Summary
Background
Inflammatory bowel diseases (IBDs) are a well-known risk factor for the development of colorectal cancer (CRC). This risk relates to different aspects of the disease, such as the duration, activity, and extension, and tends to increase in the presence of associated conditions, such as family history of CRC or some extra-intestinal manifestations. Rectal cancer (RC) in IBD has been poorly investigated.
Methods
We reviewed the scientific literature for data on the features and management of RC in the setting of IBD. Here, we provide a practical insight into the diagnosis and management of the condition.
Results
Several genetic and environmental factors promote the development of CRC, including alterations of intestinal microflora and mutations in the genes responsible for the cell cycle and for DNA mismatch repair. Dysplasia is the main evidence of a clear conversion of normal epithelium to cancer. Dysplasia is often multifocal, in contrast to sporadic CRC, which explains the tendency toward the development of synchronous and metachronous CRC in patients with IBD. Other conditions that need attention are strictures, for which the threshold for surgery must be low. Treatment of RC in patients with IBD follows the same oncologic criteria as non-IBD-related RC, but patients are often diagnosed at more advanced stages, suggesting that this is frequently overlooked. This is ultimately associated with poorer outcomes in IBD patients.
Conclusion
There is a pressing need for more data on IBD-related RC. Implementing knowledge will result in optimization of survival for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel diseases (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), are widely accepted as one of the important factors leading to colorectal cancer (CRC). IBD ranks as the third highest risk condition for CRC, behind only familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer syndrome (HNPCC) [1]. CRC has been reported to account for one sixth of UC-related deaths [2]. In addition, the problem of rectal cancer (RC) in IBD remains largely unexplored.
More recently, a reduced incidence of CRC in IBD has been observed. This is probably due to earlier diagnosis and more timely treatment of these conditions, because CRC arising in the setting of CD or UC has different features than sporadic CRC, meaning that it relies on an inflammation-driven carcinogenesis [3–5].
However, RC may still be overlooked in IBD patients, suggesting that much needs to be done in order to better understand the features and development of CRC in these patients. We herein review the characteristics of RC in patients suffering from CD or UC, and provide a practical overview of the etiology, presentation, and management of this problem. RC and CRC represent two faces of the same entity; hence, we will deal with CRC and focus on RC when specific considerations are possible and/or needed.
Colorectal cancer, rectal cancer, and IBDs
Burden of disease
The historical global risk for CRC in UC is about 7.2 % after 20 years of disease [6]. In a meta-analysis of 116 studies, Eaden [7] showed that there was an increased risk for developing CRC in patients with UC and this became appreciable after 10 years of disease.
The development of CRC in patients with IBD is related to the extension, the duration, and the severity of the disease.
The prospective surveillance data from Rutter et al. [8] have demonstrated that the median duration of UC to the diagnosis of CRC was 23.5 years (range, 11–48 years). The cumulative incidence of CRC was 0 % at 10 years, 2.5 % at 20 years, 7.6 % at 30 years, 10.8 % at 40 years, and 13.5 % at 45 years (from the onset of colitis).
Lakatos et al. [9] retrospectively reviewed the data of 723 patients with IBD from a 30-year database. They evaluated the incidence of CRC based on the duration of the disease and found the cumulative risk to be 0.6 % at 10 years (95% CI: 0.2–1.0), 5.4 % at 20 years (95% CI: 3.7–7.1), and 7.5 % at 30 years (95% CI: 4.8–10.2) [9].
The extent of disease is an important risk factor, along with UC duration, for the development of CRC. Several studies have shown a general consensus that indicates little or no increased risk of CRC in patients with proctitis or proctosigmoiditis, while the risk is intermediate in those with left-sided colitis and higher with pan-colitis [9–15]. A Danish population-based cohort study involving 1,515 patients diagnosed with UC showed that the risk of CRC in UC is higher among patients with extensive colitis (standardized incidence ratio [SIR], 1.85; 95% CI: 0.60–4.32) [13].
The severity of the disease is another risk factor for the onset of CRC. It is believed that the incidence of CRC is linked to inflammation and that anti-inflammatory treatment can reduce this risk. Recent studies have focused on this association. Gupta et al. [16] retrospectively studied a cohort of 418 patients undergoing colonoscopy surveillance for UC; they described a close relationship between histological inflammation over time and progression of advanced neoplasia (hazard ratio, 3.0; 95% CI: 1.4–6.3), which remained an independent risk factor in multivariate analysis. However, other studies found that people with quiescent disease have a similar risk of developing CRC compared with patients with active disease [9, 17].
Patients with a family history of CRC have a higher risk of developing IBD-related CRC compared with patients who do not have familiarity. Askling et al. studied 876 patients born between 1941 and 1995, and found that patients with second-degree relatives suffering from sporadic CRC had a risk two times greater than CRC incurring in IBD [18]. If the patient had a first-degree relative affected by CRC and he or she was younger than 50, the risk was up to nine times higher [18]. Velayos et al. [19] also found that family history of CRC is to be considered a relevant risk factor in patients with UC.
It is now well established that CD patients with colonic disease have a CRC risk similar to that of UC [20–23]. However, the development of dysplasia in CD can involve both the small bowel and the colon [3–5]. Jess et al. found a pooled SIR of 1.9 (1.4–2.5) [13]. Canavan et al. [24] described a relative risk of 4.5 in patients with CD colitis and a cumulative risk of 2.9 % at 10 years, 5.6 % at 20 years, and 8.3 % at 30 years. Von Roon [25] estimated a 2.44 relative risk for CRC compared with the general population, and Laukoetter et al. [26] described an incidence two to three times greater than age-matched controls. In a population-based study from the Mayo Clinic, only a slightly increased risk was found in CD, which is in marked contrast to a 40-fold increased risk of developing small bowel malignancy [27].
In CD, young age at onset is associated with an increased risk of CRC [20]. Söderlund et al.[28] found that males have a 60 % higher risk of CRC, and a greater cumulative incidence after 40 years of disease; the effect of gender was limited to patients with more than 10 years of follow-up and in those aged <45 years at diagnosis.
