Abstract
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), can develop various complications during the chronic remitting course. In the long-term course of the disease process, colorectal cancer (CRC) represents the most serious and life-threatening hazard. Evaluation of the incidence, risk factors, and confounding factors has been discussed, and surveillance methods have been established to lessen the burden on IBD patients. In this chapter, recent epidemiological trends and risk factors for CRC in IBD are discussed, and radiographic and endoscopic pictures of malignancies in UC and CD are presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Ulcerative Colitis
13.1.1 Epidemiology
Colorectal cancer (CRC) has long been a major clinical problem in ulcerative colitis (UC), and many studies have examined its incidence and risk factors. Eaden et al. conducted a meta-analysis of CRC in UC and reported that the rate of complicating CRC was 3.7 % in all UC cases, and the cumulative cancer incidence rate was 1.6 % at 10 years after onset of UC, increasing to 8.3 % after 20 years and 18 % after 30 years [1]. As disease duration increases, so does the risk of developing CRC [2, 3]. Many recent studies have reported that this risk of acquiring CRC has decreased over recent decades. In 2004, Winther et al. reported that the risk of developing CRC at 30 years after onset of UC was 2.1 % [4], while Lakatos et al. reported a risk of 7.5 % [5]. From a surveillance study of about 600 subjects, Rutter et al. reported that the cumulative CRC onset rate was 2.5 % at 20 years, 7.6 % at 30 years, and 10.8 % at 40 years [6]. The risk of acquiring CRC has thus decreased over the decades (Table 13.1). The causes of this are thought to be advances in pharmacotherapy, increased excision of premalignant lesions, and better techniques in colon resection, as well as the spread of surveillance colonoscopy (SC).
13.1.2 Risk Factors
The clinical characteristics of UC-associated CRC are similar in Western and Eastern countries. Long-standing disease represents an important risk factor, and since the age of UC onset is young, CRC occurs at a younger age (40s) than in the general population. CRC is estimated to be the cause of death in about 20 % of UC cases [7, 8]. The incidence of multiple cancers is about 30 %. Since UC is present in the background mucosa, the morphology and histological findings vary and present different appearances from general CRC. There is a high incidence of flat and invasive types. Histologically, a characteristic of these cancers is that poorly differentiated adenocarcinoma and mucinous carcinoma account for about half of cases. A relationship with the severity of colitis has been indicated, and attention has been directed to the relationship with tissue inflammation [9, 10]. Factors raising the risk of CRC have recently been considered to be disease duration, extent of the diseased colon, family history of colon cancer, concomitant primary sclerosing cholangitis, and persistence of colitis. Conversely, factors that lower the risk are reported to be prophylactic colectomy, regular examination by a doctor, SC, chemical prevention, and adherence to treatment.
13.1.3 Purpose and Targets of SC
As mentioned above, patients with long-standing UC have a high risk of complicating CRC against a background of chronic inflammation. There is also a high rate of dysplasia, which is thought to represent a precancerous lesion. For that reason, surveillance for dysplasia as a cancer marker is recommended. Regular SC is thought to be essential for early detection of cancer or dysplasia. In fact, it has been shown that with regular SC, cancer or the related dysplastic lesions can be detected and treated at an early, curable stage [11, 12].
The main purpose of surveillance is to reduce deaths from CRC by detecting and excising precancerous lesions. A study by Choi et al. [13] looked at 41 patients who developed cancer, comprising 19 in an SC group and 22 in a non-SC group. A significant difference was seen between groups in 5-year survival, confirming the efficacy of SC [13]. In a recent study by Lutgens et al., 149 patients who developed cancer were studied, including an SC group of 23 patients. This SC group included many early-stage tumors than in non-SC group (52 % vs. 24 %), the mortality rate was lower (4 % vs. 24 %), and the 5-year survival rate was significantly higher than in the non-SC patients [14]. Based on the above studies, the decrease in mortality rate was concluded to be as much as 63 %. The largest study was one by Rutter et al. at St. Mark’s Hospital [6]. As a result of long-term SC, cancer was detected in 5 % of patients, and their prognosis was shown to be fair. The method of SC used was not perfect but was judged to be effective.
13.1.4 Endoscopic Images
The macroscopic picture of CRC in UC is thought to be protruding cancer in many cases, but few accurate statistics are available. Matsumoto et al. classified and reported endoscopic findings of UC-associated CRC using a dye method [15] (Table 13.2).
