Abstract
Several samples of gastropod and pelecypod mollusk shells from three localities are selected along the Egyptian Red Sea coast. This is a study of the wall structure of some different shells of gastropods and pelecypod mollusks and their adaptive significance on evolution of the physical and chemical properties of the Red Sea water prevailing along the Egyptian shores and coasts. Analysis of gastropod and pelecypod mollusk shells of the study areas provides investigators with data to characterize the composition of these gastropod and pelecypod mollusk shells and to know the degree of human activities influence on the composition of the gastropod and pelecypod mollusk shells. Lambis truncata at Quseir and Safaga Harbors recorded the highest carbonate percentages (98.0 and 98.4%); meanwhile, Tridacna gigas at Quseir and Safaga Harbors recorded the highest organic matter contents (2.0 and 1.9%). On the other hand, Tridacna gigas recorded the highest values of organic matter in the study areas compared with the other species. The highest Ca contents were observed in Tridacna gigas and Strombus tricornis (71.1 and 69.6%) at El-Esh area, also the highest Mg was in Tridacna gigas (2.0%) at Qusier and Safaga Harbors, while the highest Sr was in Strombus tricornis at Qseir Harbor and El-Esh area (2473 and 2335 ppm). A study of the geochemistry, X-ray diffraction contents of the common gastropod and pelecypod mollusk shells from Quseir Harebour, Safaga Harbor, and El-Esh area along the Egyptian Red Sea coast was carried out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Red Sea is a semi-closed basin at the extreme end of the Indo-Pacific province; it is expected to contain some species which are quite different from closely related species in nearly seas. These include forms which are particularly living and flourishing in warm, shallow water, carbonate environments with higher salinities. The Red Sea stretch represents an ideal carbonate environment in which biostromes, carbonate sands with vegetation and reefs predominate.
Recent invertebrates mainly are benthic foraminifera, coral reefs, and Mollusca which are considered to be most important for this integrated approach. Recent invertebrates and protists, especially Mollusca and benthic foraminifera, serve as excellent indicators of the water temperature in the past and present environments (Madkour 2015). Mollusca is the second largest phylum of invertebrates in the animal kingdom, comprising about 100,000 species, about 60% of them are marine organisms (Wye 1989). Two classes of mollusks are studied here, namely Gastropoda and Pelecypod. The marine mollusks have a world-wide distribution and those chosen almost every possible living place from the rocky shores high above the tide marks to the deepest parts of the ocean. Thus, mollusks seem to represent an important and vital part of the ecology and economy of the sea. As many Indo-Pacific molluscan species are also found in the Red Sea, this work should be of value to researchers who are interested in the entire region. Molluscan shells are sensitive organisms for environmental changes (e.g., Yanko et al. 1994, 1998; Saraswat et al. 2004; Yumun 2017). Gastropoda and pelecypod mollusks are the two main classes included in this study. Gastropods are represented by Lambis truncata and Strombus tricornis and pelecypod include only Tridacana gigas. These species represent the famous types in the marine environment of the Red Sea and they are a valuable seafood source.
The use of recent invertebrates, especially molluscan shells, as bioindicators is less established; however, previous studies proved the ability of molluscan shells to monitor the environmental quality (e.g., Alve et al. 2009; Frontalini et al. 2009; Coccioni et al. 2009; Armynot du Chȃtelet et al. 2004; Bouchet et al. 2012; Dolven et al. 2013; Hess et al. 2013).
There is little published information on geochemical and mineral compositions of macro-invertebrates especially gastropod and pelecypod shells also and how to use these shells as pollution indicators along the Red Sea coast. Among them are Rinkevich and Loya (1977), Rinkevich and Loya (1979), Loya and Rinkevich (1980), Loya and Rinkevich (1987), Hanna (1990), Abd El-Salam (1993), Ouda and Obaidalla (1998), Jameson et al., (1999), Ziko et al. (2001), Mohammed and El-Sorogy (2003), Madkour (2004), Mansour et al. (2005), Madkour (2005), Madkour and Ali (2009), El-Taher and Madkour (2011), Mekawy and Madkour (2012, 2013), Mohamed et al. (2013), El-Taher and Madkour (2014), Madkour et al. (2015), and Ali et al. (2017). In addition, very little studies have been done on the nature, composition, distribution, and ecology of recent gastropod and pelecypod shells along the Egyptian Red Sea coast.