It is believed that there is a greater risk of developing CRC when at least one third of the colon is involved [23]. The risk of CRC in CD is equivalent to that in UC when comparison is matched to a similar extent of disease. This is relevant when considering RC in IBD patients. Gillen et al. [29] compared patients with extensive Crohn’s colitis and patients with extensive UC, and found that the relative risk of developing CRC was similar between the two groups: 18 for CD and 19 for UC. The cumulative risk of CRC was 8 % at 22 years for patients with CD compared with 7 % at 22 years for patients with UC [29].
As previously reported, another important factor to consider is the location of the disease. A meta-analysis of 12 hospital-based and population-based studies of CRC risk in CD [30] revealed a relative risk of 4.5 in patients with involvement of the colon, while in patients with ileal involvement alone the risk of developing CRC was not dissimilar to that of the general population.
Stahl et al. [31] showed that the involvement of the rectum in patients with CD is lower than for those patients with sporadic cancer, 17 % vs. 38 %; and conversely the location above the splenic flexure is involved in 59 % of patients with CD compared with 28 % of patients with sporadic cancer.
Other locations considered at risk of developing CRC are bypassed segments of bowel [32] and perianal fistulae [4, 33]. Particular consideration should be given to strictures. Lovasz et al. showed that patients with a stricturing phenotype had an absolute risk of 7.5 % for CRC within 10 years from diagnosis, and the risk was 19 times greater than for patients with any other disease phenotype [34]. In addition, the period between the diagnosis of CD and the development of CRC in patients with stricturing CD was much shorter than in patients with other disease phenotypes [34].
Another feature of CRC arising in the setting of CD is the higher incidence of mucinous adenocarcinoma, which occurs in 50 % of cases, compared with only 10 % of sporadic cancers [35]. This particularly aggressive histology, along with an often delayed diagnosis, explains the high incidence of cancer in an advanced stage at diagnosis compared with sporadic RC and the poor outcomes in these patients.
Pathogenesis
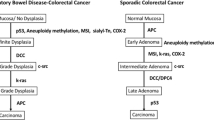
Genetic and environmental factors contribute to the pathogenesis of CRC in IBD. Many of the factors involved in the development of sporadic CRC play an important role in the development of CRC associated with IBD [36, 37; Fig. 1-5]. In IBD-related cancer, some suggested a sequence similar to that found for the adenoma-carcinoma in sporadic CRC; however, sporadic CRC occurs on one or two foci of dysplasia in the colon, whereas CRC in IBD occurs on a multifocal dysplasia, indicating a “change field effect”[38, 39]. Aneuploidy, a marker of genomic instability, has been demonstrated in 20–50 % of dysplastic lesions and in 50–90 % of cancers, and is observed in long-standing UC [38, 40–42]. The aneuploidy does not correlate morphologically with dysplasia, probably being more prevalent with respect to it. The cause of the field effect can be explained by the constant re-epithelization of the chronically inflamed and ulcerated areas of the colonic mucosa by abnormal healing clones that expand [43], as well as by environmental changes, such as the dysbiosis of the microflora, which can cause the appearance of these mutations in a number of spots [44].
The two main types of genomic instability found in CRC are chromosomal instability (CIN) and the instability of micro satellites (MSI) [45]. CIN and MSI in IBD-related CRC occur with the same frequency observed for the sporadic CRC (85 % CIN; 15 % MSI), but they differ in timing and frequency from the pattern seen with sporadic CRC [39, 40, 46, 47]. It is known that p53 mutations in IBD-related CRC occur earlier than in sporadic cancer, probably representing a key initiator event [48]. Conversely, mutations in the APC gene, considered an early event in sporadic cancer, occur late in the IBD CRC [49, 50]. However, the role of p53 remains to be further explored [51].The role of the MSI pathway is less clear. MSI due to a defective DNA-mismatch repair occurs with a variable frequency in IBD-related CRC (28–36.38 %), while the frequency of high MSI is observed in 10–15 % of sporadic CRC[52]. A study on mutational events secondary to MSI found similar rates between IBD-related and sporadic CRC [52]. Data on the methylation pathway are contradictory. Some evidence suggests a greater involvement of this pathway in the development of IBD-related CRC [53]. The hypermethylation of CpG islands is a key mechanism for epigenetic silencing of tumor suppressor genes [37, 47], and may precede dysplasia. It can be found through the colon in patients with UC [54]. p16INK4, a cell cycle inhibitor, also implicated in sporadic CRC, is commonly hypermethylated in tissues with UC, in the absence of dysplasia [37, 55, 56].
Other important elements in the pathogenesis of IBD-related CRC are intertwined with chronic inflammation, such as induction of cyclo-oxygenase-2 (COX-2), inflammatory cytokines, and chemokines. Some studies have shown that COX-2 is induced by inflammation and activated by inflammatory stimuli such as IL-1, IFN-γ, and TNF-α [57]. The expression of COX-2 is increased in about 50 % of adenomas and 85 % of adenocarcinomas [58, 59]. TNF-α is released by activated macrophages and T cells, binds to the TNF receptor (TNF-R), and has been reported to promote inflammation and cancer-associated colitis [39]. High levels of IL-6 and soluble receptor of IL-6 in patients with IBD, up-regulating the expression of anti-apoptotic factors, promote the accumulation of T cells in the lamina propria of the colon [60, 61]. The expression of IL-6 and STAT3 is increased in patients with active UC and after progression to CRC compared with controls or with patients with inactive disease [62]. IL-10 suppresses the levels of IL-6 and controls the development of IBD-related CRC in animal models. It was recently shown that patients with mutations in the receptor for IL-10 develop early and aggressive disease [63].
Oxidative stress can also contribute to the pathogenesis of CRC. It develops in response to inflammatory reactions, especially following the production of large amounts of reactive oxygen and nitrogen species (RONS) by activated neutrophils and macrophages [37, 39, 57]. The inflamed tissues of patients with UC and CD have an increased expression of nitric oxide synthase (NOS) and RONS [50, 64, 65]. RONS can interact with key genes involved in carcinogenesis, such as p53 and genes for DNA mismatch repair [37].
