Abstract
Purpose
To explore the features of filtering blebs exhibiting transconjunctival oozing via three-dimensional anterior segment optical coherence tomography (3D AS-OCT).
Methods
In this cross-sectional study, 131 eyes of 131 patients exhibiting filtering blebs were examined. Of those, 20 eyes were excluded as flat-shaped, non-functioning bleb. Transconjunctival oozing was defined as transconjunctival aqueous egress evident on the bleb surface, in the absence of any point leak observable using a slit-lamp, as confirmed by application of digital pressure. Total bleb height, the height of the fluid-filled cavity, and bleb wall thickness and density were measured using 3D AS-OCT. Patient age, the etiology of glaucoma, postoperative follow-up period, number of glaucoma medication classes prescribed, intraocular pressure (IOP), grade of bleb vascularity, and bleb parameters were compared in eyes with and without bleb oozing.
Results
Sixty (54.0 %) of 111 eyes excluding non-functioning flat blebs exhibited oozing; mean IOP value (11.7 ± 4.5 vs. 14.8 ± 4.0 mmHg) and bleb vascularity grade (1.5 ± 0.7 vs. 2.4 ± 1.0) were lower than those of eyes without oozing. Total bleb height (1.1 ± 0.4 vs. 0.9 ± 0.4 mm), bleb wall thickness (0.7 ± 0.4 vs. 0.5 ± 0.3 mm), and bleb wall density (131.3 ± 45.7 vs. 180.9 ± 39.8 optical density units) differed significantly between the two groups (oozing vs. non-oozing).
Conclusion
Transconjunctival oozing after trabeculectomy with MMC was associated with a low IOP, low-level bleb vascularity, an elevated total bleb height, a thicker bleb wall, and low bleb wall density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraocular pressure (IOP)-reducing treatment is key in prevention of progression of glaucomatous visual field defects, and trabeculectomy is one of the most common surgical interventions used to lower IOP in glaucoma patients. After trabeculectomy with administration of antimetabolites, transconjunctival oozing is often observed [1]. The oozing rate increases in a time-dependent manner, and is associated with enlargement of avascular areas within blebs [1, 2]. Although oozing can be stopped by application of autologous serum, this procedure is associated with a significant increase in IOP [3], and the presence of oozing may thus be associated with good IOP control. However, the characteristics of blebs exhibiting oozing are reportedly similar to those of leaking blebs [1], suggesting a potential association between oozing and a risk of bleb infection. Oozing is closely associated with the surgical outcomes of trabeculectomy, and, therefore, new insights into the oozing phenomenon may be useful in the clinical management of glaucoma patients.
Recently, we reported that three-dimensional anterior segment optical coherence topography (3D AS-OCT) could be used to perform detailed evaluation of the internal morphology of filtration blebs, and to identify precisely filtration openings on scleral flap margins after trabeculectomy [4, 5]. Although it is generally assumed that changes in internal bleb morphology trigger various sequelae, the particular characteristics of oozing blebs have not previously been investigated using 3D AS-OCT. In the present study, we investigated the relationship between the development of oozing and bleb characteristics, with aid of 3D AS-OCT.
Material and methods
Patients
All procedures adhered strictly to the tenets of the Declaration of Helsinki. The present cross-sectional study was approved by the institutional review board and ethics committee of Kumamoto University. A total of 131 eyes of 131 patients with blebs that exhibited filtering features for at least 3 months after trabeculectomy were examined at Kumamoto University Hospital between August 2011 and March 2013. Eyes with blebs that had point leaks, and eyes exhibiting poor IOP control (and that thus required additional surgery to treat glaucoma), were excluded from the study.
Surgical procedures
Trabeculectomy was performed as reported previously [5]. Briefly, either a fornix- or limbal-based conjunctival flap was created without removing the Tenon’s capsule, as well as a 4-mm-wide half-layer triangle scleral flap. Mitomycin C (MMC; 0.4 mg/ml) was applied onto and under the scleral flap, and under the conjunctiva, for 4 min, followed by irrigation with 200 ml of physiological saline. A deep limbal block was excised to create a fistula in the anterior chamber, and peripheral iridectomy was next performed. The scleral and conjunctival flaps were sutured using 10–0 nylon. All surgeries were performed by one of three glaucoma specialists (H.T., M.I., or T.I.).
