Abstract
Purpose
To show alterations of retinal arteriolar caliber (RAC), retinal venular caliber (RVC), retinal nerve fiber layer thickness (RNFLT), peripapillary choroidal thickness (ppCT), and central macular thickness (CMT) in acute and chronic phases of nonarteritic anterior ischemic optic neuropathy (NAION).
Methods
Forty-one eyes of 41 patients with NAION were included in this retrospective study. RAC, RVC, RNFLT, ppCT, and CMT measurements were performed via spectral-domain optical coherence tomography in the acute and chronic phases of NAION.
Results
RVC, RNFLT, ppCT, and CMT were significantly thinner in the chronic phase compared to the acute phase (p < 0.001), whereas RAC remained similar throughout the visits (p = 0.26). The visual acuity difference between the acute and chronic phases was not correlated with the changes of RAC, RVC, RNFLT, ppCT, or CMT.
Conclusions
RVC, RNFLT, ppCT, and CMT decreases in the chronic phase when compared to the acute phase of NAION, whereas RAC does not change significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ischemic optic neuropathy (ION) is the most common acute optic neuropathy in individuals older than 50 years of age [1]. Classification of ION is done as anterior or posterior, according to the segment of affected optic nerve [1, 2]. Anterior ION and posterior ION are further divided into two categories which are arteritic (i.e., related to vasculitis) and nonarteritic (i.e., not related to vasculitis) [1,2,3]. Nonarteritic anterior ION (NAION) accounts for the most of the ION cases [3].
NAION is manifested as sudden, painless, and monocular vision loss accompanied by optic disk edema [4]. Acute phase optic disk edema subsides in approximately 8 weeks and is followed by disk pallor and atrophy [5]. Although the exact pathogenesis of NAION is not fully understood, it appears to be a multifactorial disease, mainly due to a deterioration in the blood supply of the optic nerve head leading to hypoperfusion and ischemia [6, 7]. Decreased circumpapillary perfusion density, reduced peripapillary choroidal vascularity index, thinned retinal nerve fiber layer, and decreased macular ganglion cell complex thickness were reported in NAION in the recent years [8,9,10,11].
In the present study, our aim was to demonstrate the alterations of the retinal arteriolar caliber (RAC), retinal venular caliber (RVC), retinal nerve fiber layer (RNFL) thickness, peripapillary choroidal thickness (ppCT), and central macular thickness (CMT) in the acute and chronic phases of NAION. We also wanted to test the hypothesis that age or visual acuity gain may be associated with the amount of retina-choroidal thickness changes. Though some information has been identified about risk factors and clinical presentation of NAION, much remains unclear about its pathophysiology and treatment. The present study tries to fill a little gap in the huge unknown areas of NAION.
Methods
Forty-one eyes of 41 patients with non-arteritic anterior ischemic optic neuropathy (NAION) were included in this retrospective study. The medical records of the patients covered the time period between the years 2015 and 2019 in a tertiary referral center. This research was carried out in compliance with the ethical principles set out in the Helsinki Declaration and approved by the Institutional Ethical Committee.
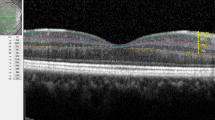
The patients had been diagnosed as NAION after detailed ophthalmological and neurological examinations. In addition, cardiovascular system, rheumatology, and infectious disease consultations were made for some of the patients to confirm the diagnosis. For the present study, ophthalmological examination records of the patients included visual acuity assessment (Snellen chart), biomicroscopy, indirect ophthalmoscopy, intra-ocular pressure, and optical coherence tomography (OCT) measurements. Visual field analysis of the patients showed inferior altitudinal defect or central scotoma. All of the patients had abnormalities on color vision in the affected eyes. The measurements were done at the acute phase (i.e., edematous disk and newly onset symptoms) and chronic phase (i.e., establishment of optic atrophy at least 2 months from the beginning of the disease) (Fig. 1). The treatment procedure was similar for all patients, including intravenous methylprednisolone followed by oral prednisone. Acetylsalicylic acid (Aspirin®) was also prescribed. The patients had been followed up at least 3 months. Exclusion criteria were any history of ocular surgery other than uneventful phacoemulsification and any ocular disease other than mild cataract or refractive errors. Patients with giant cell arteritis, optic neuritis, and other neurologic or ophthalmic disorders (e.g., diabetic retinopathy, hypertensive retinopathy, glaucoma, multiple sclerosis, optic disk drusen, etc.), which may affect optic nerve, were excluded.
