Abstract
Boring patterns of microbial endoliths were analyzed in bivalve shells collected from different environments in the Northern Bay of Safaga on the Red Sea, Egypt. A total of 20 ichnotaxa have been recognized within 64 samples and evaluated on the basis of 250 scanning electron micrographs. The analysis of microorganisms in shells from the same depth profile has been performed separately. Morphological properties of selected ichnotaxa were studied in detail, morphometric parameters established, measured, and statistically evaluated. This method was applied to determine the variability of dominant traces in view of their ichnotaxonomic treatment. Ichnogenus Rhopalia was discussed and a new ichnospecies Rhopalia spinosa was described. Formal change of name for cyanobacterial traces of Hyella and Solentia from Fasciculus to Fascichnus, and for the traces of the green alga Ostreobium from Reticulina to Ichnoreticulina were proposed on the ground of the priority rights in compliance with rules of the International Code of Zoological Nomenclature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial euendoliths constitute a diverse group of specialized microorganisms that penetrate hard calcareous substrates, such as sedimentary rocks and skeletons of live and dead animals and plants, leaving behind recognizable boring traces (Golubic et al. 1981; Golubic and Schneider 2003). Through their activity, they are able to inflict damage to their hosts (Kaehler and McQuaid 1999; Bentis et al. 2000), to contribute to primary production on coral reefs (Larkum et al. 2003), to attract grazing by gastropods, echinoderms, and fish in combined bioerosion activity and to enhance fine grain sediment production (Schneider and Torunski 1983; Tribollet et al. 2002). Through their involvement in coastal bioerosion, microbial euendoliths have been a major shaping force over geological time (Radtke et al. 1996, 1997c).
The study of microbial boring traces has generated interest as indicators of past environmental conditions and paleobathymetric estimates, especially for depths where there are few sedimentary clues (Vogel et al. 1987, 1995; Glaub and Vogel 2004). Microbial euendoliths occupy an ancient ecological niche as the euendolithic mode of life predates for ca. 109 years the evolution of skeleton bearing organisms that most frequently serve as their present-day hosts. The earliest members of the euendolithic community, which today consists of a wide spectrum of prokaryotic and eukaryotic phototrophic and organotrophic organisms, were cyanobacteria, recorded in 1500–1700 My old sediments, where they have penetrated lithified layers of carbonate stromatolites (Zhang and Golubic 1987). By the end of the Proterozoic, before the appearance of the mineralized skeletons in the fossil record, there were established assemblages of euendolithic cyanobacteria penetrating 700–800 My old ooid grains that shoaled along ancient coasts of Greenland (Knoll 1985; Knoll et al. 1986; Green et al. 1988) and of Spitsbergen (Knoll et al. 1989). These cyanobacteria represented the primary producers in otherwise barren fields of carbonate sands and were quite comparable with their modern counterparts in similar environments of the Arabian Gulf and the Bahaman Carbonate Platform (Al-Thukair and Golubic 1991a, b, 1996; Al-Thukair et al. 1994. The evidence for Proterozoic endolithic rhodophytes (Butterfield et al. 1990) is indirect by the finding of epilithic specimens of Bangia, a genus known to have an endolithic Conchocelis-stage as a part of its life cycle.
The oldest microborers were preserved in silica as body fossils just like the early microorganisms that formed microbial mats and stromatolites (e.g., Knoll 1985), but this mode of preservation became quite rare in the course of the entire Phanerozoic (Croft and George 1959), possibly restricted to particular chemical environments as in the case of the Rhynie chert (Rice et al. 2002). Equally rare are the findings of organically preserved microbial euendoliths in carbonates, sheltered inside of their borings in ca. 420 million years old crinoid ossicles (Campbell et al. 1979; Campbell 1980). Ancient boring traces are often preserved as contrasting fill of boreholes (Campbell 1982) or as weathered out natural casts (Harris et al. 1979), ranging from the Proterozoic to Pleistocene. However, a large proportion of fossil microscopic borings in carbonates remained empty.
The three-dimensional display of void fossil borings has been studied using scanning electron microscopy (SEM) of resin casts that faithfully replicate their shapes and distribution within the bored substrate. The use of basically the same preparation technique for modern and ancient boring traces (Golubic et al. 1970, 1983) provided the opportunity for direct comparisons. Empty or partially filled microborings occur throughout the entire Phanerozoic (Glaub and Vogel 2004). Modern borings with resident endoliths permitted identification of endolithic organisms, thus providing a biological interpretation of their traces. The work on modern endoliths includes the study of naturally occurring substrates such as bivalve shells and shell fragments (Günther 1990; Radtke 1993), as well as experimental studies of endolith settlements on denuded (Le Campion-Alsumard 1970) or exposed substrates (Kiene et al. 1995; Vogel et al. 2000). The older literature on microbial euendoliths is summarized by Lukas (1973) and Radtke et al. (1997b).
Microbial euendoliths offer several advantages for paleoecological applications: they are common in various calcareous substrata, where they leave recognizable, often species-specific boring traces, which conform closely to the outlines of the bodies of organisms that produced them and they often remain preserved in the fossil record. Due to their small size, entire populations can be found and studied in sand-sized sediment particles. Thus, their study easily encompasses the assessment of entire assemblages of borings and ichnocoenoses (see Vogel et al. 1995).
Boring behavior occurs in a variety of phylogenetic lineages and among organisms with different metabolic properties. Among prokaryotes it is found in phototrophic cyanobacteria and in heterotrophic bacteria. Among eukaryotes, boring behavior occurs in phototrophic red and green algae and heterotrophic protists, fungi and animals. The light-dependence of endolithic phototrophs permits their use as indication of the euphotic and dysphotic zone in paleoenvironmental settings, whereas the heterotrophs exhibit a dependence on organic nutrient sources. However, the peculiarity of the endolithic mode of life provided a selective pressure that resulted in convergent evolution and morphological similarities among unrelated organisms. This circumstance calls for more precision in describing and defining boring patterns, accompanied by morphometric data.
The present contribution presents the distribution of microborings of mostly phototrophic euendoliths in a clear water tropical setting. Selected ichnotaxa are re-evaluated and morphometrically characterized, addressing the importance of dimensions in characterizing and delimitating ichnotaxa.
Materials and methods
Field work
As a part of a comprehensive investigation of benthic flora and fauna, shells and shell fragments were sampled in various identified environments of the North Safaga Bay by SCUBA diving in 1994 and 1995. These materials were provided to us by the Paleontological Institute of the University of Vienna, Austria. For detailed documentation of different bottom facies and molluskan fauna consult Piller and Pervesler (1989). A separate trip for collecting and studying microbial endoliths followed in the year 2001.
Microboring sample preparation
The sampled shells and shell fragments were stored dry. Prior to preparation, they were examined by dissecting microscopy, and cut with a saw to a size appropriate for further preparation and scanning electron microscopy. The specimens were prepared by applying the embedding/casting procedure (Golubic et al. 1983) using the polymerizing resin Araldite as follows: They were dried in a desiccation oven and then infiltrated by concentrated acetone, followed by a transfer through a series of acetone/araldite solution into pure 4 component araldite resin mix. The infiltrated samples were cured in an oven at the proscribed polymerization temperature of 60°C for 72 h and cut open. The exposed carbonate substrate of the shell was removed by dilute HCl to expose the resin-cast microborings. In total, 64 such preparations from ten different facies types were examined, evaluating 250 SEM images.
Microbial endolith analysis
Shells collected for the study of resident endoliths were fixed in the field with 3% formaldehyde solution in environmental water. They were examined by dissecting microscopy for presence of pigmented endoliths. Carbonate was dissolved gradually by dilute HCl or Perenyi solution. The emerging microbial endoliths were mounted on microscope slides and examined using a Zeiss Universal microscope.
Morphometry
As a part of morphological characterization, quantifiable parameters were selected for each microboring type that would express the proportions, variability and provide comparative data for delimitation between similar ichnotaxa. Measurements were taken in scale from scanned SEM images using Sigma-Scan Image software (Jandel Corporation, San Rafael, CA, USA). For endolithic microorganisms, the measurements were taken using in-scale camera lucida projections from light microscope and Sigma-Scan Image software. Data were evaluated with the aid of the basic statistics program provided by the same software and Microsoft Excel. They were used for comparisons between microboring organisms and the corresponding resin casts.