Clinical examinations
Bleb vascularity was assessed via color photography of the anterior ocular segment and classified using the Moorfields Bleb Grading System (http://blebs.net/). Next, IOP was measured by Goldmann applanation tonometry performed between the hours of 1 pm and 4 pm. The post-operative IOP value of each eye was calculated as the average of three consecutive IOP measurements at the time of 3D AS-OCT assessment. Transconjunctival oozing was defined as evident transconjunctival aqueous egress on the bleb without interruption of the conjunctival tissue or the aqueous stream of the bleb wall, and was observed as described below: a semi-dry fluorescein strip was gently applied to the bleb with the patient looking downwards, and the eyeball was gently pressed with a finger (of the observer). We examined the bleb by slit-lamp microscopy under cobalt-blue illumination over the next 15 seconds. Typical transconjunctival oozing is shown in Fig. 1. Images of filtering blebs were acquired using 3D AS-OCT (Casia; Tomey, Nagoya, Japan), as described previously [4, 5]. Briefly, the examiner used a finger to elevate the upper lid, exposing the filtration bleb, and bleb scans were obtained from at least two directions (horizontal and vertical rasters). Each raster consisted of 512 A-scans of an 8 × 8-mm area, and all scans were completed within 3 s.
Typical findings in blebs exhibiting transconjunctival oozing upon slit-lamp examination. A bleb that was avascular in appearance (a) was examined using a blue lamp. A semi-dry fluorescein strip was gently applied to the bleb as the patient looked downwards, and the eyeball was gently pressed with a finger (of the observer). Immediately after blinking, multiple points at which the fluorescein became gradually diluted by aqueous oozing were evident on the surface of the bleb, without interruption of the conjunctival tissue or the aqueous stream on the bleb wall (b, early stage; c, late stage).
Three-dimensional AS-OCT image analysis
Internal bleb structures were assessed with the aid of Casia bleb assessment software, version 4.0 L (Tomey), as described previously [5]. Three different reviewers examined complete 3D images of the internal structures of all filtration blebs, and independently measured bleb parameters. When opinions differed among the reviewers, the majority decision prevailed. Filtration openings were identified by the presence of pits and/or troughs in fluid-filled cavities evident in either the horizontal or vertical rasters and the corresponding C-scan images of the scleral flap margins of the bleb (Fig. 2). Non-functioning, flat-shaped blebs without fluid-filled cavity were defined as type N and excluded from further analyses. Blebs in which filtration openings could be identified using 3D AS-OCT were termed type F blebs. Those blebs in which filtration openings could not be identified were subdivided into two groups, one of which had an elevated bleb wall that was sometimes of high reflectivity (type H), and the other filtration openings masked by a sponge-like structure exhibiting low reflectivity (type S), as described previously [5]. After identification of filtration openings, the reviewers measured bleb parameters, including the total bleb height, the height of the fluid-filled cavity, bleb wall thickness, and bleb wall density (Fig. 3). Measurements were made on horizontal images, at the center of each filtration opening, or at the center of the scleral flap when no filtration opening could be identified. The mean values of bleb parameters calculated by the three independent reviewers were used in analysis. For comparison of bleb parameters, only type F blebs were included.
Identification of filtration openings on the scleral flaps, as seen on three-dimensional anterior segment optical coherence tomographic (3D AS-OCT) images. A charge-coupled device (CCD) image (left upper panel), and 3D AS-OCT images (the other panels). The latter images are vertical (right upper panel), horizontal (left lower panel), and C-scan (right lower panel) images. The box outlined in green indicates the 8 × 8-mm area used for 3D AS-OCT imaging. Red and blue lines indicate the horizontal and vertical AS-OCT axes, respectively. The yellow line indicates the Z axis of the AS-OCT region, corresponding to that of the C-scan image. The intersection points of colored lines (red, blue, and yellow) indicate the positions of pits and troughs in fluid-filled cavities. The filtration opening on the scleral flap was identified after detailed examination of 512 scans by moving all color lines manually
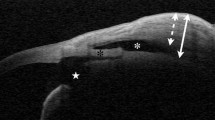
Images of filtering blebs taken in the horizontal plane and showing various bleb parameters. These are the total bleb height (a), bleb wall thickness (b), the height of the fluid-filled cavity (c), and bleb wall intensity (d). These values are shown in green, and the red lines indicate where measurements were made. A filtration opening on the margin of the scleral flap is indicated by an arrow
Statistical analysis
All of bleb parameters, IOP, patient age, and postoperative follow-up period, were compared between groups using Student’s t-test. The etiology of glaucoma and the base positions of conjunctival flaps were compared between groups using the χ2 test. The numbers of topical treatments required, and bleb vascularity grades, were compared using the Wilcoxon rank-sum test. Data were analyzed with the aid of the JMP statistical software package (version 8; SAS Institute, Cary, NC, USA).
