic adverse reactions are particularly problematic in AMD patients, because of a high risk of hypertension, stroke, and cardiovascular disease [11]. In patients who already have these complications, the risk of further exacerbation is high. Based on the results of a clinical study of ranibizumab, which is a non-selective VEGF inhibitor, the incidence of other than ocular events such as bleeding and cerebrovascular events, compared to a sham injection, was reported to be higher [18, 19]. However, during a 1- to 2-year study, evaluation of the effect of long-term use of VEGF inhibitors is difficult [20]. Pegaptanib was used continuously for ≥4 years in one clinical study, but no warnings about ocular or systemic safety were issued [21, 22]. Moreover, even in a study at doses 10 times higher than normally used clinically, no increased risk of systemic adverse reactions was observed [23].
In the present study, more than half of the patients (57.3 %) had some positive medical history, including 27 (36 %) with hypertension and 4 (5.3 %) with diabetes, but no serious complications such as thrombus formation were reported (Table 2). Although the number of cases was limited, the present study results suggest that pegaptanib maintenance therapy is a treatment method that can safely preserve, over the long term, improved VA obtained during induction therapy. For neovascular AMD patients with cardiovascular risk, given the need for long-term therapy, exposure to non-selective inhibition should ideally be minimized. The present results support the fact that maintenance therapy with pegaptanib after induction, even though booster treatment was required in at least half of the patients, can be an alternative to long-term treatment with a non-selective VEGF inhibitor. Attempts to achieve equivalent results to the effectiveness obtained with once-monthly injections of ranibizumab by decreasing the frequency of administration have not always been successful [24, 25]. This suggests that, to optimally preserve VA, regular frequent administration of a VEGF inhibitor is necessary.
In conclusion, the present study suggests that maintenance therapy with pegaptanib is potentially an effective and well-tolerated option in Japanese patients with neovascular AMD in whom induction therapy has been successful. Although the nature of this study was descriptive or exploratory, this type of study is very useful for constructing hypotheses leading to subsequent studies and for establishing novel methods of treatment [26, 27]. However, it should be noted that the results of this type of study do not demonstrate a causal response to the treatment used. For more definitive evidence, a larger-scale comparative study will need to be conducted.
References
Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205–10.
Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116:653–8.
Oshima Y, Ishibashi T, Murata T, Tahara Y, Kiyohara Y, Kubota T. Prevalence of age related maculopathy in a representative Japanese population: the Hisayama study. Br J Ophthalmol. 2001;85:1153–7.
Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9,980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111:53–61.
Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Eye Diseases Prevalence Research Group, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72.
Bressler NM, Bressler SB, Congdon NG, Ferris FL 3rd, Friedman DS, Klein R, Age-Related Eye Disease Study Research Group, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–4.
Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–60.
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, MARINA Study Group, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, ANCHOR Study Group, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Tuñón J, Ruiz-Moreno JM, Martín-Ventura JL, Blanco-Colio LM, Lorenzo O, Egido J. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv Ophthalmol. 2009;54:339–48.
Hughes MS, Sang DN. Safety and efficacy of intravitreal bevacizumab followed by pegaptanib maintenance as a treatment regimen for age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2006;37:446–54.
Farah SE. Treatment of neovascular age-related macular degeneration with pegaptanib and boosting with bevacizumab or ranibizumab as needed. Ophthalmic Surg Lasers Imaging. 2008;39:294–8.
Friberg TR, Tolentino M, Weber P, Patel S, Campbell S, Goldbaum M, LEVEL Study Group. Pegaptanib sodium as maintenance therapy in neovascular age-related macular degeneration: the LEVEL study. Br J Ophthalmol. 2010;94:1611–7.
Pfizer PRO. Interim report of Macugen PMS. http://pfizerpro.jp/cs/sv/pms/pmsC_D/generalcontents/1259675425953 (Accessed 3 Aug 2012).
Tano Y; Pegaptanib Sodium Multi-center Study Group. Pegaptanib sodium one-year treatment study for neovascular age-related macular degeneration. Nihon Ganka Gakkai Zasshi. 2008;112:590–600 (in Japanese).
