Abstract
Purpose
We investigated changes in intrableb morphology of functioning trabeculectomy blebs with anterior segment optical coherence tomography (AS-OCT) after digital ocular compression.
Methods
Sixty-eight patients who underwent fornix-based trabeculectomy were recruited from Seoul St. Mary’s Hospital. Intraocular pressure (IOP) and AS-OCT images were taken before and after ocular compression. Bleb height and wall thickness and height and length of the internal cavity were measured by AS-OCT. The hyporeflective areas and number of microcysts were checked on both vertical and horizontal images. AS-OCT parameters for IOP change, bleb morphology, and interval between surgery and examination were compared.
Results
Ocular compression yielded significant changes for all AS-OCT parameters other than maximum bleb wall thickness in the 6- to 12-month period after surgery. Correlation between IOP change and AS-OCT parameters was greatest for the horizontal and vertical length of the internal cavity (Spearman rank correlation coefficient ρ = 0.717, P < 0.0001, and ρ = 0.866, P < 0.0001, respectively). Response to ocular compression in cystic blebs was mainly enlargement of the internal cavity and increase in bleb height. However, in diffuse filtering blebs, increases in the size of the hyporeflective areas and the number of microcysts were the main findings. From 12 months postoperatively, changes in AS-OCT intrableb parameters were significantly reduced, showing no difference after ocular compression.
Conclusion
Digital ocular compression may be useful in maintaining bleb morphology when it is used for 6 months postoperatively. Response to ocular compression as assessed by bleb morphology was different.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Determination of bleb morphology after trabeculectomy provides an important clinical parameter in predicting surgical outcomes [1, 2]. To maintain the trabeculectomy bleb, digital ocular compression is commonly recommended postoperatively. Ocular compression by pressing on the globe with a finger can temporarily increase intraocular pressure (IOP), thereby forcing aqueous fluid out through the filtering site and lowering IOP. Ocular compression can also encourage the flow of aqueous fluid through the constructed fistula, maintain the outflow pathway of the bleb, and prevent formation of scar tissue [3, 4]. However, opinions regarding the use of ocular compression vary, and no consensus has been reached among physicians about using the technique. Digital ocular compression has been shown to reduce IOP in patients with a functioning bleb [5]. The effects are best achieved during the first month after surgery to avoid underfiltration with a flat bleb [6]. Moreover, previous reports have shown a decrease in IOP after ocular compression in functioning blebs 3 months to 6 years after surgery [7]. However, ocular compression was not effective in patients when ocular compression was initiated ≥3 months after surgery, and ocular compression at 6 months after surgery provided no beneficial effect on mean IOP or mean change in IOP [8].
Investigators have begun to seek more intricate visualization of the intrableb morphology. Some internal features of the filtering bleb, such as formation of a cavernous fluid-filled space and intrableb reflectivity, have been characterized by ultrasound biomicroscopy (UBM) or anterior segment optical coherence tomography (AS-OCT), and these intrableb features are thought to be related to bleb function [9–11]. However, visualization of the internal morphologic changes of trabeculectomy blebs 12 months after ocular compression surgery has not been performed prospectively.
In this study, we used AS-OCT (Zeiss Visante OCT Model 1000; Carl Zeiss Meditec, Dublin, CA, USA) to image and characterize the internal morphology of functioning blebs after digital ocular compression. We also investigated the effect of ocular compression on IOP change and its long-term effects on intrableb morphology. Through this study, we hoped to determine the role of ocular compression on the internal bleb structures and thus provide recommendations on the use of ocular compression in clinical settings.
Materials and methods
This was a prospective cross-sectional study of patients at Seoul St. Mary’s Hospital, Seoul, Korea. Primary open-angle glaucoma patients who had previously undergone trabeculectomy surgery between 2004 and 2011 were enrolled. Patients were recruited when they came for routine follow-up visits at the clinic and included in the study within a minimum of 2 weeks after trabeculectomy. Only one eye per patient was included. In each case, fornix-based blebs were created with addition of mitomycin C (0.2–0.4 mg/ml for 2–3 min). Patients with limbus-based blebs, a history of a bleb leak, or a history of bleb infection were excluded. Written informed consent was obtained from all patients, and the study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital and carried out in accordance with the tenets of the Declaration of Helsinki.