Results
Twenty eyes of 131 eyes were categorized as type N. None of the type N blebs exhibited transconjunctival oozing and were excluded from further analysis. Sixty eyes (54.0 %) of 111 eyes exhibited transconjunctival oozing. Of these, 50 eyes oozed only when digital pressure was applied. The characteristics of eyes that did and did not ooze are shown in Table 1. The postoperative IOP was significantly lower in oozing eyes (11.7 ± 4.5 vs. 14.8 ± 4.0 mm Hg; P < 0.001). The number of different glaucoma medication classes required to treat oozing eyes was significantly less than required to treat non-oozing eyes (0.4 ± 1.0 vs. 1.1 ± 1.4; P < 0.001). The ratio of blebs with the base of limbal-based conjunctival flap was significantly higher in oozing eyes compared to non-oozing eyes, (45/15 vs 43/8; P =0.034). Parameters of gender, patient age, follow-up period, or glaucoma etiology did not differ significantly between the two groups.
The extent of bleb vascularity was significantly lower in oozing eyes (1.5 ± 0.7 vs. 2.4 ± 1.0; P =0.005). Bleb types classified with the aid of 3D AS-OCT images are summarized in Table 2, and did not differ significantly between the two groups (P =0.119). The total bleb heights were 1.1 ± 0.4 and 0.9 ± 0.4 mm, the fluid-filled cavity heights 0.4 ± 0.3 and 0.4 ± 0.4 mm, the bleb wall thicknesses 0.7 ± 0.4 and 0.5 ± 0.3 mm, and the bleb wall intensities 131.3 ± 45.7 and 180.9 ± 39.8 optical density units, in eyes with and without oozing, respectively (Fig. 4). The total bleb height and bleb wall thickness of oozing eyes were significantly greater than those of non-oozing eyes (P =0.028 and P < 0.001, respectively). Bleb wall density was significantly lower in oozing eyes (P < 0.001).
Typical examples of blebs, and 3D AS-OCT images of blebs that did or did not exhibit transconjunctival oozing, are shown in Fig. 5. Blebs exhibiting transconjunctival oozing had greater avascular areas (Fig. 5a) than did non-oozing blebs (Fig. 5b). Turning to internal bleb structures visible on 3D AS-OCT, an elevated total bleb height, enhanced bleb wall thickness, and a reduced bleb wall intensity, were characteristics of oozing blebs (Fig. 5c, d shows a non-oozing bleb for comparison). Upon examination of 3D bleb images, the wall surface of an oozing bleb tended to be irregular (Fig. 5e), whereas a non-oozing bleb surface tended to be smooth (Fig. 5f).