Pegaptanib Sodium Multi-center Study Group. Long-term efficacy and safety profile of pegaptanib sodium for age-related macular degeneration with choroidal neovascularization — evaluation of extended phase II clinical trial. Nihon Ganka Gakkai Zasshi. 2011;115:122–33 (in Japanese).
Gillies MC, Wong TY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2007;356:748–9 (author’s reply 749–50).
Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009;116:362.
Wong TY, Liew G, Mitchell P. Clinical update: new treatments for age-related macular degeneration. Lancet. 2007;370:204–6.
Singerman LJ, Masonson H, Patel M, Adamis AP, Buggage R, Cunningham E, et al. Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol. 2008;92:1606–11.
Marcus DM, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Four-year safety of pegaptanib sodium in neovascular age-related macular degeneration (AMD): results of the V.I.S.I.O.N. trial. Invest Ophthalmol Vis Sci. 2008;49:E-abstract 5069.
Macugen AMD Study Group, Apte RS, Modi M, Masonson H, Patel M, Whitfield L, et al. Pegaptanib 1-year systemic safety results from a safety-pharmacokinetic trial in patients with neovascular age-related macular degeneration. Ophthalmology. 2007;114:1702–12.
Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731–9.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(43–58):e1.
Albrecht J, Meves A, Bigby M. Case reports and case series from Lancet had significant impact on medical literature. J Clin Epidemiol. 2005;58:1227–32.
Kooistra B, Dijkman B, Einhorn TA, Bhandari M. How to design a good case series. J Bone Joint Surg Am. 2009;91(Suppl 3):21–6.
Acknowledgments
The LEVEL-J Study Group was organized and sponsored by the nonprofit organization HEART (Ishikawa, Japan). The Office of the Study Group, managed by Clinical Study Support, Inc. (Aichi, Japan), was responsible for site initiation, study management, data collection, and analysis. Professional medical English editing was done by K.K. FORTE (FORTE, Inc.).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The members of “The LEVEL-J Study Group” are listed in Appendix.
Appendix
Appendix
Writing Committee (affiliations)
Tatsuro Ishibashi (Kyushu University Graduate School of Medical Sciences) *Corresponding author
Nagahisa Yoshimura (Kyoto University Graduate School of Medicine)
Mitsuko Yuzawa (Nihon University School of Medicine)
Masahito Ohji (Shiga University of Medical Science)
Susumu Ishida (Hokkaido University Graduate School of Medicine)
Study group investigators (affiliations)
Shoji Kishi (Gunma University School of Medicine)
Tomohiro Iida (Fukushima Medical University School of Medicine)
Annabelle Ayame Okada (Kyorin University School of Medicine)
Hiroko Terasaki (Nagoya University Graduate School of Medicine)
Fumio Shiraga (Kagawa University Faculty of Medicine)
Yoko Ozawa (Keio University School of Medicine)
Miki Honnda (Juntendo University Urayasu Hospital)
Muneyasu Takeda (Souen Muneyasu Eye Clinic)
Kanji Takahashi (Kansai Medical University Hirakata Hospital)
Tsunehiko Ikeda (Osaka Medical College)
Yuichiro Ogura (Nagoya City University Medical School)
Taiji Sakamoto (Kagoshima University Graduate School of Medical and Dental Sciences)
Kazuaki Kadonosono (Yokohama City University Medical Center)
Shuichi Yamamoto (Chiba University Graduate School of Medicine)
Fumi Gomi (Osaka University Graduate School of Medicine)
Yasuo Yanagi (University of Tokyo School of Medicine)
About this article
Cite this article
Ishibashi, T., on behalf of the LEVEL-J Study Group. Maintenance therapy with pegaptanib sodium for neovascular age-related macular degeneration: an exploratory study in Japanese patients (LEVEL-J study). Jpn J Ophthalmol 57, 417–423 (2013). https://doi.org/10.1007/s10384-013-0255-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-013-0255-7