Recent studies report changes in the microflora in patients with IBD. Many animal models of IBD suggested that commensal or specific bacteria may contribute to the development of IBD-related CRC. For example, adherent-invasive Escherichia coli is associated with both IBD and CRC [66, 67]. Deletion of the bacterial polyketide synthase (pks) genotoxic island from E. coli NC101 reduces the development of dysplasia and cancer, independent of inflammation [3].
Precancerous lesions and conditions
CRC develops through several stages involving inflammation and dysplasia. The latter is defined as a clear conversion of the intestinal epithelium into cancer through a precancerous condition: dysplasia. Dysplasia is defined as “an unequivocal neoplastic transformation of the epithelium excluding all reactive changes as expression of histological abnormalities suggesting that clone(s) of cells have DNA damage rendering them neoplastic and predisposed to malignancy, for this reason the correct definition of dysplasia is the histological expression of DNA damage that precedes malignancy” [68–70]. Dysplasia is classified as low-grade dysplasia (LGD) and high-grade dysplasia (HGD). Cases where the diagnosis of dysplasia appears uncertain, especially in the setting of active inflammation, are referred to as “indefinite for dysplasia” [68]. When dysplasia is diagnosed, a second evaluation of the biopsies by another pathologist is strongly indicated.
The diagnosis of dysplasia is not easy to make, especially because the histological changes secondary to inflammation are often indistinguishable from dysplasia. In addition, there is a significant interobserver variation for the grading of dysplasia, particularly for LGD [69–71]. Dysplasia may arise in a normal-appearing area of the intestinal mucosa, and in that case it is called “flat dysplasia,” or as a lesion macroscopically detectable, such sporadic adenomas, or plaques, or depressed/raised lesions, in which case it is defined as “dysplasia-associated lesion or mass” (DALM).
An adequate number of biopsies of colonic mucosa are needed to rule out the presence of dysplasia. It has been suggested that 56 nontargeted jumbo-forceps biopsies might be necessary for a 95 % confidence, and that a confidence of 90 % can be obtained with 33 biopsies [72]. Currently, it is believed that the best approach is to carry out a minimum of 32 biopsies for each endoscopic control, obtaining samples from the four quadrants every 10 cm, in addition to biopsies of suspicious lesions [73].
Dysplasia is associated with an increased risk of synchronous or metachronous CRC. In patients with UC, synchronous CRC can occur in 10–20 % of patients with flat LGD, in 42–45.5 % of patients with flat HGD, and in 33–43 % of patients with DALM [8, 74]. The risk of developing metachronous CRC is 25–32 % in patients with HGD, and highly variable in patients with LGD [75]. A recent meta-analysis estimated that the positive predictive value of flat LGD for progression to CRC was 22 %, and that patients with LGD had a risk of CRC nine times higher than those without dysplasia [76]. It is important to note that one study shows a greater progression to HGD or CRC from unifocal than multifocal LGD; however, there are no clinical features that are predictive of subsequent progression, and progression to node-positive CRC can occur in patients with flat LGD within 12 months from a previous dysplasia-free diagnosis [77–79].
Patients with the diagnosis of indefinite for dysplasia represent a poorly studied group of IBD subjects. Recently, Ullman et al. [80] reported a progression rate to HGD or CRC of 9.0 % at 5 years in a group of 56 patients considered indefinite for dysplasia, and this rate appeared intermediate between patients considered dysplasia-free and those with flat LGD. The Crohn’s and Colitis Foundation of America (CCFA) consensus guideline recommends that patients with biopsies indefinite for dysplasia are followed up with annual surveillance examinations [81]. Patients without histological evidence of dysplasia show the lowest rate of neoplastic progression [80]. Ullman et al. also reported an incidence of CRC of 1.1 % at 5 years in a study of 311 patients [80]. Examinations every 1–2 years is suggested by the CCFA Consensus conference for this group of patients [81].
The presence of inflammatory polyps correlates with an increased risk of CRC; in fact, they have no malignant potential [82] but seem to increase the risk of not recognizing dysplastic lesions, and represent an index of a previous severe inflammation. A retrospective study [19] using the Mayo Clinic centralized diagnostic index identified 188 patients with UC-associated CRC, and compared them with 1,528 gender- and disease-extent-matched controls. The presence of postinflammatory polyps remained statistically associated with CRC even after adjusting for surveillance and anti-inflammatory treatments[19]. In the follow-up case-control study by Rutter et al. [83], patients with CRC were significantly more likely to have post-inflammatory polyps than were controls.
Primary sclerosing cholangitis (PSC) is a chronic cholestatic disease and a recognized risk factor for CRC in IBD, especially in patients with UC compared with CD (80 % vs. 10 %) [84]. In particular, patients with UC complicated by PSC have a risk of CRC four times greater than those with non-PSC UC [85]. The risk remains high even after liver transplantation [85]. Kornfeld et al. [86] showed that the cumulative risk was 33 % at 20 years and 40 % at 30 years after diagnosis of UC.
In a meta-analysis of 11 studies with a total of 16,844 patients with UC, Soetikno et al. [85] showed that 21 % of patients with UC and PSC developed CRC, unlike patients without PSC, for whom the incidence of CRC was 4 %. The OR for developing dysplasia or cancer in patients with PSC was therefore 4.8 (95% CI: 3.6–6.4) [85].
Several theories have been proposed to explain the increased incidence of CRC in PSC-IBD: Some authors consider the high content of bile acids in the colon to be the cause of the increased risk of CRC in these patients[46]. Sokol et al. [87] have also observed that patients with PSC received more 5‑aminosalicylic acid (5-ASA) than patients with IBD alone, considering that the main risk factor.
It was also demonstrated that the CRC in IBD patients with PSC has different characteristics compared with CRC diagnosed in patients without PSC. CRC in PSC-IBD is diagnosed at an earlier age, is frequently located at the right colon, or at least above the splenic flexure, and the interval between the diagnosis of both diseases and the development of dysplasia or cancer is reduced [88].