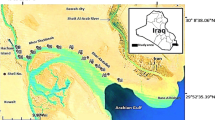
The present study focuses on areas subjected to nature, composition, and ecology of recent gastropod and pelecypod shells; they are Quseir and Safaga Harbors and El-Esh area (Fig. 1). The distribution of bottom facies at the investigated areas is shown in Figs. 2 and 3. The present study deals with the mineral composition of these skeleton and measurement of carbonate content, total organic matter content, major and minor elements of Tridacana gigas, Lambis truncate, and Strombus tricornis. These species of gastropods and pelecypods represent the famous types in the marine environment of the Red Sea. This study provides baseline information on geochemical and mineral composition of gastropod and pelecypod shells. Also, these species provides much information’s on the environment in which they live.
Study area
Quseir Harbor is located in Quseir City and is considered from old harbors on the Egyptian Red Sea coast. It is lying at latitudes 26° 05′ 02″ N to 26° 06′ 12″ N and longitudes 34° 16′ 58″ E to 34° 17′ 08″ E (Fig. 2). This harbor lies in a small by at the mouth of Wadi Ambaji. Terrigenous sediments have been transported to marine environment by Wadi Ambaji especially in the southern part of Quseir Harbor. It is observed that these sediments have a relatively large under cutting effect of the violent drive water during heavy torrents. The beach sediments are coarse sands. These sands are significant terrigenous fragments (Fig. 3). The tidal flat is very narrow and extents smoothly and slopes gently seaward. The sediments covering the bottom topography of this area are of fine sand to sandy mud. Most sediment samples have brown color. This is due to phosphate shipment operations. The system of the malaise of marine environment deterioration at Quseir area includes the spread of algal blooms, dense seagrasses, coral bleaching, and declining of productivity (Fig. 3), in addition to the poor biological activity of marine organisms especially coral reefs.
Safaga Harbor is the largest harbor on the Egyptian Red Sea coast. It is situated between latitudes 26° 43′ 42″ N and 26° 44′ 22″ N and longitudes 33° 56′ 20″ E and 33° 56′ 05″ E (Fig. 2). The intertidal zone in Safaga Harbor is extremely narrow. Shoreward, the area is skirted by high basement mountains. The beach sediments are generally coarse sands mixed with common rock-forming detritus from the surrounding formations (Figs. 2 and 3). The sediments covering the intertidal zone are fine to very fine sand sizes and rich in terrigenous constituents. On the other hand, bottom topography of Safaga Harbor is mud to sandy mud (Figs. 2 and 3). This is due to phosphate shipment, packing of cement, and other activities enter this harbor. Therefore, most sediment samples have gray to dark gray color. Some patches of seagrass were observed in tidal flat area. The diversity and distribution of marine organisms in Safaga Harbor is very little (Figs. 2 and 3). The coastal zone along the Red Sea at Safaga Town is a site for industrial development and for future establishment of dense recreational resorts. It includes Safaga Harbor on which the international trade depends. Therefore, studies in different fields are urgently needed to help managers identify anthropogenic impacts, and better assessing the needs for remediation by detecting any changes, from the existing level expected with operation of future activity.
El-Esh area is located ~ 30 km north of Hurghada between latitudes 27° 27′ 93″ N and 27°, 28′ 80″ N and longitudes 33° 38′ 00″ E and 33° 38′ 20″ E (Fig. 2). The catchment area of El-Esh is 0.48 × 106 m2 and the flushing waters drown from this area is 14,483 m3 by some wadis as obtained from the Red Sea and Gulf of Aden pilot (Anon 1980). El-Esh area is characterized by the existence of a wide intertidal zone about (300–500 m). The plenty of water drained from the wadis in the area necessitates constructing of a tunnel under the asphaltic high way (Suez - Hurghada road) allowing for water during torrent periods not to damage the way. Before the shoreline, the sands are accumulated in the form of small dunes covered by some vegetation. These dunes separate between the shore zone and sabkha evaporates. The very gentle slope of this area creates a wide sabkha basin and a wider tidal flat and the intertidal zone of this area is very long reaching there are about 1.5 km from the beach. Along the shore, there are some very small patches of mangroves observed. A prominent feature, which characterizes the seaward of the tidal flat, is the presence of more seagrasses patches and some coral reef patches, namely fringing reefs (Figs. 2 and 3) Recently, the area was subjected to some impacts by drilling of oil production where artificial tongue of sediments enter the sea to more than 500 m with pipe lines of oil production (Figs. 2 and 3).