Intraocular pressure and AS-OCT images of the bleb were taken before and after ocular compression. All IOP measurements were performed using Goldmann applanation tonometry. After IOP measurements and AS-OCT images had been taken, digital ocular massage by placing the pad of the index finger on the central cornea with the eyelid closed was performed by the examiner (MDA). Mild-to-moderate pressure was then applied to the eye by pushing in a constant fashion toward the back of the head, and a gentle massage was added. The pressure was applied for 10 s, followed by a rest of 5 s, and then applied for another 10 s. Immediately after ocular compression, IOP and AS-OCT images were again taken. AS-OCT images were taken by one examiner (HYP) according to the following procedure: Patients were asked to look down, and the upper lid was retracted to expose as much as possible of the bleb in the superior bulbar conjunctiva, with care taken to avoid pressure on the globe or bleb. Initially, AS-OCT images were obtained for each bleb in two directions: first, a radial section perpendicular to the limbus through the site of a peripheral iridectomy, and second, a section tangential to the limbus through the site of maximal bleb elevation. At least five images were obtained for each scan, and the best image was used. From the several images taken in each radial and tangential section, similar images from the same location were chosen by comparing scan location in layout images of the AS-OCT screen. According to AS-OCT findings, blebs were classified at baseline examinations as diffuse or cystic. Diffuse blebs were characterized by multiple subconjunctival fluid collections and low-to-moderate intrableb reflectivity. Cystic blebs were composed of a large subconjunctival hyporeflective space with multiloculated fluid collections of various sizes and intensities. These blebs were covered by a thin layer of conjunctiva [9].
Height, wall thickness, internal cavity height, and horizontal and vertical lengths of the internal cavity of the bleb were measured. Bleb height was defined as the maximum distance between the conjunctival surface and the sclera. The height of the internal cavity was defined as the maximum height of the region of low reflectivity in the area between the conjunctiva and sclera. The length of the internal cavity was defined as the anteroposterior linear distance of the region of low reflectivity. Bleb wall thickness was defined as the thickest area of high reflectivity within the region over the internal cavity. The presence of a hyporeflective area and the number of microcysts were also checked. A hyporeflective area was defined as reflectivity of the bleb wall being lower than that of the surrounding healthy conjunctiva or if multiple areas of low reflectivity were present in the bleb wall. A microcyst was defined as a small hyporeflective space >10 μm in diameter found in the bleb wall. The number of microcysts was counted on both vertical and horizontal images (Fig. 1).
a Slit-lamp morphology of a bleb. b Horizontal slice image of the bleb; horizontal length (HL) and height (arrow) of the internal fluid-filled cavity (asterisk). c Vertical slice image of the bleb; vertical length (VL) of the internal fluid-filled cavity (asterisk) and maximum bleb wall thickness (arrow); sclerotomy site (S) and iridectomy site (IR); microcysts (M) of >10 μm in diameter were counted in the images. d Total bleb height (BH) is the maximum distance between the internal surface of the fluid-filled cavity (asterisk) and bleb surface; hyporeflective area (arrows)
Statistical analyses were conducted with SigmaStat 2.03 statistical software (SPSS, Chicago, IL, USA) and version 10.0 SPSS (SPSS). A probability value of <0.05 was deemed to indicate statistical significance in all analyses. The Mann–Whitney test was used to compare IOP, horizontal and vertical length of the internal cavity, height of the internal cavity, total bleb height, maximum bleb wall thickness, and number of microcysts. The presence of hyporeflective areas was compared using the chi-square test. To compare groups based on the interval between surgery and examination, a post hoc test (Scheffé method) was used. The relationship between change in IOP and AS-OCT parameters was analyzed using the Spearman rank correlation.
Results
Of the 68 participants, 37 were men and 31 were women (mean age 65.3 ± 10.3 years). The interval between surgery and imaging ranged from 0.26 to 52.2 months (median 5.5 ± 0.3 months). Mean baseline IOP was 15.61 ± 5.57 mmHg. Bleb appearance showed diffuse-filtering blebs in 38 eyes (55.9%) and cystic blebs in 30 eyes (44.1%). Patient demographics and baseline characteristics are summarized in Table 1.
Changes in intrableb morphology within the AS-OCT parameters induced by ocular compression are shown in Table 2. After ocular compression, significant changes were observed in IOP (P < 0.001), internal cavity horizontal and vertical lengths (P < 0.001 and P < 0.001, respectively), internal cavity height (P = 0.020), total bleb height (P = 0.013), appearance of the hyporeflective area (P < 0.001), and number of microcysts (P < 0.001). Only the maximum bleb wall thickness did not change significantly (P = 0.218).