Colonic strictures in patients with UC are an important risk factor for CRC, and represent an indication for surgical treatment, being closely associated with the presence of dysplasia or cancer [81], especially if the stricture is causing symptoms and is located in the proximal colon [89].
As for UC, in patients with CD the presence of PSC is associated with an increased risk of CRC, especially if colitis is extensive. Two retrospective case-control studies reported conflicting results. A study from Sweden described an increased risk in patients with PSC-CD compared with non-PSC [90], whereas a study from the UK did not find any significant differences [91].
In CD, the incidence of dysplasia in CRC development was reviewed by Friedeman [23] through a surveillance study. CRC was found in 25 % of patients undergoing surgery for multifocal LGD and in 50 % of patients with HGD; none of the patients with unifocal LGD developed CRC during surveillance.
Korelitz et al. [92] reported the results of 812 biopsies of the rectum performed on 356 patients with CD: 5 % of the patients showed dysplasia, but only after four repeated biopsies. Of these, 17 % developed cancer or adenoma. Another four patients developed adenocarcinoma of the rectum without showing dysplasia at previous biopsies. There is a close relationship between inflammation, dysplasia, and cancer [93], and three studies have demonstrated the presence of severe epithelial dysplasia in 80–100 % of patients with CD-associated CRC [35, 94, 95]. Petras et al. [95] argued that this association is more limited and less frequent than CRC arising in the setting of UC. Stahl et al. [31] showed that 77 % of CD patients developed cancer in those areas known to have been involved with inflammatory disease. Severe dysplasia was detected only in 27 % of patients, questioning its role in the development of CRC [31].
Management of rectal dysplasia and cancer
Colorectal dysplasia
As discussed earlier, multifocal LGD or HGD is associated with higher rates of synchronous or metachronous CRC, advocating the need for total proctocolectomy in such cases.
The management of flat, unifocal LGD is controversial. The authors are divided between two options: a prophylactic proctocolectomy given the high risk (approximately 20 %) of synchronous CRC, or continue the surveillance, performing checks at narrower intervals (3–6 months) [81]. The decision must be individualized and should be evaluated carefully for additional risk factors.
Raised lesions are the most frequently found during endoscopic follow-up: in a retrospective study of 56 patients with dysplasia from St. Mark’s Hospital, 50 patients (89.3 %) had macroscopically detectable lesions [96]. The management of these lesions depends on their size, appearance, and the ability to remove them entirely in the course of colonoscopy. In the absence of dysplasia in the remaining parts of the colon, the risk for developing dysplasia or CRC after polypectomy seems very low after an observation period of 82 months [97, 98]. Rubin et al. [99] found similar results in a cohort study of patients followed up for 49 months. Hence, provided polypectomy is complete and biopsies show that the mucosa surrounding the lesion is dysplasia-free, endoscopic surveillance at 6 months, until other abnormal findings are observed, is a viable choice. Conversely, if the surrounding mucosa appears dysplastic, or if the polypoid lesion cannot be removed entirely, or has different characteristics from typical adenoma, the risk of a synchronous CRC appears to be unacceptably high, and a total proctocolectomy would be a safer option.
Adenomatous polyps in areas free from inflammatory disease should be considered sporadic adenomas and treated accordingly [100].
Inflammatory polyps (pseudo-polyps) are commonly observed during endoscopic investigations. Their presentation may differ, but they have no malignant potential. However, in the presence of a massive pseudopolyposis, alternative conducts could be required, ranging from a complete removal of all lesions to colectomy.
Stenosis represents a further challenge when dealing with during endoscopic examination of patients with IBD. Colonic strictures in the setting of UC or CD are best treated with surgical resection (colectomy, proctocolectomy, or segmental resection, as required).
In patients with remittent CD, colonoscopy screening at 8–10 years after diagnosis of the disease is recommended. It must be repeated at intervals of time to be determined according to the individual risk factors. In patients with PSC, active disease, family history of CRC, dysplasia, or stenosis, an annual survey is desirable [101].
The management of dysplasia in CD depends on its severity and localization. According to these features, the options of surgery or continued endoscopic surveillance are both viable. Colectomy is the option for patients with HGD or multifocal flat LGD, after the diagnosis is confirmed by a second pathologist. Multifocal LGD is the strongest indication for colectomy. In patients with rectal strictures, the eventuality of RC is not negligible, and there is no agreement concerning the ideal sampling of the lesion to rule out malignancies [102, 103]. The threshold for surgery in these patients should be low, especially when strictures are not passable with the endoscope [104].
Rectal cancer
Proctocolectomy with ileal pouch anal anastomosis (IPAA) is the procedure of choice for patients with acute or refractory-to-medical therapy UC [105]. This surgical option ensures excellent quality of life for patients and can be performed with low mortality and morbidity [106, 107]. In patients with a concomitant RC, resection must also take account of oncologic radicality. For this reason, it is essential to properly stage the disease and study it with a multidisciplinary team [108]. There are many factors that can affect eligibility for restorative surgery in UC patients with RC. For example, patients with involvement of the lower rectum may require resection of the sphincters to obtain a complete excision of the RC [109, 110], meaning abdominoperineal excision (APE), which excludes the possibility of restorative surgery. Another factor to consider is the benefit that radiotherapy offers patients with low, advanced, or aggressive RC, and its role in preventing recurrences [111–114]. However, the small bowel has high sensitivity to radiation damage, and the creation of a pouch in the center of the radiation field can impair its function.
UC patients with RC should be carefully selected for IPAA. IPAA is a complex surgical procedure that requires expertise and is associated with a higher rate of complications compared with single-stage APE with permanent ileostomy. Snelgrove et al. [115] suggested that IPAA is an effective option for young patients, who are fit, with good function of the anal sphincter, and who have RC with favorable features. Generally, IPAA should not be performed or carefully considered in patients with rectal cancer in stage T3/T4, and is proscribed in those in whom the resection margin could be involved by the tumor (R1 resection). When radiotherapy is necessary, it is advisable to perform it preoperatively rather than postoperatively. The failure rate of the procedure ranges between 14 and 18 %, higher than that of patients who receive IPAA without RC [116–123]. This finding may be due to several factors, such as poor nutrition owing to cancer or treatment that can impair healing.