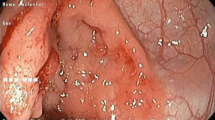
They divided cancers into protruded lesions (Figs. 13.1 and 13.2), slightly elevated lesions (Figs. 13.3, 13.4, and 13.5), flat lesions (Figs. 13.6, 13.7, 13.8, and 13.9), depressed lesion (Fig. 13.10), and mixed-type lesions (Fig. 13.2) and presented endoscopic images of each. Appropriate endoscopic classifications like this are needed. Of course, the protruded lesion called dysplasia-associated lesion or mass (DALM) (Figs. 13.2, 13.3, 13.4, and 13.5) is the most important finding [16]. DALM presents diverse morphologies, including villiform, coarsely granular, and irregular flat-elevated shapes, with margins that are often indistinct. Changes in color tone have been confirmed in flat dysplasia, but differentiation from surrounding inflammatory regenerated mucosa is difficult (Figs. 13.5, 13.6, 13.7, 13.8, and 13.9). In endoscopy, abnormalities are seen even in non-tumor areas because of the inflammation in UC, making detection and definitive diagnosis of neoplastic lesions tricky. As mentioned above, a special care is needed in stenotic portions (Figs. 13.11 and 13.12).
Protruded-type cancer in long-standing UC. Endoscopic view of a protruded-type cancer showing an irregular and nodular surface (a). Histologic picture of a protruded-type cancer with UC (b). Resected specimen shows IIa-like protrusion, and histologically, this tumor was diagnosed as well-differentiated adenocarcinoma and tumor invaded down to the subserosa (Quoted from Nishimura et al. [30], under permission of publisher)
Protruded-type cancer in UC. A 29-year-old patient with long-standing refractory UC, disease duration 11 years, total colitis. Colonoscopic view of a reddish, protruded-type cancer (Is) in the sigmoid colon (a). The lesion seemed to be soft, and the surface is covered by granular or papillary mucosa, but surrounding mucosa has abnormal granularity. Histological examination showed very well to poorly differentiated adenocarcinoma (b) with mucinous degeneration invading into the deeper portion of the submucosa and surrounding area of dysplastic epithelium
(a) Endoscopic view of a slightly elevated tumor with granular surface in the transverse colon. (b) Histologic picture of a slightly elevated lesion. Histologically, the tumor showed IIa + Is-like advanced cancer invading down to deeper parts of the muscularis propria (Quoted from Nishimura et al. [30], under permission of publisher)
Flat lesion. A 46-year-old patient; disease period, 18 years; total colitis. Surveillance colonoscopy detected a flat lesion with superficial irregularity. Histological diagnosis was a mucosal lesion with moderately to severely dysplastic epithelium highly suggestive of well-differentiated adenocarcinoma
Flat lesion. A 76-year-old patient; disease period, 4 years; left-sided type. Extremely difficult to diagnose as cancer. Endoscopic findings showed a flat lesion with low-grade inflammation and ill-defined margins. Histologically, the tumor consisted of adenocarcinoma and dysplastic epithelium in the sigmoid colon
Macroscopic picture of a flat lesion and a protrusion. A 46-year-old patient; disease period, 26 years; left-sided colitis type. Schematic illustration of the resected colon and rectum. In Rb there is a protrusion which is composed of well-differentiated adenocarcinoma with mucinous change invading into the adventitia. In Ra there showed a broad, flat lesion with well-differentiated adenocarcinoma invading into the superficial part of the submucosa
Endoscopic view of flat lesion in the upper rectum (Ra) shown in Fig. 13.8. A flat, irregular surface lesion was detected in the upper rectum, without demarcating boundary between the mucosal cancer and inflamed area (a). Histologically, a flat, well-differentiated lesion is seen in Ra (b)
(a) Endoscopic features of depressed-type cancer in UC. (b) Histologic picture of depressed-type cancer. In the descending colon, a depressed-type (IIc-like advanced) well-differentiated adenocarcinoma which invaded down to the subserosa was seen (Quoted from Nishimura et al. [30], under permission of publisher)
Stenotic-type rectal cancer associated with UC. Stenosis as long as 22 cm was shown on radiography (a). Endoscopic picture of a CRC associated with UC, stenotic lesion (b). Biopsy showed mucinous adenocarcinoma with signet ring cell carcinoma (c) (Quoted from Nishimura et al. [30], under permission of publisher)
Stenotic-type cancer in UC. A patient in their 30s; disease period, 15 years; mild persistent activity. Surveillance endoscopy revealed a tumor with severe stenosis in the descending colon (a). In the 4 years before final diagnosis, endoscopic findings revealed mild stenosis with mild inflammation without biopsy (b) (Quoted from Higashi et al. [31], under permission of publisher)
Random biopsy is a method in which two to four biopsy samples are taken from the diseased colon randomly at 10-cm intervals. If sites with findings such as DALM are identified, additional biopsy is performed for pathological investigation for the presence of dysplasia. In Japan, targeted biopsy has been performed traditionally, and its effectiveness has been demonstrated [6, 17, 18]. In Western countries, concurrent use of dye methods has also been shown to be effective. The comparative studies of Matsumoto et al. and Rutter et al. using chromoendoscopy have greatly advanced the combined use of target biopsy and chromoendoscopy [7, 18].