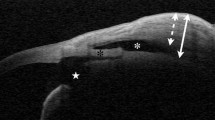
The response to ocular compression was analyzed in terms of bleb morphology (Table 3). Diffuse filtering blebs showed significant changes after ocular compression through the appearance of a hyporeflective area and in the number of microcysts. These changes were statistically significant when compared with changes in cystic blebs (P < 0.001 and P < 0.001, respectively). For cystic blebs, AS-OCT parameters changed significantly in internal cavity horizontal and vertical lengths and height and total bleb height. These parameters were significantly different from observations seen in diffuse filtering blebs. Representative photographs are shown in Figs. 2 and 3. In cystic blebs, increases in internal cavity length and height and in total bleb height were the main features after ocular massage (Fig. 2). Instead of an increase in the internal cavity and total bleb height, diffuse filtering blebs showed an increase in the size of the hyporeflective area (Fig. 3a, b) and the number of microcysts (Fig. 3c, d).
Representative images of a diffuse filtering bleb. Case 1: a Before digital ocular compression; vertical image. b After ocular compression, increase in the size of the internal fluid-filled cavity is not apparent; However, an enlarged hyporeflective area (arrows) is evident. Case 2: c Before ocular compression: horizontal image shows one microcyst (M). d After ocular compression; more microcysts are apparent
The correlation between change in IOP and quantitative intrableb AS-OCT parameters after ocular compression was assessed (Table 4). The change in IOP after ocular compression showed a significantly positive correlation with internal cavity horizontal and vertical length [Spearman rank correlation ρ = 0.717, P < 0.0001 (Fig. 4a) and ρ = 0.866, P < 0.0001 (Fig. 4b), respectively], height of internal cavity (ρ = 0.655, P < 0.0001), total bleb height [ρ = 0.464, P < 0.0001 (Fig. 4c)], and number of microcysts [ρ = 0.632, P < 0.0001 (Fig. 4d)].
a–d Relationship between intraocular pressure (IOP) change and intrableb parameters measured by anterior segment optical coherence tomography (AS-OCT). Horizontal (HL) and vertical (VL) length correlated most strongly with IOP change. e–h Further analysis according to elapsed time after trabeculectomy: AS-OCT parameters showed significant changes 6–12 months after surgery but not thereafter
Changes in intrableb morphology after ocular compression with respect to the interval between surgery and examination are shown in Table 5. Patients were divided into four groups: interval of <3 months, 3–6 months, 6–12 months, and >12 months. Changes in IOP, internal cavity horizontal and vertical lengths and height, total bleb height, and number of microcysts were significantly different. A comparison between patients in the 3- to 6-month group and those in the 6- to 12-month group showed significant differences in change in IOP (P = 0.011), internal cavity horizontal (P = 0.037) and vertical (P = 0.026) lengths, total bleb height (P = 0.048), and number of microcysts (P = 0.004). The change in intrableb AS-OCT parameters decreased as the time interval between surgery and examination increased. No significant difference in intrableb parameters was found between patients in the 6- to 12-month group and patients in the >12-month group. Changes in intrableb parameters were significantly reduced 6–12 months after surgery for internal cavity horizontal and vertical lengths (Fig. 4e, f, respectively), total bleb height (Fig. 4g), and number of microcysts (Fig. 4h).
Discussion
With the advent of AS-OCT (a noncontact, high-resolution tomographic cross-sectional method of imaging), visualizing ocular anterior segment structures has become possible. Unlike UBM, the position and orientation of the scan can be localized and visualized from the real-time camera panel. OCT also provides higher axial resolution images (18 μm with Visante vs 25 μm with UBM) [12, 13]. Such advantages translate into more accurate and reproducible images for direct and objective bleb assessment and measurement. Recent studies reported that the internal bleb morphology can be visualized and analyzed using OCT [14–17]. IOP correlated with bleb height, internal cavity height, and extent of the internal cavity. Also, low intrableb reflectivity, episcleral fluid, and microcysts were identified in the images of the mature bleb and shown to be related with bleb function and IOP [16, 18–20]. Many reports support the notion that AS-OCT is useful in identifying intrableb morphology, and intrableb morphology was shown to be an important aspect in predicting bleb function [21].
The change in intrableb morphology in response to digital ocular compression or digital ocular massage depicted by AS-OCT was significant. Bleb height and length and internal cavity height were enlarged after ocular compression, and this correlated significantly with change in IOP. Vertical and horizontal lengths of the internal cavity were especially strongly correlated with change in IOP resulting from ocular compression (ρ = 0.866 and ρ = 0.717, respectively). Bleb height and the extent of microcysts were found to be moderately correlated with IOP change (ρ = 0.655 and ρ = 0.632, respectively). Results of our study are consistent with those of previous reports that showed that larger internal fluid-filled cavities indicates better reduction in IOP. During ocular compression, IOP reduction was correlated with internal fluid-filled cavity enlargement.