The treatment of RC in patients with CD requires proctectomy and associated resections of the colon or total colectomy depending on the extent of inflammation and disease localization. The ideal option would be tumor-free resection with colorectal or coloanal anastomosis construction with adequate lymphadenectomy, and possible extension of the resection in cases of inflammation [124]. Other surgical procedures could be suited to the individual case, e. g., subtotal colectomy, total proctocolectomy with ileostomy, and palliative procedures, but IPAA is proscribed in CD owing to cancer concerns and risk of failure [5, 27, 125–129]. CD patients with RC may tolerate chemotherapy and radiation therapy well [38]. The results are the same as for patients with non-IBD-related RC in the corresponding stage [38, 40], but, overall, the prognosis is poorer according to age, because of the advanced stage of disease at diagnosis.
Conclusion
The risk of CRC in IBD patients is well known. Several factors contribute to its development, such as the duration, severity, and extent of disease, and familiarity for sporadic CRC. The onset of CRC is further increased by associated conditions, such as PSC. There is a pressing need for further data before reaching a consensus on the management of RC in IBD [130, 131]. Implementing early diagnosis would be crucial for obtaining better results (e. g., by means of biomarkers) [132].
The development of CRC is related to inflammation and dysplasia. Surveillance should be adapted to the individual patient, taking into account risk factors for RC development. Endoscopic surveillance allows one to make a diagnosis of CRC earlier in IBD, but poor patient compliance reduces its effectiveness [133].
Colonic strictures, especially in long-standing disease, are prudently managed with resection.
IBD patients who have RC must be managed following the criteria of oncologic radicality. When radiotherapy is necessary in UC with RC, it is best performed before IPAA.
The treatment of RC in patients with CD requires proctectomy and associated resections of the colon or total colectomy depending on the extent of inflammation and disease localization, but IPAA is proscribed. Neo-adjuvant and adjuvant treatments are well tolerated by patients with CD.
Abbreviations
- 5-ASA:
-
5-aminosalicylic acid
- APE:
-
Abdominoperineal excision
- CCFA:
-
Crohn’s and Colitis Foundation of America
- CD:
-
Crohn’s disease
- CI:
-
Confidence intervals
- CIN:
-
Chromosomal instability
- COX-2:
-
Cyclo-oxygenase-2
- CRC:
-
Colorectal cancer
- DALM:
-
Dysplasia-associated lesion or mass
- FAP:
-
Familial adenomatous polyposis
- HGD:
-
High-grade dysplasia
- HNPCC:
-
Hereditary non-polyposis colorectal cancer syndrome
- IBD:
-
Inflammatory bowel disease
- IPAA:
-
Ileal pouch–anal anastomosis
- LGD:
-
Low-grade dysplasia
- MSI:
-
Micro satellites instability
- NOS:
-
Nitric oxide synthase
- OR:
-
Odds ratio
- PSC:
-
Primary sclerosing cholangitis
- RC:
-
Rectal cancer
- RONS:
-
Reactive oxygen and nitrogen species
- SIR:
-
Standardized incidence ratio
- UC:
-
Ulcerative colitis
References
Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol. 2010;101:706–12.
Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ, Munkholm P, et al. Risk of intestinal cancer in inflammatory bowel disease:a population-based study from olmsted county,Minnesota. Gastroenterology. 2006;130:1039–46.
Sebastian S, Hernández V, Myrelid P, Kariv R, Tsianos E, Toruner M, Marti-Gallostra M, Spinelli A, Meulen-de Jong AE van der, Yuksel ES, Gasche C, Ardizzone S, Danese S. Colorectal cancer in inflammatory bowel disease: results of the 3rd ECCO pathogenesis scientific workshop (I). J Crohns Colitis. 2014;8(1):5–18.
Egan L, D’Inca R, Jess T, Pellino G, Carbonnel F, Bokemeyer B, Harbord M, Nunes P, Van der Woude J, Selvaggi F, Triantafillidis J. Non-colorectal intestinal tract carcinomas in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (II). J Crohns Colitis. 2014;8(1):19–30.
Selvaggi F, Pellino G, Canonico S, Sciaudone G. Systematic review of cuff and pouch cancer in patients with ileal pelvic pouch for ulcerative colitis. Inflamm Bowel Dis. 2014;20(7):1296–308.
Gyde SN, Prior O, Allan RN, et al. Colorectal cancer in ulcerative colitis:a cohort study of primary referrals from three centres. Gut. 1988;29:206–17.
Eaden J. Review article: colorectal carcinoma and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:24–30.
Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis ofa colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–8.
Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, Fischer S, Vargha P, Lakatos PL. Risk factors for ulcerative colitis-associated colorectal cancer ina Hungarian cohort of patients with ulcerative colitis: results ofa population-based study. Inflamm Bowel Dis. 2006;12:205–11.
Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis:a meta-analysis. Gut. 2001;48:526–35.
Kim BJ, Yang SK, Kim JS, Jeen YT, Choi H, Han DS, Kim HJ, Kim WH, Kim JY, Chang DK. Trends of ulcerative colitis-associated colorectal cancer in Korea: A KASID study. J Gastroenterol Hepatol. 2009;24:667–71.
Söderlund S, Brandt L, Lapidus A, Karlén P, Broström O, Löfberg R, Ekbom A, Askling J. Decreasing time-trends of colorectal cancer ina large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561–1157, quiz 1818–9.
Jess T, Horváth-Puhó E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment:a Danish population-based cohort study. Am J Gastroenterol. 2013;108:1869–76.
Potack J, Itzkowitz SH. Colorectal Cancer in Inflammatory Bowel Disease. Gut Liver. 2008;2:61–73.
Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33.
Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105.
Smith MP, Loe RH. Sclerosing cholangitis. Review of recent case reports and associated diseases and four new cases. Am J Surg. 1965;110:239–46.
Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Colorectal cancer rates among first-degree relatives of patients with inflammatory bowel disease:a population-based cohort study. Lancet. 2001;357:262–6.