Materials and methods
Field works
Three of the gastropod and pelecypod shells in the Red Sea were selected for this study. Gastropods are represented by Lambis truncata and Strombus tricornis, and Bivalves include only Tridacana gigas (Plate 1). The specimens were identified according to Wye (1989). All species were photographed in situ using a Canon Power Shot A80 at each location. Gastropoda and pelecypod shell specimens were collected by Scuba diving in all areas under study (Figs. 1, 2, and 3). The specimens of gastropod and pelecypod shells were cleaned and the soft tissue was removed on the beach of the area from which the shells were collected. The empty pelecypod and gastropod shells were soaked in water for 15–30 min in order to kill any clinging algae then left to dry in air. Oceanographic parameters that control the coastal features of the Red Sea, such as salinity, temperature, pH, and turbidity values were determined on a water sample overlying each sampling site.
Laboratory analyses
All geochemical analysis was carried out on gastropod and pelecypod shells in the National Institute of Oceanography and Fisheries, Red Sea, and Alexandria Branches. Ten grams of each prepared sub-samples of all collected samples was ground using an agate mortar (Retsch Mortar), passed through an 80 mesh sieve and kept in dry, clean bag waiting for analysis. To determine the carbonate content, 1 g of each prepared sample was treated by (1 N HCL acid), filtered and washed several times by distilled water, dried and re-weight in order to calculate the percentage of carbonate content of the samples (Mansour et al. 1997 and Basaham and El-Sayed 1998). One gram of each crude sample was burned to 550 °C for about 2 h. Organic matter content of the sediments was determined by sequential weight loss at 550 °C (Brenner and Binford 1988).
Total phosphorus content was calculated; about 0.5 g of each prepared sample was digested with 10 ml of conc. HCL acid at 250 °C. The residue was diluted with distilled water to about 25 ml and shacked vigorously, and then the solution was filtered to remove any solid remains. By the same method, blank was also prepared. The filtrate solution was taken and titrated by using ascorbic acid-molybdenum blue method (American Public Health Association “APHA” 1995).
Major elements, calcium, magnesium, strontium, sodium, and potassium, were determined using Atomic Absorption Spectrophotometer (AAS-GBC-932 Ver. 1.1) at the National Institute of Oceanography and Fisheries, Red Sea branch.0.5 g of the prepared ground sample was completely digested in a Teflon by using a mixture of conc. Nitric (HNO3), perchloric (HClO4), and hydrofluoric (HF) acids have the ratio 3:2:1 according to (Chester et al. 1994). The results were expressed in parts per million.
The mineralogy of the selected samples was determined using the X-ray diffraction technique. About 10 g of each sample was grounded using agate mortar to less than 80 mesh size to be ready for the X-ray diffraction. Quantitative method was applied to determine the carbonate and the non-carbonate minerals (about 12 minerals were recorded) by using the copper slide technique between 20 and 60 A° according to Hardy and Tuker (1988). The obtained values are used to calculate the carbonate and non-carbonate mineral percentages.