The outlines of cast borings are assumed to conform closely to the outline of the organism with certain limitations. In tubular casts, for example, the variation of diameters is often expressed as alternating swellings and constrictions, which may reflect the positions of cells and crosswalls respectively, whereas their frequency along a tubular cast may be the function of cell length. Casts approaching isodiametric morphology are measured in terms of longer versus shorter diameter, whereas the third dimension is the depth of boring from the substrate surface. A total of 20 recognized ichnotaxa have been analyzed in 64 samples, and evaluated on the basis of 250 SEM photomicrographs, four of them in detail. The holotype and the Ph.D. thesis material is hosted in the Institute of Geology and Paleontology, Johann Wolfgang Goethe-University, Frankfurt am Main, Germany (GPI, Bo 7).
Data presentation
The variations are expressed as means ± standard deviation (n) with the number of measurements in parentheses. The parameters are paired and displayed graphically as crosses with the mean value positioned in the intersection and the arms of one standard deviation on each side. The graphs were constructed with the Microsoft Excel software. Graphic scale bars are provided with photomicrographs.
Environmental setting
The Northern Bay of Safaga was selected for this actuo-paleontological investigation because it typifies a highly structured, tropical shallow-marine environment, and because it was subject of one of the few comprehensive interdisciplinary studies of carbonate sedimentary environments on modern tropical coasts. These studies provided detailed bottom topography and identified bottom facies (Piller and Pervesler 1989), sedimentary facies and mineralogy (Piller and Mansour 1990), and microfacies (Piller 1994). Furthermore, it compiled a database for investigation of different organismal groups, e.g. foraminifers (Haunold et al. 1997), corals (Kleemann 1992; Riegl and Piller 1997), hard substrate mollusks (Zuschin and Piller 1997), echinoids (Nebelsick 1992), and coralline red algae (Piller and Rasser 1996; Rasser and Piller 1997).
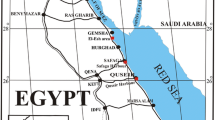
The Northern Bay of Safaga on the Egyptian coast of the Red Sea is a tectonically and structurally complex carbonate-dominated coastal system (Fig. 1). The northern part of the bay is an extended area of shallows interrupted by intertidal exposed shoals. On the west side, a separate 30–40 m deep mud-covered basin is connected by a narrow channel to the Southern Safaga Bay. The bay opens to the east toward the open sea with depths down to 80 m protected by a coral reef. The prevalent current flows from the open sea through the southwest channel and passes through a spectrum of hard- to soft-bottom sedimentary facies, providing good circulation throughout the bay. The water temperature ranges between 21 and 29°C and the salinity between 40 and 46‰ (Grill and Zuschin 2001). Tidal oscillations due to celestial causes are negligible, and minor sea level variation depends on prevailing wind direction.
Location map with general bathymetry and sampling sites. Densely stippled fields in the right map are intertidal areas. AM = aerial mast; H = Safaga Hotel (modified after Piller and Pervesler 1989)
Different benthic sedimentary facies were identified and carefully mapped by Piller and Pervesler (1989). A quantitative assessment of molluskan assemblages (biocoenoses versus thanatocoenoses) has been subsequently carried out in the bay and correlated with sedimentary environments (Zuschin et al. 2000). The work was followed up with correlations between molluskan populations, bathymetry and the habitats within the coral reef ecosystem with reference to literature data (Zuschin et al. 2001). The present study deals with microborings in shells collected in the context of the above studies, complemented by an analysis of microboring organisms.
Results
Distribution of microborings and microbial endoliths in shells
The environments under study range from intertidal pools on the limestone coast to a depth of 60 m. Microborings in molluskan shells were studied in a variety of habitats shown along an idealized bathymetric transect (Fig. 2). From the coast outward, shells were collected in rockpools on exposed rocky intertidal zone and shallow subtidal from 0 to 0.5 m depth (D4-2), in protected shallow environments with periodically exposed sand bars and flats between mangroves (A5-3), in protected rocky intertidal zone (A1-6), and in seagrass stands with gentle currents down to a depth of 1 m (A1-5). Deeper subtidal sampling sites include seagrass meadows on sandy ground with mild current down to a depth of 6 m (B5-8), a coral reef at the NE shelf edge at 15 m depth exposed to strong currents (C12-1), the base of the coral reef at 27 m depth surrounded by sandy bottom in front of the small island Tubya Al-Bayda (B1-1), and a deep channel at the same depth with strong current, lined with muddy sand (A11-2). The transect ends with a quiet 50 m deep depression, covered by muddy sand (B7-1) and by current-exposed hard ground with sand patches at 60 m depth on the outer slope of the reef (C1-5). Microbial endoliths extracted from the shells and analyzed separately are listed in Table 1.
In the intertidal pools and shallow coastal ranges less than 1 m deep, the most common euendolithic traces were colonies of Fascichnus (former Fasciculus, see below). Different ichnospecies were observed, especially Fascichnus dactylus and to a lesser extent F. parvus and F. frutex (Fig. 3A and B). These traces, produced by the coccoid cyanobacteria Solentia and Hyella, were subject of a detailed morphometric analysis (see below). Another common boring, which dominates in the intertidal ranges, is Eurygonum nodosum, a trace produced by the heterocystous cyanobacterium Mastigocoleus testarum. The organism is characterized by true branching and heterocysts (Golubic et al. 1996), properties also noticeable on borings (Fig. 3C and D, arrows).
Boring patterns of phototrophic microbial endoliths representative of four depth ranges from 0 to 60 m. The scale in all pictures is 30 μm. A Fascichnus frutex, very shallow, 1 m water depth, sand bottom and seagrass (sample A1/5, Pitar sp.). B Detail of (A) showing few branchings. C Eurygonum nodosum showing branched tunnels with intercalated swellings indicating the positions of heterocysts (arrows), 6 m water depth, seagrass meadow (sample B5/8, Callista sp.). D Eurygonum nodosum with heterocyst swellings (arrows), the large rounded casts are formed by the cyanobacterium Cyanosaccus piriformis, 27 m water depth (sample B1/1). E Rhopalia catenata from 27 m water depth; note the monopodial branching order, indicating apical growth (sample B1/1). F Detail from the center of the radiating system showing avoidance behavior at contact. G Cavernula pediculata, a solitary boring of a green algal Codiolum-stage in a shell of Laevicardium sp., 27 m water depth, reef bottom habitat (sample B1/1). H Dense network of Ichnoreticulina elegans, 50 m in a shell of Astarte sp.; note the presence of thin, straight "exploratory" filaments. They are a part of the same Ichnoreticulina colony (sample B7/1)
In wider ranges of habitats in shallow illuminated waters, the most conspicuous is the presence of large borings of Rhopalia catenata (Fig. 3E and F); a trace produced at least in part by the chlorophyte Phaeophila engleri. This trace is also found occasionally in deeper ranges at 15 m and down to 27 m. Solitary Cavernula pediculata (Fig. 3G), produced by endolithic Codiolum-stages of green algae, occurred at all depths from 6 to 60 m. The deepest collections of shells taken between 27 and 60 m were dominated by Ichnoreticulina (former Reticulina) elegans (Figs. 3H and 7), borings produced by the variable and omnipresent siphonal chlorophyte Ostreobium quekettii, accompanied occasionally by colonies of Rhopalia spinosa isp. nov. (see below).
Large boring traces classified as Fascichnus grandis, produced by rhizoids of the green alga Acetabularia were found occasionally in shallow subtidal ranges, as well as sphaeroid borings which were produced by cyanobacteria of the genus Cyanosaccus (listed as ‘Sphaeroid form’ in Fig. 2; see Radtke 1993). The trace Scolecia filosa (Fig. 3D, right) refers to thin tunnels close to 1 μm in diameter formed by the cyanobacterium Plectonema (Leptolyngbya) terebrans. The organism is present in all depths, increasing in frequency in deeper sites. It is sometimes difficult to distinguish from fungal hyphae of similar size.