Velayos FS, Loftus EV, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: a case-control study. Gastroenterology. 2006;130:1941–9.
Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–9.
Jess T, Winther KV, Munkholm P, Langholz E, Binder V. Intestinal and extra-intestinal cancer in Crohn’s disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Aliment Pharmacol Ther. 2004;19:287–93.
Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease:a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–9.
Friedman S, Rubin PH, Bodian C, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn’s colitis: results ofa surveillance program spanning 25 years. Clin Gastroenterol Hepatol. 2008;6:993–8.
Canavan C, Abrams KR, Meta-analysis MJ. colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–104.
Roon AC von, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum. 2007;50:839–55.
Laukoetter MG, Mennigen R, Hannig CM, et al. Intestinal cancer risk in Crohn’s disease:a meta-analysis. J Gastrointest Surg. 2011;15:576–83.
Selvaggi F, Pellino G. Pouch-related fistula and intraoperative tricks to prevent it. Tech Coloproctol. 2015;19:63–7.
Söderlund S, Granath F, Broström O, Karlén P, Löfberg R, Ekbom A, et al. Inflammatory bowel disease confersa lower risk of colorectal cancer to females than males. Gastroenterology. 2010;138((5):1697–703.
Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease:a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–2.
Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–104.
Stahl TJ, Schoetz DJ, Roberts PL, et al. Crohn’s disease and carcinoma: Increasing justification for surveillance? Dis Colon Rectum. 1992;35:850–6.
Greenstein AJ, Sachar D, Pucillo A, Kreel I, Geller S, Janowitz HD, Aufses A Jr. Cancer in Crohn’s disease after diversionary surgery. A report of seven carcinomas occurring in excluded bowel. Am J Surg. 1978;135:86–90.
Connell WR, Sheffield JP, Kamm MA, Ritchie JK, Hawley PR, Lennard-Jones JE. Lower gastrointestinal malignancy in Crohn’s disease. Gut. 1994;35:347–52.
Lovasz BD, Lakatos L, Golovics PA, David G, Pandur T, Erdelyi Z, et al. Risk of colorectal cancer in Crohn’s disease patients with colonic involvement and stenosing disease in a population-based cohort from Hungary. J Gastrointestin Liver Dis. 2013;22(3):265–8.
Hamilton SR. Colorectal carcinoma in patients with Crohn’s disease. Gastroenterology. 1985;89:398–407.
Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–37.
Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405–10.
Green S, Stock RG, Greenstein AJ. Rectal cancer and inflammatory bowel disease: natural history and implication for radiation therapy. Int J Radiol Oncol Biol Phys. 1999;44:835–40.
Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16.
Slater G, Greenstein A, Aufses AH Jr.. Anal carcinoma in patients with Crohn’s disease. Ann Surg. 1984;199:348–50.
Befrits R, Hammarberg C, Rubio C, Jaramillo E, Tribukait B. DNA aneuploidy and histologic dysplasia in long-standing ulcerative colitis. A 10-year follow-up study. Dis Colon Rectum. 1994;37:313–9, discussion 319-320.
Willenbucher RF, Zelman SJ, Ferrell LD, Moore DH, Waldman FM. Chromosomal alterations in ulcerative colitis-related neoplastic progression. Gastroenterology. 1997;113:791–801.
Lyda MH, Noffsinger A, Belli J, et al. Micro satellite instability and k‑ras mutations in patients with ulcerative colitis. Hum Pathol. 2000;31(6):655–71.
Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G7–G17.
Hardy RG, Meltzer SJ, Jankowski JA. ABC of colorectal cancer. Molecular basis for risk factors. BMJ. 2000;321:886–9.
Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937–47.
Itzkowitz S. Colon carcinogenesis in inflammatory bowel disease: applying molecular genetics to clinical practice. J Clin Gastroenterol. 2003;36:70–4, discussion S94–96.
Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, et al. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;36(2):542–50.
Fogt F, Vortemeyer AO, Goldman H, Giordano TJ, Merino MJ, Zhuang Z. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29(2):131–6.
Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis:a cancer prone-chronic inflammatory disease. Cancer Res. 2000;60(13):3333–7.
Romano M, Cuomo A, Tuccillo C, Salerno R, Rocco A, Staibano S, Mascolo M, Sciaudone G, Mucherino C, Giuliani A, Riegler G, Nardone G, Del Vecchio BC, Selvaggi F. Vascular endothelial growth factor and cyclooxygenase-2 are overexpressed in ileal pouch-anal anastomosis. Dis Colon Rectum. 2007;50(5):650–9.
Svrcek M, El-Bchiri J, Chalastanis A, Capel E, Dumont S, Buhard O, Oliveira C, Seruca R, Bossard C, Mosnier JF, et al. Specific clinical and biological features characterize inflammatory bowel disease associated colorectal cancers showing microsatellite instability. J Clin Oncol. 2007;25:4231–8.
Schulmann K, Mori Y, Croog V, Yin J, Olaru A, Sterian A, et al. Molecular phenotype of inflammatory bowel disease-associated neoplasm with microsatellite instability. Gastroenterology. 2005;129(1):74–85.
Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61(9):3573–7.
Fleisher AS, Esteller M, Harpaz N, Leytin A, Rashid A, Xu Y, et al. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesion is associated with hypermethylation and diminished expression of DNA mismatchrepair gene, hMLH1. Cancer Res. 2000;60(17):4864–8.
Hsieh CJ, Klump B, Holzmann K, Borchard F, Gregor M, Porschen R. Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with long-standing and extensive ulcerative colitis. Cancer Res. 1998;58:3942–5.
Azer SA. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur J Gastroenterol Hepatol. 2013;25:271–81.
Elzagheid A, Emaetig F, Alkikhia L, Buhmeida A, Syrjänen K, El-Faitori O, Latto M, Collan Y, Pyrhönen S. High cyclooxygenase-2 expression is associated with advanced stages in colorectal cancer. Anticancer Res. 2013;33:3137–43.
Marnett LJ, DuBois RN. COX-2:a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80.
Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signalling suppresses T‑cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8.
McLoughlin RM, Witowski J, Robson RL, Wilkinson TS, Hurst SM, Williams AS, et al. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598–607.