Results and discussion
Climate and hydrographic influences
Temperature, humidity, rainfall, and wind are of paramount significance in determining the nature of marine biological systems in all parts of the world including the Red Sea. Understanding the circulation of water in the area is critical to determine how nutrients, sediment, contaminants, and other water-borne materials are transported. Winds, tides, and currents drive circulation in the area. Over the whole year winds, the NW to NE trend predominates, but only in rare cases, the southern directions occur. Wind velocity usually ranges between 6.39 and 9.2 km/h with an average of 8.3 km/h in summer and between 6.01 and 11.57 km/h with an average of 9.43 km/h in winter (Table 1). Because of the nearly permanent air and water turbulence, a complete mixing of the water column occurs and no stratification is developed inside the water body. This is reflected also by the values of temperature and salinity, which show no significant differences between surface and bottom waters (Piller and Pervesler 1989). Humidity ranges from 56.4 to 62 with an average of 59.2% in summer and varies between 54.5 and 69.2 with an average of 61.8% in winter (Meteorological station of National Institute of Oceanography and Fisheries (NIOF), Red Sea branch (Table 1). Sunlight and warmth seem to be as essential to the production of the bright and varied colors of thee molluscan shells as they are to the growth of thick and ornamented shells.
Generally, molluscan shells of the Egyptian Red Sea coast are indicative of warm, shallow water, carbonate environments with hypersaline conditions. The molluscan shells are commonly large thick, opaque and mostly colored thus corroborating the fact that warm shallow water, tropical shells are generally more colored, thicker, large-sized, and ornamented than those of the cold water (Ouda and Obaidalla 1998) (Table 2).
Geochemistry
Carbonate
Typical molluscan shells contain three or more layers. The outermost layer is usually a chitinous periostracum. The inner layers (two or more) are calcareous but can contain up to 9% organic matter (with an average content 2 to 3%) (Hare and Abeleson 1964). Generally, calcium carbonate contains three major elements (calcium, carbon, and oxygen), two minor elements (magnesium and strontium), and a considerable number of trace elements whose concentrations exceed 1 ppm (Milliman 1974). Pelecypods inhabit nearly every marine environment. They are probably benthic carbonate contributor to modern shallow sediments. Pelecypods tend to deposit their calcium carbonate shells more slowly than gastropods, but absolute growth is strongly dependent upon the nature of the environment and substrate (Swan 1952). Most quite-water pelecypods have thin shells while those mollusks living in higher energy environments have thicker shells. Similarly, mollusks living in very gold waters usually have thin and chalky shells. Perhaps the fastest measured rate of pelecypods carbonate deposition is that of Tridacna gigas which deposits 23 g of CaCo3/100cm2/year (Bonham 1965). The total carbonate content in gastropod and pelecypod mollusk shells of the study areas varies between 95.61% in Tridacna gigas at Quseir Harbor and 98.41% in Lambis truncata at Safaga Harbor (Table 3; Fig. 3). According to Nicols (1967), pelecypods are probably the major benthic carbonate contributor to modern shallow marine sediments. They tend to deposit their calcium carbonate shells more slowly than do gastropods.
Organic matter
Layers of organic matrix between the crystalline layers give the shells increased flexibility needed in environmental stresses (Wainwright 1969). The organic matter is mainly derived from the autolysis of dead cells or actively excreted by diverse organisms as benthic algae, copepods, and sea urchins, as well as planktic species (Kenneth 1988). The distribution of total organic matter varies from 0.3% in Strombus tricornis at Safaga Harbor to 2.0% in Tridacna gigas at the same area (Table 3; Fig. 4). Based on the organic matter, content plays a significant role in the marine environment. Therefore, the organic materials are present essentially cooperated with the mud fraction that considered the scavengers for the trace metals. There is no correlation between TOM and the variables except to magnesium content (+ 0.62) and quartz (− 0.79) Table 4.
Major elements
Calcium
Calcium is considered an essential element for the marine carbonates where calcium is the main in aragonite and Mg-calcite. Calcium level ranges from 55.2% in Strombus tricornis at Safaga Harbor to 71.05% in Tridacna gigas at El-Esh area (Table 3; Fig. 4). According to Madkour (2004), molluscan shells have high values of calcium contents compared with foraminferal tests. This is due to molluscan shells are mainly composed of aragonite. Milliman (1974) found that Ca content in Sorites is 35.2%, while (Goreau and Goreau 1960a) found that Acropora and Porites have Ca levels of 38.8 and 39.4%, respectively. Hanna (1990) recorded that the Ca content in hard corals ranges from 48.2% ± 0.1 in Acropora aspera to 63% ± 0.2 in Fungia serrata at the western Egyptian Red Sea coastal area in front of Al-Ghardaqa. Ziko et al. (2001) pointed that the Ca content in some recent shells ranges from 40.8 in Turbo (Batillus) radiatus to 55.2% in Cypraea staphylaea along the Red Sea coast.