The retinal arteriolar caliber (RAC), retinal venular caliber (RVC), retinal nerve fiber layer (RNFL) thickness, peripapillary choroidal thickness (ppCT), and central macular thickness (CMT) measurements were performed by the help of spectral-domain optical coherence tomography (SD-OCT, Spectralis, Heidelberg, Germany). Measurements of the retinal vessel caliber (RAC and RVC) were taken using manual caliber tools on the optic disk screen provided by the SD-OCT software. First, superior and inferior temporal retinal arterioles and venules passing through an area one-half to one-disk diameter from the optic disk margin were measured. Then, the average caliber values of superior and inferior temporal retinal vessels were calculated for each eye. The average and quadrantal RNFL thickness measurements were calculated automatically by the SD-OCT device. The ppCT was measured from the outer part of the hyper-reflective line corresponding to the retinal pigment epithelium to the inner surface of the sclera in the peripapillary region at approximately 1.0 mm from the nasal optic disk margin. In all relevant SD-OCT records, the chorio-scleral interface was clearly visualized. The CMT signified the mean thickness of a 1-mm-diameter circular area at the foveal center. The ocular measurements including choroidal thickness were taken during the same period of the day (between 10:00 and 12:00 a.m.) in order to avoid diurnal variations.
The Statistical Package of the Social Sciences (SPSS, version 17.0, Chicago, IL) was used for statistical analysis. ‘p’ values lower than 0.05 were accepted as statistically significant. Visual acuity values were converted from Snellen to logMAR for statistical analysis. Paired samples t test was used for comparison of the parameters at acute and chronic disease stages. The Pearson correlation analysis was performed in order to detect associations between age, visual acuity gain, and retina-choroidal thickness changes. The ppCT, RAC, and RVC in both acute and chronic phases were measured by 2 different researchers (SA, GP). The average of their measurements was recorded. The interobserver correlation for all the 6 sets of data was > 0.90.
Results
A total of 41 patients (24 male, 17 female) was included in this study. The mean age of the patients was 61.2 ± 8.9 years (range 46–86 years). The mean time period between the measurements at the acute and chronic stages of the disease was 3.1 ± 1.0 months (range 2–5 months). Right eyes were affected in 18 cases (44%), and left eyes were affected in 23 cases (56%). Pseudo-Foster Kennedy syndrome was noticed in 13 patients (32%).
The mean visual acuity values at the acute and chronic disease stages were 0.98 ± 0.65 logMAR and 0.66 ± 0.60 logMAR, respectively (p < 0.001). At the last visit, the visual acuity remained the same in 22 eyes (54%), increased in 17 eyes (41%), and decreased in 2 eyes (5%). The mean intra-ocular pressure values at the acute and chronic disease stages were 15.3 ± 2.7 mmHg and 15.0 ± 2.9 mmHg, respectively (p = 0.37).
The mean retinal arteriole caliber (RAC), retinal venule caliber (RVC), peripapillary choroidal thickness (ppCT), and central macular thickness (CMT) measurements of the patients at the acute and chronic stages are shown in Table 1. The RVC, ppCT, and CMT were significantly thinner in the chronic stages compared to the acute stage (p < 0.001), whereas RAC remained similar throughout the visits (p = 0.26).
The mean average peripapillary RNFL measurements at the acute and chronic disease stages were 225.3 ± 72.1 µm and 71.9 ± 21.5 µm, respectively (p < 0.001). The mean quadrantal peripapillary RNFL thickness (i.e., inferior, superior, nasal, and temporal quadrants) measurements of the patients in the acute and chronic phases were statistically significantly different as shown in Table 2.
The correlations of age and the retina-choroidal thickness changes at the visits were analyzed. Age was not found to be correlated with RAC change (r = 0.02, p = 0.92), RVC change (r = 0.23, p = 0.15), average RNFL thickness change (r = 0.12, p = 0.45), ppCT change (r = 0.09, p = 0.59), and CMT change (r = − 0.12, p = 0.49) at the acute and chronic phases of the disease.