Bleb wall thickness has been reported to be negatively correlated with IOP [22]. Singh et al. [17] hypothesized that bleb wall thickening was the hallmark of blebs in which IOP was successfully controlled. However, we found no change in bleb wall thickness induced by ocular compression. The bleb wall consists of the conjunctiva and subconjunctival tissue. In failed encapsulated blebs, the bleb wall can be thicker owing to excessive subconjunctival scarring. Therefore, it does not seem likely that bleb wall thickness correlates with bleb function. We also think that this is due to the difference in fornix-based and limbus-based trabeculectomy blebs. Previous reports showing that bleb wall thickness increased with good bleb function included more cases of fornix-based blebs, which seem to form more diffuse blebs with thicker walls than do limbus-based blebs [23]. In our study, the maximum bleb wall thickness of diffuse filtering blebs increased in response to ocular compression than that of cystic blebs. However, this finding was not statistically significant. To rule out the influence of the surgical techniques, we included only fornix-based blebs treated by one surgeon. However, differences between fornix- and limbus-based blebs need to be further investigated.
The low to medium reflectivities in OCT images are features of functioning blebs. Histological studies on functioning blebs show that collagen in the conjunctival substantia propia was widely spaced and the subepithelial connective tissue loosely arranged [24]. This is a contrast feature that shows high reflectivity in encapsulated blebs, in which the bleb wall is composed of dense collagenous connective tissue [25]. In our study, the size of hyporeflective areas increased significantly after ocular compression. This change was more apparent in diffuse filtering blebs. Under a slit lamp, diffuse filtering and cystic blebs have different clinical features, as does intrableb morphology shown by AS-OCT or UBM [9]. Cystic blebs and diffuse filtering blebs had different responses to ocular compression, which in cystic blebs enlarged the internal fluid-filled cavity, and internal cavity length and height changed significantly. Total bleb height also increased significantly. In contrast, in the diffuse filtering blebs, as mentioned earlier, enlargement of the hyporeflective areas was apparent. After ocular compression, an increase in the number of microcysts without a significant increase in the extent of the internal cavity was observed. The diffuse filtering blebs showed change in IOP, as evidenced by an increase in the size of hyporeflective areas or in the number of microcysts in response to ocular compression. These findings demonstrate that different responses to ocular compression may be due to differences in bleb morphology. Moreover, this is consistent with results that showed that the crude dimensions of the internal cavity or the total bleb do not reflect the extent of aqueous drainage in diffuse filtering blebs [17, 22].
Measurements were performed at different time points after trabeculectomy, leading us to examine the response of intrableb morphology to ocular compression at those different time points. IOP change after ocular compression was significant in trabeculectomy blebs <6 months postoperatively. However, >6 months posttrabeculectomy, all intrableb parameters measured by AS-OCT were unchanged when compared with blebs measured 3–6 months posttrabeculectomy. The effect of ocular compression in maintaining the function of the bleb is still a matter of debate, and most studies show ocular compression to be effective in changing IOP at 1–3 months postoperatively. One report showed ocular compression had little to no success in lowering IOP at 6 months after surgery [8]. By using AS-OCT, we found effective changes in intrableb structure induced by ocular compression that corresponded to the amount of IOP change when ocular compression was performed for 6 months after surgery. Ocular compression did not induce changes in intrableb structures beyond 6 months after surgery; thus, using ocular compression thereafter may have little effect on the bleb.
In conclusion, ours is the first study to use AS-OCT to evaluate changes in internal bleb morphology after digital ocular compression. At <6 months after surgery, all intrableb parameters other than wall thickness changed after ocular compression. Digital ocular compression may be useful in maintaining bleb morphology when it is used during the first 6 months after surgery. Also, we found that diffuse filtering blebs responded to ocular compression with an increased size of the hyporeflective areas and an increase in the number of microcysts. In contrast, cystic blebs enlarged the internal cavity and total bleb height in response to ocular compression. This may lead to a better understanding of the effects of digital ocular compression in fornix-based trabeculectomy and of structural changes in various types of bleb morphology.
References
Cantor LB, Mantravadi A, WuDunn D, Swamynathan K, Cortes A. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading Scale. J Glaucoma. 2003;12:266–71.