Magnesium
Magnesium is one of the major elements in some marine invertebrate’s especially foraminiferal tests. Magnesium is associated with calcium and is essential in the dolomites and high Mg-calcite. Mg concentration in molluscan shells changes from 0.06% in Lambis truncata at Quseir Harbor to 0.24% in Tridacna gigas at the same area (Table 3; Fig. 4). Abd el Aal and Hassan (1988) found that Mg content in Strombus tricornis is 0.12% of Hurghada area. Ramadan and Shata (1993) pointed that Mg concentration range between 118 and 707 ppm, averaging 288 ppm in Anadara diluvii from Mediterranean coast. Shata and Hassan (2000) found that Mg content of bivalve shells varies from 252.7 ppm in family Venerida to 510.0 ppm in family Arcidae at the Eastern Harbor of Alexandria. Ziko et al. (2001) illustrated that the Mg content in some recent shells ranges from 0.75% in Chama pacifica at Gebel Zeit to 3.11% in Amphistegina lessonii, at El-Hamrawein area. According to Madkour (2004), foraminiferal tests have the highest values of Mg content compared with coral reefs and molluscan shells along the Egyptian Red Sea coast. This is because benthic foraminfera are composed of Mg-calcite with rarely aragonite (Hemleben et al. 1986).
Strontium
The presence of Sr++ is linked with the crystallization of aragonite. On the other hand, Sr can be related to the rate of growth of the molluscan shells (Milliman 1974). According to Thompson and Livingston (1970), strontium decreases with increasing temperature in the some aragonitic molluscan shells and increases with increasing temperature in some calcite species, but in most organic carbonates, the strontium content remains more or less constant with temperature. The Sr concentration in molluscan shells varies from 1686.42 ppm in Lambis truncata at Quseir Harbor to 2335 ppm in Strombus tricornis at El-Esh area (Table 3; Fig. 4). Ramadan and Shata (1993) stated that, strontium shows a somewhat different mode of variation, whereas its concentration varies from 3000 to 8000 ppm, averaging 5000 ppm in the shells of Anadara diliuvii collected from the beach of Port Said. They also added that, the irregular variations of the Sr++; and its apparent increase through the age stages of the studied shells can be interpreted on basis of the degree of crystallization of aragonite. Ziko et al. (2001) recorded that, the strontium content in the recent shells ranges from 1141 ppm in Clanculus (Clanculus) pharaonius at Gebel Zeit to 2833 ppm in Cypraea staphylaea at El-Hamrawein area. According to Madkour (2004), coral reef species recorded the maximum values in strontium content of the areas under study, compared with forminferal tests and molluscan shells. This is because all scleractinians are composed of aragonitic.
Sodium
The occurrence of alkaline cations (Na and K) is extremely little in marine carbonate especially recent shells. The variation of sodium levels in molluscan shells is from 0.4% in Strombus tricornis at Safaga Harbor to 1.04% in Lambis truncata at El-Esh area (Table 3; Fig. 4). According to Milliman (1974), sodium contents in Acropora and Porites are 0.44 and 0.43%, respectively. In comparison, coral reefs and recent shells have higher values of sodium content than foraminiferal tests (Madkour 2004). This is probably due to the effect of the environmental condition surrounding these organisms.
Potassium
The potassium level in molluscan shells ranges from 0.01% in Tridacna gigas at Safaga Harbor to 0.08% in Strombus tricornis at Quseir Harbor (Table 3; Fig. 4). Madkour (2004) recorded that foraminiferal tests have high values of potassium content compared with coral reefs and molluscan shells. This variation, probably the source, is rich of K element; therefore, the difference in percentage of the same species from place to place is found. Madkour (2004) recorded the major elements (Ca, Mg, Na, and K) in molluscan shell species at the same areas and those values were close to these values. Elemental composition depends strongly upon the mineralogy of the skeleton of molluscan shells.