The associations of visual acuity change and the retina-choroidal thickness changes at the acute and chronic phases were also analyzed. Visual acuity change was not correlated with RAC change (r = 0.24, p = 0.14), RVC change (r = − 0.10, p = 0.56), average RNFL thickness change (r = 0.01, p = 0.95), ppCT change (r = − 0.05, p = 0.80), and CMT change (r = − 0.03, p = 0.87).
Discussion
The outcomes of the present study show that the thickness of retina and choroid is decreased in the chronic phase compared to acute phase of NAION. Different from previously published research, this study showed that RVC is decreased, whereas RAC remained similar in the course of time. In addition, age was not found to be associated with the degree of retina-choroidal thickness alteration between the two visits. Another new finding was that visual acuity difference between the acute and chronic phases was not correlated with RAC change, RVC change, average RNFL change, ppCT change, or CMT change. Also, choroidal thickness change between acute and chronic phases of the disease was firstly evaluated in the present study.
The SD-OCT is a useful tool for monitoring RNFL thickness alterations following NAION. The thinning of RNFL after several months from the onset of NAION is shown in several studies [12,13,14]. In chronic stage of NAION, peripapillary RNFL atrophy is usually seen in all quadrants, but in some cases specific quadrantal RNFL damage could be seen [14,15,16]. In the present study, all RNFL quadrants were atrophic at the chronic stage, but were consistent with the ISNT rule. In the acute phase, superior quadrant RNFL edema was more marked, which was consistent with the inferior altitudinal visual field defect commonly seen in NAION.
In this study, the enlarged retinal venules observed in the acute phase of NAION might be due to constriction of central retinal vein by edematous optic disk. Since arteries are more resistant to compression compared to veins, caliber alteration of retinal arterioles throughout resolution was not observed in the present study. Remond et al. reported that acute NAION is associated with enlargement of both retinal arterioles and venules, while resolution NAION yielded normalization of those values [17]. As expected in most before-after studies, we did not use a control group in this study, since confounding factors (i.e., systemic hypertension, diabetes, etc.) might affect retinal vessel calibers in different individuals.
Several studies reported that mean ppCT was greater in NAION eyes compared to control group [18,19,20,21]. They suggested that a thicker choroid was not the effect, but the cause of NAION [18,19,20,21]. They hypothesized that a thick choroid may constrict optic nerve and its vessels [20, 21]. In addition to those published outcomes, we found that acute phase NAION is associated with thicker choroid compared to chronic phase. In contrast to those suggestions, one might think that thicker choroid is a compensatory mechanism in the acute phase of the disease according to the outcomes of the present study.
Macular edema can accompany NAION [22, 23]. Type of macular edema seen in NAION is usually subretinal fluid accumulation. In parallel to our outcomes, it was reported that submacular fluid is resorbed in the chronic phase of NAION [22, 23]. Since vision loss in NAION is due to optic nerve axonal ischemia, resolution of macular edema in the chronic phase was not found to be associated with visual acuity gain in the present study.
The present study has some limitations. Since it is a retrospective study, it has inferior level of evidence compared to prospective studies. Measurements with OCT angiography might improve the study, but our clinic did not have this device. The lack of correlation of the visual field tests with the SD-OCT measurements was another limitation, since our archive records of visual field analysis only included types of scotomas (i.e., inferior altitudinal defect, central scotoma).
In conclusion, the RVC, RNFL thickness, ppCT, and CMT decreased in the chronic phase when compared to acute phase of NAION, whereas RAC did not change significantly. Visual acuity change was not associated with the amount of retina-choroidal thickness reduction from the acute phase to the chronic phase (in approximately 3 months). Those outcomes may be helpful for determining the timing and type of future therapeutic approach in NAION. Future studies having larger sample size and longer follow-up times should be needed to confirm the outcomes of the present research.