Wells AP, Crowston JG, Marks J, Kirwan JF, Smith G, Clarke JC, et al. A pilot study of a system for grading of drainage blebs after glaucoma surgery. J Glaucoma. 2004;13:454–60.
Parrow KA, Shin DH. Enhancing filtration in the early postoperative trabeculectomy refractory to digital massage. Ophthalmic Surg. 1990;21:401–3.
Traverso CE, Greenidge KC, Spaeth GL, Wilson RP. Focal pressure: a new method to encourage filtration after trabeculectomy. Ophthalmic Surg. 1984;15:62–5.
Kane H, Gaasterland DE, Monsour M. Response of filtered eyes to digital ocular pressure. Ophthalmology. 1997;104:202–6.
Gouws P, Buys YM, Rachmiel R, Trope GE, Fresco BB. Finger massage versus a novel massage device after trabeculectomy. Can J Ophthalmol. 2008;43:222–4.
Kane H. What we know about digital ocular massage. Ophthalmology. 1998;5:65–73.
Henderer JD, Heeg MC, Spaeth GL, Moster MR, Myers JS, Schmidt CM Jr, et al. A randomized trial of the long-term effects of digital ocular compression in the late postoperative period. J Glaucoma. 2001;10:266–70.
Leung CK, Yick DW, Kwong YY, Li FC, Leung DY, Mohamed S, et al. Analysis of bleb morphology after trabeculectomy with Visante anterior segment optical coherence tomography. Br J Ophthalmol. 2007;91:340–4.
Nakano N, Hangai M, Nakanishi H, Inoue R, Unoki N, Hirose F, et al. Early trabeculectomy bleb walls on anterior-segment optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248:1173–82.
Tominaga A, Miki A, Yamazaki Y, Matsushita K, Otori Y. The assessment of the filtering bleb function with anterior segment optical coherence tomography. J Glaucoma. 2010;19:551–5.
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81.
Izatt JA, Hee MR, Swanson EA, Lin CP, Huang D, Schuman JS, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112:1584–9.
Singh M, See JL, Aquino MC, Thean LS, Chew PT. High-definition imaging of trabeculectomy blebs using spectral domain optical coherence tomography adapted for the anterior segment. Clin Exp Ophthalmol. 2009;37:345–51.
Singh M, Aung T, Friedman DS, Zheng C, Foster PJ, Nolan WP, et al. Anterior segment optical coherence tomography imaging of trabeculectomy blebs before and after laser suture lysis. Am J Ophthalmol. 2007;143:873–5.
Theelen T, Wesseling P, Keunen JE, Klevering BJ. A pilot study on slit lamp-adapted optical coherence tomography imaging of trabeculectomy filtering blebs. Graefes Arch Clin Exp Ophthalmol. 2007;245:877–82.
Singh M, Chew PT, Friedman DS, Nolan WP, See JL, Smith SD, et al. Imaging of trabeculectomy blebs using anterior segment optical coherence tomography. Ophthalmology. 2007;114:47–53.
Aptel F, Dumas S, Denis P. Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep sclerectomy with new collagen implant. Eur J Ophthalmol. 2009;19:223–30.
Savini G, Zanini M, Barboni P. Filtering blebs imaging by optical coherence tomography. Clin Exp Ophthalmol. 2005;33:483–9.
Miura M, Kawana K, Iwasaki T, Kiuchi T, Oshika T, Mori H, et al. Three-dimensional anterior segment optical coherence tomography of filtering blebs after trabeculectomy. J Glaucoma. 2008;17:193–6.
Nakano N, Hangai M, Nakanishi H, Inoue R, Unoki N, Hirose F, et al. Early trabeculectomy bleb walls on anterior-segment optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;8:1173–82.
Tominaga A, Miki A, Yamazaki Y, Matsushita K, Otori Y. The assessment of the filtering bleb function with anterior segment optical coherence tomography. J Glaucoma. 2010;8:551–5.
Wells AP, Cordeiro MF, Bunce C, Khaw PT. Cystic bleb formation and related complications in limbus-versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology. 2003;110:2192–7.
Powers TP, Stewart WC, Stroman GA. Ultrastructural features of filtration blebs with different clinical appearances. Ophthalmic Surg Lasers. 1996;27:790–4.
Addicks EM, Quigley HA, Green WR, Robin AL. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983;101:795–8.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Park, HY.L., Ahn, M.D. Imaging of trabeculectomy blebs with Visante anterior segment optical coherence tomography after digital ocular compression. Jpn J Ophthalmol 56, 38–45 (2012). https://doi.org/10.1007/s10384-011-0101-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-011-0101-8