Typical examples of blebs with (a, c, e) and without (b, d, f) transconjunctival oozing. The upper (a, b), middle (c, d), and lower images (e, f) show photographs of blebs, horizontal slices, and reconstructed 3D images acquired using three-dimensional anterior segment optical coherence tomography, respectively. Blebs exhibiting transconjunctival oozing had greater extents of avascular area (a) than did blebs lacking oozing (b). If transconjunctival oozing was present, the bleb height was relatively great, and the bleb wall thick and of low intensity, with a sponge-like appearance (in part) (c). In contrast, the height of blebs lacking transconjunctival oozing was relatively low, and the bleb wall relatively thin and of high intensity (d). The wall surfaces of oozing blebs tended to be irregular (e), whereas the wall surfaces of blebs that were not oozing tended to be smooth (f)
Discussion
In the present study, we first identified oozing-dependent differences in the internal structures of filtering blebs, with the aid of 3D AS-OCT. As oozing was positively correlated with good IOP control both in the present study (Table 1) and past studies [1, 3], examination of the characteristics of oozing blebs may yield valuable insights into the optimal clinical management of glaucoma patients. In a prospective study, Hu et al. found that the prevalence of oozing upon application of digital pressure was 29.0 % within 1 year and 38.2 % within 3 years after trabeculectomy (with application of antimetabolites) [2]. Anand et al. reported that the prevalence of oozing was 59.0 % over an average observational period of 609 days after trabeculectomy [6]. Although the oozing prevalence noted in the present study could not be compared with those of past studies because of the cross-sectional design of the present work, we hypothesize that transconjunctival oozing is not rare, being in fact rather common after trabeculectomy with application of antimetabolites.
In the present study, the total height of oozing blebs was relatively great (Fig. 3a), possibly reflecting enhanced aqueous outflow into such blebs. Although it remains unclear whether an increased outflow into a bleb is directly associated with oozing, it is reasonable to suggest that flat non-functioning blebs may not ooze, because of a lack of aqueous humor under the bleb wall. Indeed, 20 blebs classified as type N were non-oozing blebs. In contrast, the height of the fluid-filled cavity did not differ significantly between the two groups (Fig. 3b). Thus, we suggest that the height of that cavity may not indicate either whether oozing is in play or the status (good or bad) of IOP control.
During transconjunctival oozing, fluid is thought to egress through the bleb wall. Thus, it was important to evaluate bleb wall parameters. Notably, the wall thickness was higher and the wall density lower in oozing compared to non-oozing blebs (Fig. 3c, d). This may be because a thin, high-density bleb wall, composed of a dense mixture of cells and extracellular matrix, is less permeable to aqueous humor. As is true of the well-studied trabecular meshwork, which is physiologically important in terms of aqueous outflow, an increase in empty space within the bleb wall may encourage oozing of aqueous humor through that wall. If this is true, bioactive molecules (including growth factors and cytokines) in the aqueous humor might induce the observed characteristic changes in the bleb wall. For example, proinflammatory cytokines attract inflammatory cells, and transforming growth factor-β induces contraction of such cells and also collagen gels [7]. Thus, eyes expressing high levels of bioactive molecules in a viscous aqueous matrix develop thick and high-density bleb walls inhibiting transconjunctival oozing, with a possible concomitant loss of effective IOP control. Interestingly, we previously found that a high level of monocyte chemotactic protein-1, a proinflammatory cytokine, in aqueous humor was a significant prognostic factor in terms of surgical failure of trabeculectomy in open-angle glaucoma patients [8]. Although the detailed effects of the bioactive molecules of aqueous humor on the bleb wall remain elusive, both bleb wall parameters and the nature of the aqueous humor may be important in terms of good IOP control.
Earlier studies found that oozing was exhibited predominantly by thin blebs [2]; these data are not in complete agreement with those of the present study. However, the apparent discrepancy may be linguistic in nature, being possibly based on differences in the definition of bleb wall “thickness”. Hu et al. categorized bleb walls according to whether the scleral incision lines or nylon sutures of the scleral flap were observable under the conjunctival flap [2]. In contrast, we defined the bleb wall as the tissue between the fluid-filled cavity and the bleb surface, and measured the real wall thickness on 3D AS-OCT images. As shown in Fig. 5, the wall of an oozing bleb was thick by our definition, but the wall was more transparent than the “thinner” wall of a non-oozing bleb, which may have been classified as a “thin” bleb by Hu et al. (cited above). Thus, we hypothesize that the wall of an oozing bleb is composed of less dense sponge-like tissue, which is both more transparent and sometimes thicker than the wall of a non-oozing bleb. This emphasizes the clinical importance of bleb imaging using 3D AS-OCT, and shows that use of this modality can clarify the nature of internal bleb structures that are often difficult to comprehend upon conventional slit-lamp observations.