Fungi and other heterotrophic euendoliths were less prominent in these shallow waters dominated by phototrophs. Spherical sporangial swellings of Saccomorpha sphaerula could be identified by their small size and the network of interconnecting hyphae. There were few representatives of other fungal traces such as Saccomorpha clava and Orthogonum fusiferum (Fig. 3D, upper left corner).
In shells that were intensively bored, the traces were crowded, possibly by cumulative action of several generations of borers. In such samples, it was difficult to ascertain which swellings belong to phototrophic and which to heterotrophic organisms. The differential diagnosis relies on interconnection between swelling and borings of hyphae, which is exclusively a fungal property. Such unidentified forms were listed informally as ‘clustered globules’; ‘echinoid form’ (probably foraminiferan trace) and ‘pygmy form’ (see Radtke 1993).
The distribution of microboring organisms extracted from shells and analyzed separately (Table 1) is largely consistent with the identified boring patterns. Several species of Hyella could be identified, which explains the enormous size variability of Fascichnus traces. Similarly, the analysis confirmed the morphological plasticity of Ostreobium quekettii, including formation of bag-like swellings, which were observed among Ichnoreticulina traces (see below).
Vertical zonation of microborings in the studied samples of both, euendoliths and their traces was not sharp. It was expressed by a gradual shift in dominance, rather than by the presence or absence of indicative species (Table 1 and the list in Fig. 2).
Systematic ichnology
Fascichnus nom. nov.
We are obliged to propose a change of the name Fasciculus Radtke 1991, for traces of the cyanobacteria Hyella and Solentia to be called Fascichnus in accordance with the International Code of Zoological Nomenclature (ICZN 1999), acknowledging the priority for the fossil ctenophore Fasciculus vesanus from the Cambrian Burgess Shale (Simonetta and Della Cave 1978).
Type ichnospecies: Fascichnus dactylus Radtke 1991.
Etymology: From the fusion of words fascis (lat.) = bundle and ikhnos (gr.) = trace.
Original diagnosis: Bundles of tunnels penetrate from a single point of entry and separate inward, perpendicular to oblique to the surface of the substrate (modified after Radtke 1991, translated from German).
Description: Each colony consists of a few, up to 150 tubular borings fairly uniform in diameter forming a hemispherically expanding bush. The tunnels are straight to slightly curved with rounded tips and recurring slight constrictions. The initial point of entry is small, in cast preparation usually concealed beneath the diverging galleries. Juvenile colonies consist of mostly unbranched tunnels. As the tunnels elongate, and the colony expands deeper into the substrate, their density is maintained by repeated branching.
Remarks: According to the original description, the ichnospecies within this genus are distinguished on the basis of tunnel diameters (Radtke 1991: 79). Additional morphotypic properties are provided in the description of each ichnospecies. The criteria for such distinctions, including morphometric parameters, applicable to the ichnogenus Fascichnus can now be summarized as follows: (a) shape and directionality of the galleries, e.g., straight or bent; (b) branch type, frequency (density) and angles, e.g., lateral, forked; (c) constancy of the diameter, e.g., proximal versus distal or maximum versus minimum (swelling versus constriction). The length of the galleries is not diagnostic as it depends on the age of the boring and is usually correlated with the colony size.
Boring patterns of Fascichnus ispp., covering different size ranges. The scale in all pictures is 30 μm. A A colony of F. parvus, most galleries penetrates perpendicular (upright) to the surface (sample B5/8, seagrass meadow, 6 m). B F. parvus, radiating galleries of slightly larger dimensions (sample B5/8). C F. dactylus, galleries with slightly `swollen' tips giving them a club-shaped appearance (sample A1/6, intertidal). D F. dactylus, a display of long unbranched galleries (sample A5/3, intertidal, Mangrove). E F. dactylus, a colony showing preference for deep penetration into the shell. The central tunnels are longer and wider (sample A5/3, intertidal, Mangrove). F F. dactylus, a large colony on the edge of the bored shell, surrounded by smaller borings of Rhopalia catenata and Saccomorpha sphaerula (sample C12/1, exposed reef, 15 m). G F. frutex, a cluster of massive upright galleries. There is considerable size difference among tunnels of the same colony with central ones being wider (sample A5/3, intertidal, Mangrove). H A different cluster of F. frutex. Note the attenuating tips of the largest galleries. I A view down the tunnel casts of F. frutex together with fine borings of Scolecia filosa (sample A1/6, intertidal)
Display of maximum vs. minimum diameters of galleries in different Fascichnus ispp. populations. The means are in the intersections of the cross diagrams with arms of one standard deviation. The values are in μm. Note that populations range with considerable overlap of data (95% of individual measurements are distributed over a field of two standard deviations on each side of the mean)
Diameters of tunnels are variable. However, the variability within each colony (intra-colonial) is always narrower than that between colonies (inter-colonial) implying size-specificity of the respective boring microorganism. The observed size discrepancies between different naturally occurring Fascichnus populations are considerable and the end members are vastly distinct, which prompted originally a description of several species distinguishable by the diameter of the galleries (Radtke 1991). The sizes were given specifically for F. parvus (Fig. 4A and B): 1.5–3 μm, for F. dactylus (Fig. 4C–F): 4–9 μm, and for F. frutex (Figs. 3A and B, and 4G–I): 11–25 μm. F. grandis is a much larger trace known to be formed by the rhizoids of the macroscopic dasycladacean chlorophyte Acetabularia (Radtke et al. 1997a).
In the present study, we have recorded the size ranges for individual colonies in order to determine the intra-population and inter-population variability and possibly provide a support for taxonomic characterization. The results of measurements of maximal (convexities) and minimal (constrictions) diameters of tunnels for 32 populations are displayed graphically in Fig. 5 (data in Appendix 1, asterisk marks the colonies illustrated in Figs. 3 and 4).
Diagram of size distribution in R. catenata and R. spinosa isp. nov.: Display of measurements of diameters of swellings (x-axis, maximum diameter) against constrictions between them (y-axis, minimum diameter). Both forms were originally classified within the ichnospecies R. catenata. Data are presented as cross diagrams with means at the intersection of crosses with `arms' of one standard deviation on each side of the mean
Boring system of Rhopalia spinosa isp. nov.; scale bar in all pictures is 30 μm. A Overview of a young expanding system forming a network of irregularly branched galleries (sample B7/1, protected basin, 50 m). B Detail showing spine-like attachments of galleries with the substrate surface. C Another boring system; note the unbranched exploratory tunnels backed by increasingly branched ones with swellings. D Details of the latter. E Spiny filaments with irregularly shaped swellings. F Filaments forming a loop. G R. spinosa isp. nov. – holotype; Paris Basin: Villiers-St.-Frédéric II, Middle Eocene, Lutetium. H Cluster of swellings turning globular in shape. The borings are sunk deeper into the substrate, but remain attached to the surface with longer attachments (sample N – 3 m water depth, LSI, Bahamas). I Detail from the margin of a central cluster with long attachments and arches (sample F – 10 m reef, LSI, Bahamas)
The values for all measured populations show a significant overlap, considering that about 95% measurements in each population scatter within a field of at least two standard deviations on each side of the mean. Similar continuous distributions of populational measures are also characteristics of populations of cyanobacteria that produce these boring patterns. Accordingly, the taxonomic nomenclature refers here to size ranges within a continuum of values, and not to distinct taxonomic entities separated by hiatuses.
Rhopalia catenata Radtke 1991
Original diagnosis: A system of straight branched tunnels extending parallel to the substrate surface (shell) from a single point of origin, with spherical to clavately or ellipsoidal swellings located at branch points or terminally and linked with the substrate surface with thin rhizoidal connections (modified after Radtke 1991, translated from German).
Description: The system of boring galleries expanding immediately beneath and parallel to the substrate surface, radiating from the initial point of the entry along three to six main tunnels of 1st order. The tunnels are distally cylindrical; developing intermittent swellings in its middle parts, which increase in size toward the boring's origin. Branches of the 2nd order originate from the swellings, at a certain distance from the growing tip at angles between 50° and 80°, forming a feather-like monopodial system. The branches are mostly alternate, occasionally opposite. The pattern is repeated to form branches of the 3rd order (Fig. 3E). All tunnels are straight or gently arched in the direction of the growth. They are circular in cross section and of fairly uniform diameter.