Li Y, Haar C de, Chen M, Deuring J, Gerrits MM, Smits R, et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59(2):227–32.
Glocker EO, Kotlarz D, Boztung K, Gertz EM, Schäffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–45.
Kimura H, Hokari R, Miura S, Shigematsu T, Hirokawa M, Akiba Y, Kurose I, Higuchi H, Fujimori H, Tsuzuki Y, et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180–7.
Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut. 1995;36:718–23.
Swidsinski A, Kilkin M, Kerjaschki D, Schreiber S, Ortner M, Weber j, et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115(2):281–6.
Darfeuille-Michaud A, Neut C, Lederman E, Di Martino P, Desreumaux P, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology. 1998;115(6):1405–13.
Riddell RH. Premalignant and early malignant lesions in the gastrointestinal tract. Definitions, terminology, and problems. Am J Gastroenterol. 1996;91:864–72.
Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127–32.
Odze RD, Tomaszewski JE, Furth EE, Feldman MD, Diallo R, Poremba C, et al. Variability in the diagnosis of dysplasia in ulcerative colitis by dynamic telepathology. Oncol Rep. 2006;16:1123–9.
Odze RD, Goldblum J, Noffsinger A, Alsaigh N, Rybicki LA, Fogt F. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod Pathol. 2002;15:379–86.
Rubin CE, Haggitt RC, Burmer GC, et al. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611–20.
Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21.
Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71–4.
Farraye FA, Odze DR, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74.
Thomas T, Abrams KA, Robinson RJ, et al. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007;25:657–68.
Ullman T, Croog V, Harpaz N, et al. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–9.
Choi CH, Ignjatovic-Wilson A, Askari A, Lee GH, Warusavitarne J, Moorghen M, Thomas-Gibson S, Saunders BP, Rutter MD, Graham TA. Hart AL Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol. 2015;110:1461–71.
Choi CH, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M, Thomas-Gibson S, Saunders BP, Graham TA, Hart AL. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol. 2015;110:1022–34.
Ullman TA, Croog V, Harpaz N, et al. Progression to colorectal neoplasia in ulcerative colitis: effect of 5‑aminosalicylic acid. Clin Gastroenterol Hepatol. 2008;6(11):1225–30.
Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21.
Kelly JK, Gabos S. The pathogenesis of inflammatory polyps. Dis Colon Rectum. 1987;30:251–4.
Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813–6.
Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–6.
Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis:a meta-analysis. Gastrointest Endosc. 2002;56:48–54.
Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522–5.
Sokol H, Cosnes J, Chazouilleres O, Beaugerie L, Tiret E, Poupon R, Seksik P. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497–503.
Thackeray EW, Charatcharoenwitthaya P, Elfaki D, Sinakos E, Lindor KD. Colon neoplasms develop early in the course of inflammatory bowel disease and primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2011;9:52–6.
Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992;33:938–41.
Lindström L, Lapidus A, Ost Å, Bergquist A. Increased risk of colorectal cancer and dysplasia in patients with Crohn’s colitis and primary sclerosing cholangitis. Dis Colon Rectum. 2011;54((11):1392–7.
Broden B, Halliday J, Aryasingha S, Sharifi Y, Checchin D, Warren BF, et al. Risk for colorectal neoplasia in patients with colonic Crohn’s disease and concomitant primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2012;10((3):303–8.
Korelitz BI, Lauwers GY, Sommers SC. Rectal mucosal dysplasia in Crohn’s disease. Gut. 1990;12:1382–6.
Igarashi T, Akimoto S, Otasiro Y, Hanyu F, Nagasako K. Precancerous lesions of the colon and rectum. Gan Kagaku Ryoho. 1989;16:1639–44.
Richards ME, Rickert RR, Nance FC. Crohn’s diseaseassociated carcinoma. A poorly recognized complication of inflammatory bowel disease. Ann Surg. 1989;209:764–73.
Petras RE, Mir-Madjlessi SH, Farmer RG. Crohn’s disease and intestinal carcinoma:a report of 11 cases with emphasis on associated epithelial dysplasia. Gastroenterology. 1987;93:1307–14.
Rutter MD, Saunders BP, Wilkinson KH, et al. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334–9.
Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis. Gastroenterology. 1999;117:1288–94, discussion 1488–1491.
Odze RD, Farraye FA, Hecht JL, et al. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004;2:534–41.
Rubin PH, Friedman S, Harpaz N, et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295–300.
Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology. 2003;124:544–60.
Kiran RP, Khoury W, Church JM, Lavery IC, Fazio VW, Remzi FH. Colorectal cancer complicating inflammatory bowel disease: similarities and differences between Crohn’s disease and ulcerative colitis based on three decades of experience. Ann Surg. 2010;252(2010):330–5.
Rieder F, Bruyn JR de, Pham BT, Katsanos K, Annese V, Higgins PD, Magro F, Dotan I. Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis. 2014;1;8(10):1166–78, Oct.
Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J Crohns Colitis. doi:10.1093/ecco-jcc/jjw055.
Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology. 2003;124:544–60.
Pellino G, Selvaggi F. From colon-sparing techniques to pelvic ileal pouch: history and evolution of surgery for ulcerative colitis. Eur Surg. 2015;47:81–90.
McLeod RS, Baxter NN. Quality of life of patients with inflammatory bowel disease after surgery. World J Surg. 1998;22:375–81.
Seidel SA, Newman M, Sharp KW. Ileoanal pouch versus ileostomy: is therea difference in quality of life? Am Surg. 2000;66:540–6.
Feroci F, Lenzi E, Baraghini M, Cantafio S, Scatizzi M. General surgeons’ views on Oncologic Multidisciplinary Group meetings as part of colorectal cancer care. Updates Surg. 2012;64(4):273–8.
Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Soreide O. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48–58.
Schroen AT, Cress RD. Use of surgical procedures and adjuvant therapy in rectal cancer treatment:a population-based study. Ann Surg. 2001;234:641–51.