In the present study, oozing blebs had low vascularity scores, in agreement with the data of prior studies [1, 2]. If the vascular area of the bleb wall is extensive, such tissue can deliver high numbers of proinflammatory cells and vessel-derived bioactive molecules, enhancing wound healing and strengthening the epithelial barrier after trabeculectomy. Thus, the association of oozing with low bleb vascularity is considered to reflect a direct correlation between the two features. Bleb characteristics, including vascularity, have been reported to be similar in leaking and oozing blebs [1]. In addition, blebs with limbal-based conjunctival flap were more frequently found in oozing eyes in the present study. One large trial analyzed 439 trabeculectomy sites in 347 patients, and reported that bleb-related infections occurred more frequently in limbal-based than in fornix-based trabeculectomy during a 4-year observation after trabeculectomy, though the difference was statistically marginal (P = 0.054) [9]. In contrast, another large study that included 908 eyes of 908 Japanese patients reported that the cumulative probability of bleb-related infection was not different at a 2.5-year follow up [10]. Thus, the effect of the base of conjunctival flap remains controversial. As several lines of evidence suggest that bleb leakage is a significant risk factor for development of infection [9–12], oozing may potentially be (positively) prognostic in terms of the development of blebitis. Thus, it may be important to identify the clinical characteristics of blebs in patients at high risk of this condition. However, it remains unclear whether oozing from the bleb surface should be treated to reduce aqueous egression, because oozing is associated with good IOP control after trabeculectomy.
In conclusion, transconjunctival oozing after trabeculectomy (with application of MMC) was associated with a low IOP, low bleb vascularity, the base of conjunctival flap, an elevated total bleb height, an enhanced bleb wall thickness, and a low bleb wall density. These data bear on the impact of oozing on IOP control and emphasize the clinical importance of imaging internal bleb structures using 3D AS-OCT.
References
Matsuo H, Tomidokoro A, Suzuki Y, Shirato S, Araie M (2002) Late-onset transconjunctival oozing and point leak of aqueous humor from filtering bleb after trabeculectomy. Am J Ophthalmol 133:456–462
Hu CY, Matsuo H, Tomita G et al (2003) Clinical characteristics and leakage of functioning blebs after trabeculectomy with mitomycin-C in primary glaucoma patients. Ophthalmology 110:345–352
Matsuo H, Tomidokoro A, Tomita G, Araie M (2005) Topical application of autologous serum for the treatment of late-onset aqueous oozing or point-leak through filtering bleb. Eye (Lond) 19:23–28
Kojima S, Inoue T, Kawaji T, Tanihara H (2014) Filtration bleb revision guided by 3-dimensional anterior segment optical coherence tomography. J Glaucoma 23:312–315
Inoue T, Matsumura R, Kuroda U, Nakashima K, Kawaji T, Tanihara H (2012) Precise identification of filtration openings on the scleral flap by three-dimensional anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci 53:8288–8294
Anand N, Arora S, Clowes M (2006) Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol 90:175–180
Meyer-Ter-Vehn T, Gebhardt S, Sebald W et al (2006) p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci 47:1500–1509
Inoue T, Kawaji T, Tanihara H (2014) Monocyte chemotactic protein-1 level in the aqueous humour as a prognostic factor for the outcome of trabeculectomy. Clin Exp Ophthalmol 42:334–341
Solus JF, Jampel HD, Tracey PA et al (2012) Comparison of limbus-based and fornix-based trabeculectomy: success, bleb-related complications, and bleb morphology. Ophthalmology 119:703–711
Yamamoto T, Sawada A, Mayama C et al (2014) The 5-year incidence of bleb-related infection and its risk factors after filtering surgeries with adjunctive mitomycin C: Collaborative Bleb-Related Infection Incidence and Treatment Study 2. Ophthalmology 121:1001–1006
Song A, Scott IU, Flynn HW Jr, Budenz DL (2002) Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. Ophthalmology 109:985–991
Soltau JB, Rothman RF, Budenz DL et al (2000) Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol 118:338–342
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant Numbers 23390403, 23659814, and 23791994. The funding organization had no role in the design or conduct of this research.
Conflict of interest
None.
Financial interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakashima, KI., Inoue, T., Fukushima, A. et al. Evaluation of filtering blebs exhibiting transconjunctival oozing using anterior segment optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 253, 439–445 (2015). https://doi.org/10.1007/s00417-014-2872-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2872-3