Distal parts of branches are straight, with rounded, slightly inflated tips (7.8±1.1 μm; n=50), whereas the proximal parts develop series of spindle-shaped swellings that gradually increase in size to an average of 17.7±2.6 μm (n=103) in width and 33.7±4.8 μm (n=103) in length. Swellings are separated from each other by short narrower segments (6.7±1.0 μm wide; n=140). Connections with the substrate surface arise from the swellings and are short and cylindrical, 3.0±0.4 μm wide and 6–9 μm long (n=22). In cast preparations, they are often covered by the tunnels, thus observable only in lateral or oblique panoramic view. The tunnels exhibit avoidance behavior at contact, deflecting sideways or more frequently deeper into the substrate, underpassing earlier borings (Fig. 3F). Such boring behavior permits determination of the sequential appearance of individual borings in crowded boring complexes.
Remarks: The boring galleries of R. catenata are produced among others by the septate chlorophyte Phaeophila engleri. They reflect a differentiation of the organism in the course of its development, and exhibit the timing in the onset of branches and development of swellings. The measured parameter exhibit normal distribution, thus their variability can be expressed by basic statistics. Intra-colonial size variability is narrower than variability between colonies (Fig. 6, right), but the fields of actual data overlap.
The original diagnosis of R. catenata describes the presence of ‘rhizoidal connections’ to the substrate surface, however, this feature is not always expressed, or it is hidden beneath the cast microboring. These connections correspond to long, sometimes undulate hairs produced by the cells of P. engleri, which are not always expressed in these organisms. In addition, the algal filaments are sometimes partially sunk into the substrate so that the hairs are free in the water and are not part of the boring.
The original description of this ichnospecies and the illustrations designated as holotype Radtke 1991: pl. 9, Figs. 1 and 2 and paratypes Radtke 1991: pl. 9, Figs. 3 and 4 present forms with more advanced development of swellings, presumably belonging to a more mature system then shown in Fig. 3E and F of the Safaga samples. But the original description also included much smaller and morphologically quite different forms. In the present study, these smaller forms were encountered in sufficiently large populations to study in detail the development of their boring systems. The measurements of three such populations are presented in Fig. 6 (data in Appendix 2), left in comparison with two populations to the right, which we consider typical R. catenata. These forms are sufficiently different, so that they needed to be classified as separate species. Based on morphological and morphometric properties and branching patterns we propose that these smaller forms be classified as a new ichnospecies R. spinosa isp. nov. Radtke 1991: pl. 9, Figs. 5 and 6: former paratype, now Rhopalia. spinosa isp. nov., see below). The diagnosis of the ichnogenus Rhopalia and its type ichnospecies do not need changing, as they represent well the typical forms.
Rhopalia spinosa isp. nov., Fig. 7.
Etymology: spinosus (lat.) = thorny, prickly, spiny.
Diagnosis: The system develops parallel to the substrate surface, extending from the point of the initial entry by several irregularly branched segmented tunnels. Laterally widened tunnel segments, triangular or polygonal in outline are interconnected by narrow passages. They are connected to the substrate surface by numerous spiny attachments.
Holotype: GPI Bo 7/56 from Paris Basin: Villiers-St.-Frédéric II, Middle Eocene, Lutetium (Fig. 7G).
Occurrences:
-
Fossil: Paris Basin, Villiers-St.-Frédéric I, Middle Eocene, Lutetium (Radtke 1991: pl. 9, Figs. 5 and 6; Bo 7/50).
-
Paris Basin, Auvers-St.-Georges I, Oligocene (Radtke 1991: Tab. 19; Bo 7/71);
-
Recent: Lee Stocking Island (LSI), Bahamas, mostly locality F=10 m (Fig. 8G–I, see also Radtke 1993: Fig. 6)
-
Cozumel, Mexico, samples 22 and 70 (Günther 1990).
Description: The boring system is formed by irregularly meandering, frequently branched tunnels (Fig. 7A), each comprised of triangular segments, and connected to the substrate surfaces by tapering appendages reminiscent of ‘feet of a centipede’ (Fig. 7B). The attachments are 1–1.5 μm in diameter 5.7±1.9 μm (n=54) long, 6–20 μm distant from each other, i.e., they may reach a density of 50–150 branches per mm of the filament length. The attachments taper distally, sometimes to a sharp point. Less commonly, they widen like a pinhead immediately before exiting the substrate (Fig. 7C and D).
In developed boring systems of R. spinosa we could distinguish three concentric regions. The peripheral region consists of mostly unbranched tunnels growing outward in a fan-like fashion (Fig. 7C, lower left). The region behind features frequently ramified filaments forming a network of intermediate density (Fig. 7C, center). The density of tunnels increases toward the center of the colony, where the borings penetrate deeper into the substrate and bear spherical or bag-like swellings (Fig. 7C, upper right). The branching order is not clearly discernible in this ichnotaxon.
The tunnels vary in diameter forming slightly dorsoventrally flattened swellings interconnected by narrower cylindrical sections (2.86±0.57 μm, n=54 in diameter). Swellings are spindle-shaped or triangular in outline, widening in the distal direction. The swellings (initially 5.9±1.17 μm, n=52 wide) increase in size and frequency toward the proximal (older) parts of the system (Fig. 7D). Increased frequency of branching results in a denser boring network. The borings show avoidance, bridging over at the points of interference (Fig. 7E). Branches commonly originate from isodiametric swellings, which act as distribution knots of the system. The branching is initially symmetrical dichotomous or trichotomous, diverging at 45–90° angles (Fig. 7C), but the symmetry is lost with increased branching frequency and density. Tunnels frequently change direction between subsequent branch points forming irregular loops (Fig. 7F) or produce a zig-zag pattern of alternate and opposite branches (Fig. 7G).
Swellings increase in size and frequency from the periphery to the central region of the system where they become rounded densely crowded and up to 25 μm in diameter. They also penetrate deeper into the substrate (Fig. 7H). As the system becomes more three-dimensional, the numerous attachments become longer but maintain the characteristic ‘spiny’ appearance to the trace (Fig. 7I).
Remarks: In deeper subtidal ranges, R. spinosa is frequently encountered with other similarly complex boring systems of R. catenata and I. elegans. R. spinosa shares some properties with R. catenata, e.g., the horizontal radiating expansion, and concentric differentiation of boring patterns, but it is substantially smaller (see Fig. 6, data in Appendix 2) and distinctive by its dense branching pattern and the spiny appearance. The connections with the surface are shared by all these forms but they are different in size, shape, frequency, and orientation. Survey of a large number of samples did not produce any evidence of transitions between these ichnotaxa and the detailed morphology of R. spinosa is sufficiently prominent to grant a description as a separate ichnospecies.
Ichnoreticulina nom. nov.
We are obliged to change the name of the ichnogenus Reticulina Radtke 1991, on the grounds of the priority rights, which belong to a subgenus of the ostracode Carinocythereis described by Bassiouni (1969).
Etymology: Combination of attribute ikhnos (gr.) = trace and reticulum (lat.) = small net.
Type ichnospecies: I. elegans Radtke 1991.
Original diagnosis: Densely interwoven net-like system of distal galleries with thin straight main tunnels, parallel to the substrate surface from which originate repeatedly forked, zig-zag-shaped side branches (modified after Radtke 1991, translated from German).
Description: The ichnogenus with its type ichnospecies I. elegans describes the boring traces of the marine siphonalean green alga Ostreobium quekettii Bornet et Flahault emend. Lukas 1973. The original description (Radtke 1991: 68) lists a number of morphologically different features: (a) straight, repeatedly dichotomously branched main galleries (4–5 μm in diameter), extending close to the substrate surface, (b) slightly thinner (2–3 μm wide), zig-zag-shaped galleries with (c) antler-like terminal swellings, forming tiers of dense networks and (d) swellings in the course of the main tunnels up to 8 μm wide. The presence of larger swellings up to 25 μm reported for O. quekettii could not been confirmed for the trace. High density branching created an appearance of a network, which originally inspired the name “Reticulina”. However, no anastomoses between branches exist in either Ostreobium (Lukas 1973, 1974) or Ichnoreticulina (Radtke 1991), because the branches consistently avoid contact with each other. The observed variants in trace morphology reflect a complex boring behavior that changes in the course of the development of the organism.