Minor elements (phosphorus content)
The abnormal increasing of the phosphorus content causes flushing for many undesired species as red algae which termed the coral killer and other species of macro-algae in coral reef areas such as Quseir and Safaga Harbors. On the other hand, the shortage of phosphorus content causes emaciation in the living biota. Schelske et al. (1985) stated that, the proportion of total phosphorus inputs increased with increased disposal and domestic sewage into the areas closed to urban localities. The P concentration in molluscan shells is from 34.44 ppm in Lambis truncata at El-Esh area to 4286 ppm in Tridacna gigas at Quseir Harbor. Generally, bivalve species (Tridacna gigas) recorded high values compared with gastropod species (Lambis truncata and Strombus tricornis) in all study areas (Table 3; Fig. 4). In comparison between coral reefs and molluscan shells for phosphorus content, coral reefs recorded high concentrations particularly in Quseir and Safaga Harbors (Madkour 2004). This is due to larger quantities of phosphates carried into the seawater in these harbors from which the phosphates are exported to other areas. Most organisms especially coral reefs are affected from increasing of the phosphorus content. The highest amount of dead corals in Quseir Harbor and this is associated with increasing of P content. Nutrient enrichment of coastal waters enables algae to thrive, overgrow, and kill some organisms in the marine environment.
X-ray diffraction analysis
The composition of mollusks can change greatly with environment as well as in response to various organic phenomena such as the composition of the organic matrix (Hare 1963). Generally, mollusks with mixed mineralogies aragonite content increase with decreasing salinity (Eisma 1966). Aragonite content also can increase with increasing water temperature (Milliman 1974). The X-ray analysis indicates that the gross mineralogy of molluscan shells of the study areas is nearly similar and comprises primarily carbonates minerals (Table 3). Aragonite has values ranges from 50.1% in lambis truncate at El-Esh area and 87.5% in Tridacna gigas at Quseir Harbor (Table 3; Fig. 4). The values of aragonite content in gastropoda and pelecypod skeletons at the study areas differ from species to another and depends on the nature of the area.
Variation in composition with mineralogy
According to Milliman (1974), numerous exceptions exist in the rules governing the mineralogical and chemical data of gastropods and pelecypods. Elemental composition depends strongly upon the mineralogy of the skeleton. Cations with large ionic radii such as strontium and to a lesser extent barium lead and uranium tend to be more concentrated in aragonite than in calcite. Elements with small ionic radii such as (magnesium, manganese, iron, nickel, and phosphorus) prefer calcite. The relation between radius and mineralogy seems logical in view of the lattice structure of aragonite and calcite. Strontium values are three to five times higher in most aragonite than in calcite. On the other hand, magnesium values within aragonite are uniformly low never more than 0.5% by weight and generally less than 0.25%. Magnesium concentrations in calcite can be either high or low but seldom in between.
Conclusions
The molluscan shells exhibited high concentrations of phosphorus from Quseir Harbor compared with the study areas. This is due to larger quantities of phosphates carried into the seawater in this area. Quseir Harbor exhibited the greatest amount of dead organisms associated with increased phosphorus concentrations. Quseir Harbor is the oldest port to ship phosphate on the Egyptian Red Sea coast in the past.
The results of analysis are used as fingerprints to assess polluted gastropod and pelecypod mollusk shells and to identify anthropogenic impacts and geochemical composition better assess of the need for remediation. Mineralogy is the most important parameter in determining the elemental composition of a carbonate; other factors such as phylogeny, the environment, mode of living, ontogeny, and biochemical and physiological considerations are also important. The shell-wall structure of the investigated gastropoda and pelecypods are characterized by the presence of aragonitic materials either in form of complex crossed lamellar structures or as simple prismatic structures especially in pelecypod skeletons. The aragonitic wall structure of the Red Sea shells is in contrast with the calcite wall structure recognized among some recent shells from the Mediterranean and Black Seas. Further research and assessment of gastropod and pelecypod ecosystems in the Egyptian Red Sea are needed to ensure a sound basis for environmental and resource management.