13.2 Crohn’s Disease
13.2.1 Epidemiology
As in UC, CRC or small intestinal cancer also occurs in Crohn’s disease (CD) patients (Figs. 13.13, 13.14, and 13.15). The relative risk (RR) of developing intestinal cancer increases with longer disease duration (Tables 13.3 and 13.4). Although this has been reported in many studies, the degree of rise in RR differs greatly depending on the population in each study [19–23]. Ekbom et al. reported an RR of 2.5 [19], whereas the regional studies of Persson et al., Munkholm et al., Fireman et al., and Jess et al. [20–23] did not find high RRs. In a study by Gillen et al. however, the RRs of CD and UC were both high, at about the same level [24]. A comparison of cancer incidence revealed rates of 7 % (20 years) in UC and 8 % (20 years) in CD, 18 and 19 times higher, respectively, than the risk in a healthy population of the same age composition. High RR was also supported in a recent meta-analysis by Canavan et al. [25]. Friedman et al. recently reported the results of continuous colon SC in 259 CD patients starting in 1980 [26, 27]. Cancer or dysplasia was diagnosed in initial (7 %) and later tests in 48 patients (adding more 14 %). SC is thus extremely important.
Ileal cancer in CD. A patient in their 40s at diagnosis; disease period, 28 years. Intestinal cancer at the ileocolonic anastomosis with distant metastasis and ileus. Per-oral double-balloon endoscopy revealed segmental tumor formation at the ileocolonic anastomosis (a). Radiographic picture of the stenosis (b)
Ileal cancer in CD. A patient in their 30s at diagnosis, with ileitis-type CD; disease duration, 23 years. A barium meal study was conducted after ileus. Multiple severe ileal stenoses were detected, but lesions seemed to be caused by Crohn’s disease itself, and diagnosis of cancer was not possible preoperatively (Quoted from Higashi et al. [32], under permission of publisher)
Resected specimen of the patient as Fig. 13.14. At the stenotic site, flat cancer (a) with restricted shallow ulcer formation was evident. At the bypass surgery site (b), a cancer with ill-defined margins of shallow ulceration with mild stenosis was found. Upper arrow efferent loop, lower arrow afferent loop (Quoted from Higashi et al. [32], under permission of publisher)
Our recent reports have shown a marked increase in the number of cancers detected in CD [28, 29]. The most common sites of CRC development in Japanese CD patients are from the lower rectum to anus (Figs. 13.16, 13.17, 13.18, and 13.19). A large majority of these patients have a history of anal fistula [28, 29]. Thus, in Japan, most malignant intestinal cancers associated with CD occur in the rectum or anal area, and surgical biopsy under anesthesia in the area of the anal fistula in the asymptomatic interval is thought to be effective for surveillance
Anal canal cancer in CD. A patient in their 40s at diagnosis. Advanced rectal cancer was detected from symptomatic change. Radiography shows advanced cancer in Rb (a) and endoscopic picture of the rectal tumor (b) (Quoted from Yao et al. [33], under permission of publisher)
Rectal cancer in CD diagnosed by endoscopy. Rectal stenosis and irregular mucosa were found and diagnosed as mucinous adenocarcinoma. The cancer had invaded down to the subserosa (Quoted from Futami et al. [34], under permission of publisher)
Anal cancer in CD diagnosed by surgical biopsy in a patient with long-standing anorectal fistula. The patient had ileocolitis and a history of fistula (arrow). Histology showed well-differentiated adenocarcinoma with mucinous changes (Quoted from Futami et al. [34], under permission of publisher)
(a) Protruded rectal cancer in CD. Disease period, 28 years, with long-standing ileocolitis. Endoscopic view of a protruding-type cancer with lobulation in the rectum and closeup view showing coral-like or villous appearance (a). Macroscopic picture of a resected specimen showing a protruded tumor with villous surface and surrounding coarse mucosa (b). Histologic pictures (c). Protruded carcinoma in the anorectal area with well-differentiated mucinous carcinoma subserosal invasion, and in the surrounding area dysplastic epithelium is seen (Quoted from Futami et al. [34], under permission of publisher)
References
Eaden J, Abrams KR, Mayberry JF, et al. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35.