The present study compares a number of SEM images and provides new observations of resin-cast borings in shells from a variety of environmental settings and depths ranging from 6 to 60 m in order to explore the relations among different boring morphotypes and, if possible, to relate them to developmental stages of the organism that makes them.
Module 1 – proximal: The boring is organized by straight, sparsely branched tunnels that extend over relatively long distances inside the substrate, narrowing very gradually in distal direction (Fig. 8A and B). They show only minor irregularities, which appear to be diagnostic of this ichnotaxon and, by inference, of its biological maker. The irregularities consist of intermittent sections of the tunnel with undulations (Figs. 8A and B, u), or increases in volume to form irregular swellings (s). These long, straight “primary” tunnels produce two types of branches. The 1st order branches are dichotomous with minor reduction in diameter (d); they occur at low frequency (less then one branch per mm of filament length). The 2nd order branches are arranged lateral, with significantly reduced diameters (p). They occur at higher frequencies (three to five branches per mm of filament length) and depart at approximately right angles, alternating or, less commonly, opposing each other. These 2nd order branches are more frequently characterized by undulations and irregular diameter changes. This branching pattern may continue for several branching orders upward, without major changes in appearance, but both the branching frequency and irregularities generally increase toward higher orders. The Module 1 types of boring bears some resemblance to borings of heterotrophic endolithic organisms in substrates from deep sea. Both have been observed with dichotomous and lateral branching.
Module 2 – distal: The boring pattern at the higher branching orders which constitute the Module 2 is quite distinct. It is characterized by high-frequency branching that produce dense two- and three-dimensional networks. Three basic types of tunnels could be distinguished. The pattern is usually initiated by tunnels progressing in a characteristic zig-zag pattern (Fig. 8A–C, z). This pattern seems to be generated by a series of aborted dichotomous ramifications in a plane parallel to the substrate surface (Fig. 8C, z). A terminal fork is initiated, but only one side continues to grow at an angle. The branches alternate with each subsequent fork initiation, and the angle changes into a zig-zag pattern. A combination of frequent alternating and opposing branching results in the formation of dense horizontal networks of slightly dorsoventrally flattened borings (Figs. 3H and 8A, n). The borings may become progressively finer down to submicron dimensions (Fig. 8C). The endolith consistently avoids the contact with the existing tunnels by lateral deflection or bridging the existing tunnels by deflecting deeper into the substrate (Fig. 8D). In the process, these borings have the tendency to exploit and fill the available space immediately underneath the substrate, and only then move deeper. The horizontal branching patterns of the distal Module 2 that form dense two-dimensional ‘networks’ are quite unique, and are the best diagnostic features for I. elegans.
Boring patterns of Ichnoreticulina elegans, scale bar in all pictures is 20 μm. A/B Panoramic view of two adjacent frames showing boring trace morphotypes at different developmental stages of the trace; d dichotomous branch of the 1st order filament; p positions of perpendicular lateral branches of 2nd order filaments; u undulating filaments; and n networks of higher order filaments; s swellings; z zig-zag-shape tunnels (sample C12/1, exposed reef, 15 m, in Chlamys sp.). C Detail image of two-dimensional network fields positioned between forked and laterally branched filaments of lower orders, note the tapering fine branches (sample C12/1, exposed reef, 15 m). D A detail showing two size ranges of network producing filaments, and a thin arched tunnel connecting separate network fields (sample C12/1). E A series of branches with arches. The branches are one-side or opposite and depart at right angles, each branching perpendicular to the substrate surface (sample B1/1). F Formation of irregularly shaped swellings throughout a filament network (sample B1/1). G A filament with arches and a lateral swelling (sample A11/2, channel, 27 m). H An oblique view of deep network of borings with arches and swellings; note the flat forked tapering tunnels at the expanding periphery of the colony (lower part of the picture toward the viewer; sample C1/5, reef slope, 60 m)
The third category of distal branching results in formation of series of arches up to 20 μm deep into the substrate each spanning a distance of 15–40 μm (Fig. 8E). The arches are formed by branching perpendicular rather than parallel to the substrate surface, with one branch directed toward the substrate surface and the other forming an arch to the next branch point and another surface connection. These bridges may connect areas with horizontal branching patterns (Fig. 8D), or represent multiple exits of Ostreobium filaments from the substrate (Fig. 8E). Arched tunnels that form repeated contacts with the substrate surface are also a frequent feature in borings of heterotrophic endoliths.
Module 3 – swellings: Bag-like widening and swellings occur in developed and presumably mature Ichnoreticulina systems. They are variable in shape and size. Their role as Ostreobium sporangia has been postulated, but not proven. The swellings occur intercalary or terminally (Fig. 8F), often on short lateral branches (Fig. 8G). The formation of bag-like swellings appears to be a relatively late development as it occurs in large systems, which also penetrate deeper into the substrate and form elaborate three-dimensional structures (Fig. 8H). Bag formation appears to be triggered by some particular, presently unknown environmental condition, as suggested by the occasional presence of clusters of equally spaced swellings of approximately same size (Fig. 8F). Extensive development of swellings can profoundly alter the appearance of the entire trace. Solitary terminally positioned swellings in the Module 3 type borings (as in Fig. 8G) can be confused with the fungal trace classified as Saccomorpha terminalis (Radtke 1991: pl. 14, Fig. 4). Serially arranged swellings are similar to those of rhodophytes (Conchocelis-stages), septate chlorophytes (Eugomontia sacculata) and endolithic fungi.
Remarks: The complexity of boring patterns of O. quekettii is considerable, but not amenable for quantitative morphometric characterization of its various traces because of the excessive variability of each morphotype (compare, e.g., the networks in Fig. 8D). In order to refine the description and facilitate the identification of exceedingly complex boring systems we have introduced a modular treatment. The morphotypes are organized in modules, which correspond to changes in boring strategy in the course of growth and development of the organism. Each module can be characterized by one or more morphotypes, and each usually occupies a predictable position in the boring system in relation to the development of the organism. We propose this treatment as intraspecific ichno-categories to avoid descriptions of morphotypes and their variants as separate disconnected ichnotaxa. Each of the identified boring patterns or morphotype is thereby oriented within a complex system. Each can be contrasted to similar borings produced by unrelated organisms.
The variability of O. quekettii is not exhausted with its euendolithic habitat. The organism is known to exit carbonate substrate and live as cryptoendolith in the skeletal pore spaces of corals (Schroeder 1972) and to participate in construction of modern stromatolites (Feldmann 1997). It is the most complex, but also the most frequent and widely distributed of all phototrophic endoliths. In clear oligotrophic tropical seas it has been documented at a depth of 300 m (Vogel et al. 1996), and is the main phototrophic constituent on carbonate rocks at depths between 100 and 150 m responsible for their green color (S. Golubic, unpublished). O. quekettii has to date been detected in virtually every tropical reef coral that was examined by the authors (see also Lukas 1973). For these reasons, it was important to encompass the entire spectrum of the variability of its complex boring trace.
The organism is a siphonalean green alga, which exhibits a tree-like branching mode when it grows and penetrates carbonate substrates (see Lukas 1974: Fig. 1). The filaments gain both in length and in diameter as they grow, while delivering consecutive orders of ever finer branches. Using the same analogy of a tree, one could view the tree trunk as proximal and the terminal branches as its distal parts. The spread of borings follows initially the surface of the substrate, which in the shells results in a predominantly two-dimensional display. A three-dimensional display is introduced at the advanced stages of the substrate colonization featuring considerable complexity of the boring system of this ichnotaxon when mature. Different boring patterns correlate with developmental stages. They have been analyzed in the context of modules and illustrated in Fig. 8.