References
Biousse V, Newman NJ (2015) Ischemic optic neuropathies. N Engl J Med 372:2428–2436
Hayreh SS (2013) Ischemic optic neuropathies—where are we now? Graefes Arch Clin Exp Ophthalmol 251:1873–1884
Morrow MJ (2019) Ischemic optic neuropathy. Continuum (Minneap Minn) 25:1215–1235
Berry S, Lin WV, Sadaka A, Lee AG (2017) Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain 9:23–28
Hayreh SS, Zimmerman MB (2007) Optic disc edema in non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol 245:1107–1121
Patel HR, Margo CE (2017) Pathology of ischemic optic neuropathy. Arch Pathol Lab Med 141:162–166
Peeler C, Cestari DM (2016) Non-arteritic anterior ischemic optic neuropathy (NAION): a review and update on animal models. Semin Ophthalmol 31:99–106
Mastropasqua R, Agnifili L, Borrelli E, Fasanella V, Brescia L, Di Antonio L, Mastropasqua L (2018) Optical coherence tomography angiography of the peripapillary retina in normal-tension glaucoma and chronic nonarteritic anterior ischemic optic neuropathy. Curr Eye Res 43:778–784
Pellegrini M, Giannaccare G, Bernabei F, Moscardelli F, Schiavi C, Campos EC (2019) Choroidal vascular changes in arteritic and nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 205:43–49
Duman R, Yavas GF, Veliyev I, Dogan M, Duman R (2019) Structural changes of macula and optic disk of the fellow eye in patients with nonarteritic anterior ischemic optic neuropathy. Int Ophthalmol 39:1293–1298
Sun MH, Liao YJ (2017) Structure-function analysis of nonarteritic anterior ischemic optic neuropathy and age-related differences in outcome. J Neuroophthalmol 37:258–264
Fard MA, Afzali M, Abdi P, Yasseri M, Ebrahimi KB, Moghimi S (2016) Comparison of the pattern of macular ganglion cell-inner plexiform layer defect between ischemic optic neuropathy and open-angle glaucoma. Investig Ophthalmol Vis Sci 57:1011–1016
Saito H, Tomidokoro A, Tomita G, Araie M, Wakakura M (2008) Optic disc and peripapillary morphology in unilateral nonarteritic anterior ischemic optic neuropathy and age- and refraction-matched normals. Ophthalmology 115:1585–1590
Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun AA, Barboni P (2008) Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol 246:641–647
Quigley HA, Miller NR, Green WR (1985) The pattern of optic nerve fiber loss in anterior ischemic optic neuropathy. Am J Ophthalmol 100:769–776
Saito H, Tomidokoro A, Sugimoto E et al (2006) Optic disc topography and peripapillary retinal nerve fiber layer thickness in nonarteritic ischemic optic neuropathy and open-angle glaucoma. Ophthalmology 113:1340–1344
Remond P, Aptel F, Cunnac P et al (2019) Retinal vessel phenotype in patients with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 208:178–184
Pérez-Sarriegui A, Muñoz-Negrete FJ, Noval S, De Juan V, Rebolleda G (2018) Automated evaluation of choroidal thickness and minimum rim width thickness in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol 38:7–12
Nagia L, Huisingh C, Johnstone J et al (2016) Peripapillary pachychoroid in nonarteritic anterior ischemic optic neuropathy. Investig Ophthalmol Vis Sci 57:4679–4685
Fard MA, Abdi P, Kasaei A, Soltani Mogaddam R, Afzali M, Moghimi S (2015) Peripapillary choroidal thickness in nonarteritic anterior ischemic optic neuropathy. Investig Ophthalmol Vis Sci 56:3027–3033
Nikkhah H, Feizi M, Abedi N, Karimi S, Yaseri M, Esfandiari H (2020) Choroidal thickness in acute non-arteritic anterior ischemic optic neuropathy. J Ophthalmic Vis Res 15:59–68
Tomsak RL, Zakov ZN (1998) Nonarteritic anterior ischemic optic neuropathy with macular edema: visual improvement and fluorescein angiographic characteristics. J Neuroophthalmol 18:166–168
Hedges TR 3rd, Vuong LN, Gonzalez-Garcia AO, Mendoza-Santiesteban CE, Amaro-Quierza ML (2008) Subretinal fluid from anterior ischemic optic neuropathy demonstrated by optical coherence tomography. Arch Ophthalmol 126:812–815
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
N/A since it is a retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akbulut, S., Pekel, G., Pekel, E. et al. Alterations in retinal and choroidal thickness following nonarteritic anterior ischemic optic neuropathy. Int Ophthalmol 41, 2723–2728 (2021). https://doi.org/10.1007/s10792-021-01829-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01829-7