Gilat T, Fireman Z, Grossman A, et al. Colorectal cancer in patients with ulcerative colitis. A population study in central Israel. Gastroenterology. 1988;94:870–7.
Lennard-Jones JE, Melville DM, Morson BC, et al. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31:800–6.
Winther KV, Jess T, Langholtz E, et al. Long-term risk of cancer in ulcerative colitis: a population based cohort study from Copenhagen county. Clin Gastroenterol Hepatol. 2004;2:1088–95.
Lakatos L, Mester G, Erdelyi Z, et al. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian Cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205–11.
Rutter M, Saunders B, Wilkinson K, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–8.
Gyde SN, Prior P, Allan RN, et al. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29:206–17.
Higashi D, Futami K, Ishibashi Y, et al. Clinical course of colorectal cancer in patients with ulcerative colitis. Anticancer Res. 2011;31:2499–504.
Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105.
Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9.
Rutter MD, Saunders BP, Wilkinson KH, et al. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334–9.
Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (updated from 2002). Gut. 2010;59:666–89.
Choi PM, Nugent FW, Schoetz DJ, et al. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–24.
Lutgens MW, Oldenburg B, Siersema PD, et al. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. Br J Cancer. 2009;101:1671–5.
Matsumoto T, Iwao Y, Igarashi M, et al. Endoscopic and chromoendoscopic atlas featuring dysplastic lesions in surveillance colonoscopy for patients with long-standing ulcerative colitis. Inflamm Bowel Dis. 2008;14:259–64.
Blackstone MO, Riddell RH, Rogers BHG, et al. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366–74.
Rutter M, Saunders BP, Schofield G, et al. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–60.
Matsumoto T, Nakamura S, Jo Y, et al. Chromoscopy might improve diagnostic accuracy in cancer surveillance for ulcerative colitis. Am J Gastroenterol. 2003;98:1827–33.
Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–9.
Persson PG, Karlen P, Bernell O, et al. Crohn’s disease and cancer: a population based cohort study. Gastroenterology. 1994;107:1675–9.
Munkholm P, Langholz E, Davidsen M, Binder V. Intestinal cancer risk and mortality in patients with Crohn’s disease. Gastroenterology. 1993;105:1716–23.
Fireman Z, Grossman A, Lilos P, et al. Intestinal cancer in patients with Crohn’s disease. Scand J Gastroenterol. 1989;24:346–50.
Jess T, Winther KV, Munkholm P, et al. Intestinal and extra-intestinal cancer in Crohn’s disease: follow-up of a population-based cohort in Copenhagen county, Denmark. Aliment Pharmacol Ther. 2004;19:287–93.
Gillen CD, Walmsley RS, Prior P, et al. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1507–8.
Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–104.
Friedman S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn’s colitis. Gastroenterology. 2001;120:820–6.
Friedman S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn’s colitis: results of a surveillance program spanning 25 years. Clin Gastroenterol Hepatol. 2008;6:993–8.
Yano Y, Matsui T, Uno H, et al. Risks and clinical features of colorectal cancer complicating Crohn’s disease in Japanese patients. J Gastroenterol Hepatol. 2008;23:1683–8.
Yano Y, Matsui T, Hirai F, Okado Y, et al. Cancer risk in Japanese Crohn’s disease patients: investigation of the standardized incidence ratio. J Gastroenterol Hepatol. 2013;28:1300–5.
Nishimura T, Matsui T, Hirai F, et al. Radiographic diagnosis of ulcerative colitis associated neoplasia. Stomach Intestine 2008;43: 1281–92.
Higashi D, Futami K, Ishibashi Y, et al. Clinical course of colorectal cancer in patients with ulcerative colitis. Anticancer Res 2011;31:2499–504.
Higashi D, Futami K, Kojima D, et al. Cancer of the small intestine in patients with Crohn’s disease. Anticancer Res. 2013;33:2977–80.
Yao S, Iwashita A, Nishimura T, et al. Colo-rectal cancer complicated with Crohn’s disease. Stomach Intestine 2002;37:1047–58.
Futami K, Higashi D, Egawa Y et al. Intestinal cancer in patients with Crohn’s disease: Diagnosis, treatment and prognosis. Anticancer Res. 2013;33:2977–80.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Matsui, T. (2015). Malignancies: Colitic Cancer and Small Bowel Cancer (Intestinal Cancer) in IBD. In: Kim, W., Cheon, J. (eds) Atlas of Inflammatory Bowel Diseases. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39423-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-642-39423-2_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39422-5
Online ISBN: 978-3-642-39423-2
eBook Packages: MedicineMedicine (R0)