Selvaggi F, Fucini C, Pellino G, Sciaudone G, Maretto I, Mondi I, Bartolini N, Caminati F, Pucciarelli S. Outcome and prognostic factors of local recurrent rectal cancer:a pooled analysis of 150 patients. Tech Coloproctol. 2015;19:135–44.
Frykholm GJ, Glimelius B, Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results ofa randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36:564–72.
Glimelius B, Gronberg H, Jarhult J, Wallgren A, Cavallin-Stahl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol. 2003;42:476–92.
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, Krieken JH van, Leer JW, Velde CJ van de. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46.
Snelgrove R, Brown CJ, O’Connor BI, Huang H, Victor JC, et al. Proctocolectomy for colorectal cancer – is the ileal pouch anal anastomosisa safe alternative to permanent ileostomy? Int J Colorectal Dis. 2014;29:1485–91.
Merchea A, Wolff BG, Dozois EJ, Abdelsattar ZM, Harmsen WS, Larson DW. Clinical features and oncologic outcomes in patients with rectal cancer and ulcerative colitis:a single-institution experience. Dis Colon Rectum. 2012;55:881–5.
Gorfine SR, Harris MT, Bub DS, Bauer JJ. Restorative proctocolectomy for ulcerative colitis complicated by colorectal cancer. Dis Colon Rectum. 2004;47:1377–85.
Radice E, Nelson H, Devine RM, Dozois RR, Nivatvongs S, Pemberton JH, Wolff BG, Fozard BJ, Ilstrup D. Ileal pouchanal anastomosis in patients with colorectal cancer: long-term functional and oncologic outcomes. Dis Colon Rectum. 1998;41:11–7.
Pellino G, Selvaggi F. Outcomes of Salvage Surgery for Ileal Pouch Complications and Dysfunctions. The Experience ofa Referral Centre and Review of Literature. J Crohns Colitis. doi:10.1093/ecco-jcc/jjv066.
Pellino G, Sciaudone G, Candilio G, De Fatico GS, Landino I, Canonico S, Selvaggi F. Restorative proctocolectomy with ileal pouch-anal anastomosis is safe and effective in selected very elderly patients suffering from ulcerative colitis. Int J Surg. 2014;12(Suppl 2):S56–9.
Pellino G, Sciaudone G, Miele E, Candilio G, De Fatico GS, Riegler G, Staiano A, Canonico S, Selvaggi F. Functional outcomes and quality of life after restorative proctocolectomy in paediatric patients:a case-control study. Gastroenterol Res Pract. 2014;2014:340341 doi:10.1155/2014/340341.
Selvaggi F, Pellino G, Canonico S, Sciaudone G. Is omitting pouchography before ileostomy takedown safe after negative clinical examination in asymptomatic patients with pelvic ileal pouch? An observational study. Tech Coloproctol. 2012;16:415–20.
Pellino G, Sciaudone G, Canonico S, Selvaggi F. Role of ileostomy in restorative proctocolectomy. World J Gastroenterol. 2012;18:1703–7.
Greenstein AJ. Cancer in inflammatory bowel disease. Mt Sinai J Med. 2000;67:227–40.
Fornaro R, Secco GB, Picori E, Stabilini C, Frascio M, Ricci B, Mandolino F, De Salvo L, Gianetta E. Surgical treatment of Crohn’s disease complications. Our experience. G Chir. 2006;27:21–6.
Fornaro R, Frascio M, Stabilini C, Sticchi C, Barberis A, Denegri A, Ricci B, Mandolino F, Lazzara F, Gianetta E. Crohn’s disease surgery: problems of postoperative recurrence. Chir Ital. 2008;60:761–81.
Fornaro R, Frascio M, Denegri A, Stabilini C, Imperatore M, Mandolino F, Lazzara F, Gianetta E. Crohn’s disease and cancer. Ann Ital Chir. 2009;80:119–25.
Selvaggi F, Sciaudone G, Limongelli P, Di Stazio C, Guadagni I, Pellino G, De Rosa M, Riegler G. The effect of pelvic septic complications on function and quality of life after ileal pouch-anal anastomosis:a single center experience. Am Surg. 2010;76:428–35.
Pellino G, Sciaudone G, Selvaggi F, Riegler G. Delayed diagnosis is influenced by the clinical pattern of Crohn’s disease and affects treatment outcomes and quality of life in the long term:a cross-sectional study of 361 patients in Southern Italy. Eur J Gastroenterol Hepatol. 2015;27:175–8.
Pellino G, Selvaggi F, Ghezzi G, Corona D, Riegler G, Delaini GG. A think tank of the Italian society of colorectal surgery (SICCR) on the surgical treatment of inflammatory bowel disease using the Delphi method: Crohn’s disease. Tech Coloproctol. 2015;19(10):639–51. doi:10.1007/s10151-015-1368-4.
Selvaggi F, Pellino G, Ghezzi G, Corona D, Riegler G, Delaini GG. A think tank of the Italian Society of Colorectal Surgery (SICCR) on the surgical treatment of inflammatory bowel disease using the Delphi method: ulcerative colitis. Tech Coloproctol. 2015;19(10):627–38. doi:10.1007/s10151-015-1367-5.
Cacciola NA, Calabrese C, Malapelle U, et al. UbcH10 expression can predict prognosis and sensitivity to the antineoplastic treatment for colorectal cancer patients. Mol Carcinog. 2015;27 doi:10.1002/mc.22322.
Riegler G, Bossa F, Caserta L, Pera A, Tonelli F, Sturniolo GC, Oliva L, Contessini Avesani E, Poggioli G, IG-IBD Group. Colorectal cancer and high grade dysplasia complicating ulcerative colitis in Italy. A retrospective co-operative IG-IBD study. Dig Liver Dis. 2003;35:628–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Pellino, R. Marcellinaro, G. Sciaudone, A. Reginelli, P. Esposito, G. Riegler, S. Canonico, V. Villanacci, and F. Selvaggi declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pellino, G., Marcellinaro, R., Sciaudone, G. et al. Large bowel cancer in the setting of inflammatory bowel disease. Eur Surg 48, 191–202 (2016). https://doi.org/10.1007/s10353-016-0434-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-016-0434-0