References
Abd El-Aal, A. A. and Hassan, A. K., 1988. Chemical composition and wall structure of some gastropod shells from the Mediterranean Sea, Suez-Canal and Red Sea, Egypt. Bull. Inst. Oceanogr. & Fish., ARE, 14 (3): 155–166
Abdel–Salam, H. A (1993) Impact of landfilling on coral reef ecosystem of South Magawish, Hurghada, Red Sea. J Egyp Ger Soc Zool 11(D):187–197
Ali MY, Madkour HA, Mansour AM, Alharbi WR, El-Taher A (2017) Invertebrate shells (mollusca, foraminifera) as pollution indicators, Red Sea Coast, Egypt. J Afr Earth Sci 133(2017):74–85
Alve E, Lepland A, Magnusson J, Backer-Owe K (2009) Monitoring strategies for re-establishment of ecological reference conditions: possibilities and limitations. Mar Pollut Bull 59:297–310
Anon (1980) Red Sea and Gulf of Aden Pilot. 12Th (ed.), Publ. Hydrographic of the Navy, Somerset, London, 284 p
APHA (1995) Standard methods for the examination of water and wastewater. 25(ed). American Public Health Association, Am. Water works Ass. Water Env. Federation. Washington, DC. 1200p
Armynot du Chȃtelet E, Debenay JP, Soulard R (2004) Foraminiferal proxies for pollution monitoring in moderately polluted harbors. Environ Pollut 127:27–40
Basaham AS and El-Sayed MA (1998) Distribution and phase association of some major and trace elements in the Arabian Gulf sediments. Estuarine Coast and Shelf Sci., Academic Press Limited, 46;Pp: 185 – 194
Bonham K (1965) Growth rate of the giant clam Tridacna gigas at Bikini Atoll as revealed by radioautography. Science 149. No. 3681, 300–302
Bouchet VMP, Alve E, Rygg B, Telford RJ (2012) Benthic foraminifera provide a promising tool for ecological quality assessment of marine waters. Ecol Indic 23:66–75
Brenner M, Binford MW (1988) Relationships between concentrations of sedimentary variables and trophic state in Florida Lakes. Can J Fish Aquat Sci 45:294–300
Chester R, Lin FG, Basaham AS (1994) Trace metals solid state speciation changes associated with the down-column fluxes of oceanic particulates. J Geol Soci, London 151:351–360
Coccioni R, Frontalini F, Marsili A, Mana D (2009) Benthic foraminifera and trace element distribution: a case-study from the heavily polluted lagoon of Venice (Italy). Mar Pollut Bull 59:257–267
Dolven JK, Alve E, Rygg B, Magnussen J (2013) Defining past ecological statusand in situ reference conditions using benthic foraminifera: a case study from the Oslofjord, Norway. Ecol Indic 29:219–233
Eisma D (1966) The influence of salinity on mollusk shell mineralogy: a discussion. J Geo 74:89–94
El-Taher AM, Madkour HA (2011) Distribution and environmental impacts of metals and natural radionuclides in marine sediments in-front of different wadies mouth along the Egyptian Red Sea coast. Appl Radiat Isot 69:550–558
El-Taher AM, Madkour HA (2014) Environmental and radio-ecological studies on shallow marine sediments from harbour areas along the Red Sea coast of Egypt for identification of anthropogenic impacts. Isot Environ Health Stud 50(1):120–133
Frontalini F, Buosi C, Da Pelo S, Coccioni R, Cherchi A, Bucci C (2009) Benthic foraminifera as bioindicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Mar Pollut Bull 58:858–877
Goreau TF, Goreau NI (1960) Distribution of labeled carbon in reef-building corals with and without zooxanthellae. Science 131:668–669
Hanna RG (1990) Coral reef skeletons as toxic metals indicators. Trade Waste, Technical Services Branch, Poll. Control. Water Board, NSW: 1–9
Hare PE (1963) Amino acids in the proteins from aragonite and calcite in the shells of Mytilus californianus. Science 139:216–217