Discussion
Ichnology of microboring traces in hard calcareous substrates has made major advances during the past half of a century and found application in paleoecological, specifically paleobathymetric reconstructions of ancient sedimentary basins and reefs (e.g., Vogel et al. 1987; Schmidt 1990; Radtke 1991, 1998; Glaub 1994; Balog 1996, 1997; Hofmann 1996; Glaub and Bundschuh 1997; Vogel and Marincovich 2004). A successful application depends on proper identification of the organism that made the trace as well as on the knowledge of its environmental requirements. Such information is being sought by actuo-paleontological studies of microborings and their makers in modern environments, with the added requirement that the information be expressed by properties that are preservable in the fossil record (e.g., Golubic et al. 1975; Günther 1990; Radtke 1993; Vogel et al. 2000). For this reason, characterization and identification of boring traces cannot and should not be completely independent from identification of the organisms that produced them, but neither should these identities be interchangeable. A boring trace is but one of many characteristics that identify an euendolithic microorganism, the specificity of which may vary from case to case. The same euendolithic microorganism may produce different traces in the course of its development or change the boring behavior under different environmental conditions. Conversely, similar boring patterns may have evolved convergently in organisms that are unrelated and/or metabolically different. Nevertheless, subtle distinctions may exist and be recognized as diagnostic. For paleoenvironmental interpretation, the distinction between traces of phototrophs versus organotrophs is or particular importance. In view of the richness of information derived from the study of microborings in modern environments, the current rule of the ICZN (1999), requiring that ichnotaxa be described exclusively on the basis of fragmentary fossil information is restrictive, it does not serve the needs of the field of ichnology and should be changed.
With this contribution, we have started a closer morphological and morphometric evaluation of microbial traces by identifying quantifiable morphometric parameters for common microboring types, and taking measurements at the level of field populations. The obtained data are statistically supported and graphically displayed for comparisons (Figs. 5 and 6).
In the case of the measured Fascichnus populations (Fig. 5 and Appendix 1), the results show a surprising display of diameter values in a continuous spectrum, in which each colony is well defined by relatively narrow normal distribution of sizes, but the neighboring colonies show significant overlap and the end member populations a substantial size difference. This pattern of data display is reminiscent of ring species (e.g., Irwin et al. 2001) which poses objective problem for taxonomic delimitation. The observed Fascichnus diversity was paralleled by high diversity of euendoliths that produce such trace. Seven species of Hyella and one Solentia were identified in the shells following acid extraction (Table 1). Although Fascichnus colonies show some distinctive features in addition to size, the distinction between the above cyanobacterial genera was not possible on the basis of the shape of their borings alone.
Early descriptions of microboring ichnotaxa were based on few isolated observations and were therefore conceived broadly. The ichnospecies Rhopalia catenata (Radtke 1991), for example, included forms which were different from the type. After comparative modern material became available, the description of these forms could be complemented by new observations and supported by morphometric evaluations (Fig. 6). These forms are now described separately as a new ichnospecies R. spinosa.
The very concept of species in prokaryotes is currently under scrutiny, however the models for speciation by replacement of clonal strains in the process of establishing microbial ecological niches (Cohan 2002; Konstantinidis and Tiedje 2005), are consistent with the pattern observed for cyanobacteria and their traces.
Complex boring patterns, on the other hand, are common among eukaryotic euendoliths and they often reflect changes in the course of the development of the boring organism. These can be challenging for ichnological treatment, because they frequently unite morphologically different components. For example, an orderly sequence in apical growth and monopodial branching of septate euendolithic chlorophytes is reflected in their borings (Fig. 3E).
Entire systems of borings often show concentric arrangements, reflecting differentiation stages of the boring organism from exploratory filaments on the periphery of the colony, over a zone of increased filament density to a reproductive center with sporangial swellings (Fig. 7C). Morphological differentiation in the course of the life cycle includes reproduction, both in Rhopalia spinosa (Fig. 7H) and in Ichnoreticulina elegans (Fig. 8F–H).
We have opted to describe, measure, and taxonomically treat different components of a complex boring pattern as separate intraspecific modules. The position of these units in reference to the entire boring system was thereby determined and, when possible, related to particular stages in the course of the development and/or functional differentiation of the boring organism. In the observed cases, the boring patterns change gradually from the periphery toward the point of origin (entry into the substrate), which corresponds, in terms of timing, a shift from younger to older parts of the organism. The observed changes in boring patterns, described as modules may be distinct but interconnected.
The most complex and varied boring behavior was observed in the case of the siphonalean chlorophyte Ostreobium quekettii with a dual euendolithic and cryptoendolithic mode of existence. An array of boring patterns different in shape, dimensions, and orientation could be summarized as modules within the complex trace Ichnoreticulina (Fig. 8). The variability in filament forms occurred at different size ranges within the same organism, comparable with the branching of a tree (e.g., Fig. 8D), which sets limits to practical use of measurements for determinative purposes beyond a simple orientation provided by scale bars. This complex morphology appears also to respond to the architecture of the bored substrate and to the extent the substrate has already been exploited. Thus, it is only loosely associated with changes in the course of the development of the organism.
Distribution of microborings and their makers in Safaga Bay is similar to that in other tropical settings such as the Bahamas (Radtke 1993; Vogel et al. 2000), Arabian Gulf (Al-Thukair and Golubic 1991a, b), Polynesian coral reefs (Radtke et al. 1997c; Chazottes et al. 1995), and Great Barrier Reef of Australia (Vogel et al. 2000). The findings support the notion of pantropical if not global distribution of the most common microbial endoliths and their boring patterns. As in other clear tropical waters, the microboring depth distribution is not sharply zonal, instead it shows gradual shift in dominance among competing microborers. The outcome of this competition is more clearly expressed in very shallow waters in favor of Fascichnus and in deepest ranges in favor of Ichnoreticulina.
Conclusions
Description of microborings as ichnotaxa provides needed references for paleoenvironmental and stratigraphic comparisons by removing the obligation to identify the organisms, which as a rule do not fossilize. However, actuo-paleontological studies, which link the traces to their biological identity, are equally important by providing paleoenvironmental content to these references by analogy. Morphometric characterization adds further precision to descriptions and confidence to comparisons.
The results show that a significant proportion of boring morphotypes described as ichnotaxa are not separated by hiatuses. In the case of the continuous speciation of microborers, this condition is objective. In such cases the established ichnotaxa describe ranges within a continuum of forms appropriately by convention.
Complex boring patterns, which correlate with development, life cycle or with the response of microborers to environmental conditions, are best described as intraspecific categories (modules) which can be evaluated separately, in relation to each other to similar borings, as well as in relation to entire boring systems and ichnocoenoses.
References
Al-Thukair AA, Golubic S (1991a) New endolithic cyanobacteria from the Arabian Gulf. I. Hyella immanis sp. nov. J Phycol 27:766–780
Al-Thukair AA, Golubic S (1991b) Five new Hyella species from the Arabian Gulf. In: Hickel B, Anagnostidis K, Komárek J (eds) Cyanophyta/Cyanobacteria–morphology, taxonomy, ecology. Algologic Stud 64:167–197
Al-Thukair AA, Golubic S (1996) Characterization of Hyella caespitosa var. arbuscula var. nov. (Cyanophyta, Cyanobacteria) from shoaling ooid sand grains, Arabian Gulf. Nova Hedwigia Bh 112:83–91
Al-Thukair AA, Golubic S, Rosen G (1994) New euendolithic cyanobacteria from the Bahama Bank and the Arabian Gulf: Hyella racemus sp. nov. J Phycol 30:764–769
Balog S-J (1996) Boring thallophytes in some Permian and Triassic reefs: Bathymetry and bioerosion. In: Reitner J, Neuweiler F, Gunkel F (eds) Global and regional controls on biogenic sedimentation. I. Reef evolution. Research Reports. Göttinger Arb Geol Paläont Sb 2:305–309
Balog S-J (1997) Mikroendolithen im Capitan Riff Komplex (New Mexico, USA). Courier Forschinst Senckenberg 201:47–55
Bassiouni MA (1969) Ostracoden aus dem Eozän von Ägypten. I Trachyleberidinae. Geol Jb 87:383–426
Bentis CJ, Kaufman L, Golubic S (2000) Endolithic fungi in reef-building corals (Order: Scleractinia) are common, cosmopolitan, and potentially pathogenic. Biol Bull 198:254–260
Butterfield NJ, Knoll AH, Swett K (1990) A bangiophyte red alga from the Proterozoic of Arctic Canada. Science 250:104–107
Campbell SE (1980) Palaeoconchocelis starmachii, a carbonate boring microfossil from the Upper Silurian of Poland (425 million years old): Implications for the evolution of the Bangiaceae (Rhodophyta). Phycologia 19:25–36
Campbell SE (1982) Precambrian endoliths discovered. Nature 299:429–431
Campbell SE, Kazmierczak J, Golubic S (1979) Palaeoconchocelis starmachii gen. n., sp. n., an endolithic Rhodophyte (Bangiaceae) from the Silurian of Poland. Act Palaeont Pol 24:405–408
Chazottes V, Le Campion-Alsumard T, Peyrot-Clausade M (1995) Bioerosion rates on coral reefs: Interactions between macroborers, microborers and grazers (Moorea, French Polynesia). Palaeogeogr Palaeoclimatol Palaeoecol 113:189–198
Cohan FM (2002) What are bacterial species? Ann Rev Microbiol 56:457–487
Croft WN, George EA (1959) Blue-green algae from the Middle Devonian of Rhynie, Aberdeenshire. Bull Brit Mus (Nat Hist) Geol 3:341–353
Feldmann M (1997) Stromatolitic laminae formation and carbonate precipitation associated with microbial mats from modern Bahamian environments. Facies 36:200–203
Glaub I (1994) Mikrobohrspuren in ausgewählten Ablagerungsr äumen des europäischen Jura und der Unterkreide. Courier Forschinst Senckenberg 174:1–324
Glaub I, Bundschuh M (1997) Comparative studies on Silurian and Jurassic/Lower Cretaceous microborings. Courier Forschinst Senckenberg 201:123–135
Glaub I, Vogel K (2004) The stratigraphic record of microborings. Fossils Strata 51:126–135
Golubic S, Schneider J (2003) Microbial endoliths as internal biofilms. In: Krumbein WE, Dornieden T, Volkmann M (eds) Fossil and recent biofilms. Kluwer, Dordrecht, pp 249–263
Golubic S, Brent G, Le Campion-Alsumard T (1970) Scanning electron microscopy of endolithic algae and fungi using a multipurpose casting-embedding technique. Lethaia 3:203–209
Golubic S, Perkins RD, Lukas KJ (1975) Boring microorganisms and microborings in carbonate substrates. In: Frey RW (ed) The study of trace fossils. Springer, Berlin, pp 229–259
Golubic S, Friedmann I, Schneider J (1981) The lithobiontic ecological niche, with special reference to microorganisms. J Sed Petrol 51:475–478
Golubic S, Campbell SE, Spaeth C (1983) Kunstharzausgüsse fossiler Mikroben-Bohrgänge. Präparator 29:197–200
Golubic S, Hernandez-Marine M, Hoffmann L (1996) Developmental aspects of branching in filamentous Cyanophyta/Cyanobacteria. Algologic Stud 83:303–329
Green JW, Knoll AH, Swett K (1988) Microfossils from oolites and pisolites of the upper Proterozoic Eleonore Bay group, central east Greenland. J Paleont 62:835–852
Grill B, Zuschin M (2001) Modern shallow- to deep-water bivalve death assemblages in the Red Sea—ecology and biogeography. Palaeogeogr Palaeoclimatol Palaeoecol 168:75–96
Günther A (1990) Distribution and bathymetric zonation of shell-boring endoliths in recent reef and shelf environments: Cozumel, Yucatan (Mexico). Facies 22:233–262
Harris PM, Halley RB, Lukas KJ (1979) Endolith microborings and their preservation in Holocene–Pleistocene (Bahama-Florida) ooids. Geology 7:216–220
Haunold TG, Baal C, Piller WE (1997) Benthic Foraminiferan associations in the Northern Bay of Safaga, Red Sea, Egypt. Mar Micropaleont 29:185–210
Hofmann K (1996) Die mikro-endolithischen Spurenfossilien der borealen Oberkreide Nordwest-Europas. Geol Jb A 136:1–153
International Commission on Zoological Nomenclature [ICZN] (1999) International code of zoological nomenclature. International Trust for Zoological Nomenclature, Natural History Museum, London, XXIX + pp 306
Irwin DE, Bensch S, Price TD (2001) Speciation in a ring. Nature 409:333–337
Kaehler S, McQuaid CD (1999) Lethal and sub-lethal effects of phototrophic endoliths attacking the shell of the intertidal mussel Perna perna. Mar Biol 135:497–503
Kiene WE, Radtke G, Gektidis M, Golubic S, Vogel K (1995) Factors controlling the distribution of microborers in Bahamian reef environments. In: Schuhmacher H, Kiene WE, Dullo W-Chr (eds) Factors controlling Holocene reef growth: An interdisciplinary approach. Facies 32:174–188
Kleemann K (1992) Coral communities and coral–bivalve associations in the Northern Red Sea Bay at Safaga, Egypt. Facies 26:1–10
Knoll AH (1985) Exceptional preservation of photosynthetic organisms in silicified carbonates and silicified peats. Philos Trans R Soc Lond B 311:111–122
Knoll AH, Golubic S, Green J, Swett K (1986) Organically preserved microbial endoliths from the Late Proterozoic of East Greenland. Nature 321:856–857
Knoll AH, Swett K, Burghardt E (1989) Paleoenvironmental distribution of microfossils and stromatolites in the upper Proterozoic Backlundtoppen Formation, Spitsbergen. J Paleont 63:129–145
Konstantinidis KT, Tiedje JM (2005) Genomic insights that advance the species definition for prokaryotes. PNAS 102:2567–2572
Larkum AWD, Koch EMW, Kühl M (2003) Diffusive boundary layers and photosynthesis of the epilithic algal community of coral reefs. Mar Biol 142:1073–1082
Le Campion-Alsumard T (1970) Cyanophycées marines endolithes colonisant les surfaces rocheuse dénudées (Étages supralittoral et mediolittoral de la région de Marseille). Schweiz Z Hydrol 32:552–558
Lukas KJ (1973) Taxonomy and ecology of the endolithic microflora of reef corals, with a review of the literature on endolithic microphytes. Unpublished Ph.D. Thesis, University of Rhode Island, pp 159
Lukas KJ (1974) Two species of the Chlorophyte genus Ostreobium from skeletons of Atlantic and Caribbean reef corals. J Phycol 10:331–335
Nebelsick JH (1992) The Northern Bay of Safaga (Red Sea, Egypt): An actuopalaeontological approach. III Distribution of echinoids. Beitr Paläont Österr 17:5–79
Piller WE (1994) The Northern Bay of Safaga (Red Sea, Egypt): An actuopalaeontological approach. IV Thin section analysis. Beitr Paläont Österr 18:1–73
Piller WE, Mansour A (1990) The Northern Bay of Safaga (Red Sea, Egypt): An actuopalaeontological approach. II Sediment analysis and sedimentary facies. Beitr Paläont Österr 16:1–102
Piller WE, Pervesler P (1989) The Northern Bay of Safaga (Red Sea, Egypt): An actuopalaeontological approach. I Topography and bottom facies. Beitr Paläont Österr 15:103–147
Piller WE, Rasser M (1996) Rhodolith formation induced by reef erosion in the Red Sea, Egypt. Coral Reef 15:191–198
Radtke G (1991) Die mikroendolithischen Spurenfossilien im Alt-Tertiär West-Europas und ihre palökologische Bedeutung. Courier Forschinst Senckenberg 138:1–185
Radtke G (1993) The distribution of microborings in molluscan shells from recent reef environments at Lee Stocking Island, Bahamas. Facies 29:81–92
Radtke G (1998) Mikroendolithische Bohrspuren in Gastropoden-Schalen von Sieblos/Rhön (Unter-Oligozän). In: Martini E, Rothe P (eds) Die alttertiäre Fossillagerstätte Sieblos an der Wasserkuppe/Rhön. Geol Abh Hessen 104:143–155
Radtke G, Le Campion-Alsumard T, Golubic S (1996) The bioerosional notch along tropical limestone coasts. Algologic Stud 83:469–482
Radtke G, Gektidis M, Golubic S, Hofmann K, Kiene WE, Le Campion-Alsumard T (1997a) The identity of an endolithic alga: Ostreobium brabantium Weber-van Bosse is recognized as carbonate-penetrating rhizoids of Acetabularia (Chlorophyta, Dasycladales). Courier Forschinst Senckenberg 201:341–347
Radtke G, Hofmann K, Golubic S (1997b) A bibliographic overview of micro- and macroscopic bioerosion. In: Betzler C, Hüssner H (eds) Vogel-Festschrift. Courier Forschinst Senckenberg 201:307–340
Radtke G, Le Campion-Alsumard T, Golubic S (1997c) Microbial assemblages involved in tropical coastal bioerosion: An Atlantic–Pacific comparison. Proc 8th Int Coral Reef Symp 2:1825–1830
Rasser M, Piller WE (1997) Depth distribution of calcareous encrusting associations in the Northern Red Sea (Safaga, Egypt) and their geological implications. Proc 8th Int Coral Reef Symp 1:743–748
Rice CM, Trewin NH, Anderson LI (2002) Geological setting of the early Devonian Rhynie cherts, Aberdeenshire, Scotland: An early terrestrial hot spring ecosystem. J Geol Soc Lond 159:203–214
Riegl Z, Piller WE (1997) Distribution and environmental control of coral assemblages in Northern Safaga Bay (Red Sea, Egypt). Facies 36:141–162
Schmidt H (1990) Mikrobohrspuren in Fossilien der triassischen Hallstätter Kalke und ihre bathymetrische Bedeutung. Facies 23:109–120
Schneider J, Torunski H (1983) Biokarst on limestone coasts, morphogenesis and sediment production. Mar Ecol 4:45–63
Schroeder JH (1972) Calcified algal filaments of an endolithic alga in Recent Bermuda reefs. N Jb Geol Paläont Mh 1:16–33
Simonetta AM, Della Cave L (1978) Notes on new and strange Burgess Shale fossils (Middle Cambrian of British Columbia). Att Soc Toscana Sci Nat Mem A 85:87–90
Tribollet A, Decherf G, Hutchings PA, Peyrot-Clausade M (2002) Large-scale spatial variability in bioerosion of experimental coral substrates on the Great Barrier Reef (Australia): Importance of microbrers. Coral Reefs 21:424–430
Vogel K, Golubic S, Brett CE (1987) Endolith associations and their relation to facies distribution in the Middle Devonian of New York State, USA. Lethaia 20:263–290
Vogel K, Bundschuh M, Glaub I, Hofmann K, Radtke G, Schmidt H (1995) Hard substrate ichnocoenoses and their relations to light intensity and marine bathymetry. N Jb Geol Paläont Abh 195:49–61
Vogel K, Kiene WE, Gektidis M, Radtke G (1996) Scientific results from investigations of microbial borers and bioerosion in reef environments. In: Reitner J, Neuweiler F, Gunkel F (eds) Global and regional controls on biogenic sedimentation. I. Reef evolution. Research Reports. Göttinger Arb Geol Paläont Sb 2:139–143
Vogel K, Gektidis M, Golubic S, Kiene WE, Radtke G (2000) Experimental studies on microbial bioerosion at Lee Stocking Island, Bahamas and One Tree Island, Great Barrier Reef, Autralia: Implications for paleoecological reconstructions. Lethaia 33:190–204
Vogel K, Marincovich L Jr (2004) Palaeobathymetric implications of microborings in Tertiary strata of Alaska, USA. Palaeogeogr Palaeoclimatol Palaeoecol 206:1–20
Zhang Y, Golubic S (1987) Endolithic microfossils (Cyanophyta) from Early Proterozoic stromatolites, Hebei, China. Acta Micropal Sin 4:1–12
Zuschin M, Piller WE (1997) Molluscan hard substrate associations in the Northern Red Sea. PSZNI Mar Ecol 18:361–378
Zuschin M, Hohenegger J, Steininger FF (2000) A comparison of living and dead molluscs on coral reef associated hard substrata in the northern Red Sea— implications for the fossil record. Palaeogeogr Palaeoclimatol Palaeoecol 159:167–190
Zuschin M, Hohenegger J, Steininger FF (2001) Molluscan assemblages on coral reefs and associated hard substrata in the northern Red Sea. Coral Reefs 20:107–116
Acknowledgements
This contribution is dedicated to the memory of Erik Flügel, who recognized early the significance of endolithic trace fossils as facies indicators and promoted this then still young branch of paleontology. We thank M. Zuschin (University of Vienna) and W. Piller (University of Graz) for the sample collection. K. Vogel (University of Frankfurt) is thanked for his continuing support and encouragement of the research on microborings and microbial endoliths as for his guidance of the Project `Morphometric Characterization of Microendolithic Ichnotaxa' (Vo 90/17-1), and to the Deutsche Forschungsgemeinschaft for the financial support of this research grant. For the international and interdisciplinary cooperation we thank for the support by the Alexander-von-Humboldt Foundation and Hanse Institute for Advanced Studies to S. Golubic. B. Holstein (University of Frankfurt) is thanked for statistic calculations, Ms. O. Sagert (University of Frankfurt) for sample preparations and J. Dengler (HLUG) for technical aid in furnishing of illustrations. J. Schneider (University of Göttingen) and M. Wisshak (IPAL, University of Erlangen) are cordially thanked for their exhaustive critical review which helped significantly in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Dimensions of Fascichnus borings
Diameter (max.) | Constriction | ||
|---|---|---|---|
Mean ± SD | n | Mean ± SD | n |
3.05±0.30 | 43 | 2.21±0.22 | 43* |
3.80±0.46 | 14 | 3.51±0.34 | 10 |
3.86±0.27 | 10 | 3.86±0.54 | 6 |
4.09±0.45 | 27 | 3.01±0.29 | 27 |
4.31±0.56 | 8 | 4.04±0.47 | 14 |
4.53±0.66 | 24 | 4.10±0.65 | 21 |
4.63±0.47 | 96 | 3.67±0.40 | 18 |
4.63±0.48 | 32 | 3.58±0.34 | 32 |
4.99±0.39 | 46 | 3.55±0.30 | 35 |
5.02±0.35 | 5 | 4.57±0.35 | 9 |
5.10±0.50 | 17 | 4.59±0.41 | 16 |
6.34±0.56 | 20 | 5.66±0.50 | 19 |
6.38±0.57 | 10 | 5.56±0.45 | 32 |
6.54±0.83 | 14 | 6.25±1.04 | 13 |
7.46±0.81 | 13 | 6.43±0.41 | 11 |
7.50±0.78 | 22 | 6.52±0.87 | 21* |
8.33±2.11 | 12 | 6.65±0.94 | 16 |
8.57±0.72 | 14 | 8.54±0.53 | 6 |
9.60±1.34 | 26 | 8.37±0.37 | 25 |
10.57±0.76 | 8 | 8.33±0.78 | 8 |
10.69±1.17 | 34 | 7.21±0.83 | 27 |
11.60±1.44 | 22 | 8.18±1.12 | 54 |
11.82±1.17 | 34 | 11.28±1.41 | 11 |
12.43±1.35 | 25 | 11.84±1.11 | 23 |
13.70±1.15 | 17 | 10.28±0.79 | 25 |
13.90±1.04 | 12 | 11.76±1.59 | 12* |
13.99±1.85 | 29 | 8.93±1.34 | 33 |
15.31±1.25 | 27 | 11.03±1.39 | 22 |
18.88±2.00 | 40 | 12.07±1.56 | 50 |
19.82±1.76 | 42 | 12.01±2.23 | 40 |
24.11±3.88 | 13 | 16.35±3.07 | 11* |
Appendix 2: Dimensions of two systems of branched Rhopalia borings
Diameter (max.) | Constriction | |||
|---|---|---|---|---|
Mean ± SD | n | Mean ± SD | n | |
R. catenata | 17.70±2.60 | 103 | 6.70±1.00 | 98 |
15.19±2.26 | 24 | 7.25±0.92 | 27 | |
R. spinosa | 9.55±1.26 | 30 | 3.84±0.53 | 30 |
5.90±1.17 | 52 | 2.86±0.57 | 54 | |
5.70±1.91 | 45 | 1.16±0.36 | 36 | |
Rights and permissions
About this article
Cite this article
Radtke, G., Golubic, S. Microborings in mollusk shells, Bay of Safaga, Egypt: Morphometry and ichnology. Facies 51, 118–134 (2005). https://doi.org/10.1007/s10347-005-0016-02
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10347-005-0016-02