Hare PE, Abeleson PH (1964) Comparative biochemistry of the amino acids in molluscan shells structures (abst.) Geol Soc Am Special paper 82:84
Hardy R and Tucker M (1988) X-ray powder diffraction of sediments.In: Tucker, M. (ed). Techniques in sedimentology. 191–228, Oxford-London (black Well)
Hemleben C, Anderson OR, Berthold W, Spindler M (1986) Calcification and chamber formation in foraminifera: a brief overview. In: Lead beater, B.S.C., Riding, R. (Eds.), Biomineralization in lower plants and animals. The systematic Association. Spec. Publ. London 30:237–249
Hess S, Alve E, Trannum HC, Norlin K (2013) Benthic foraminiferal responses to water-based drill cuttings and natural sediment burial: results from a mesocosm experiment. Mar Micropaleontol 101:1–9
Jameson SC, Ammar MSA, Saadall E, Mostafa HM, Riegl B (1999) A coral damage index and its application to diving sites in the Egyptian Red Sea. Coral Reefs 18:333–339
Kenneth, P. 1988. Sediment transport and depositional processes. Postgraduate Research Instit. For sed. Univ. of Reading. White kenights, 397p
Loya Y, Rinkevich B (1980) Effects of oil pollution on coral reef communities. Mar Ecol Prog Ser 3:167–180
Loya Y and Rinkevich B (1987) Effects petroleum hydrocarbons on corals. Human impacts on coral reefs: Facts and Recommendation, 91–102
Madkour, H. A., 2004. Geochemical and environmental studies of recent marine sediments and some invertebrates of the Red Sea, Egypt PhD Thesis South Valley Univ Qena 317p
Madkour HA (2005) Distribution and relationships of heavy metals in the Giant Clam (Tridacna maxima) and associated sediments from different sites in the Egyptian Red Sea coast. Egypt J Aquat Res 31(2):45–59
Madkour HA (2015) Detection of damaged areas due to tourism development along the Egyptian Red Sea coast using GIS, remote sensing and foraminifera. State of the Art, National Institute of Ocenography and Fishers. Red Sea Branch, p. 148
Madkour HA, Ali MY (2009) Heavy metals in the benthic foraminifera from the coastal lagoons, Red Sea, Egypt: indicators of anthropogenic impact on environment (case study). Environ Geol 58:543–553
Madkour HA, Abdelhalim MA, Obirikorang KA, Mohamed AW, Ahmed AN and El-Taher AM (2015) Environmental implications of surface sediments from coastal lagoons in the Red Sea coast. Journal of Environmental Biology, Vol. 36, 1421–1427, November 2015
Mansour AM, Nawar AH, Mohamed AM (1997) Recent intertidal sediments and negative impact of human activities, Red Sea coast, Egypt. Egyptian Jour of Geo, 41/2A:239–272
Mansour, A. M., Nawar, A. H., and Madkour, H. A., 2005. Metals concentration of recent invertebrates along the Red Sea Coast of Egypt: a tool for monitoring environmental hazards. Sedimentology of Egypt, vol. 13. p. 171–185
Mekawy MS, Madkour HA (2012) Studies on the Indo-Pacific Tridacnidae (Tridacna maxim) from the northern Red Sea, Egypt. Int J Geosci 3:1089–1095
Mekawy, M. S., and Madkour, H. A., 2013. Oxygen and carbon stable isotope analysis on some Egyptian Tridacna maxima as an environmental and climatic indicator. Egypt Jour Paleontol, 13: 173–183. ISSN 1687–4986
Milliman JD (1974) Marine carbonates. Springer, Berlin Heidelberg New York 375 p
Mohamed AW and El-Sorogy AS (2003) Scleractinian corals as pollution indicators, Red Sea coast, Egypt. N. Jb. Geol. Paläont. Mh., XXX-XXX; Stuttgart, 1–15
Mohamed AM, Madkour HA, El-Taher A (2013) Recent benthic foraminifera in the saline pool and its surrounding areas at ras Shukier and Gulf of Suez, Egypt. Indian J Geo-Marine Sci 42(3):293–299
Nicols, D., 1967. Some characteristics of cold water marine Pelecypods. Jour. Pal., 41/6: 1330–1340
Ouda K and Obaidalla N (1998) Ecology and distribution of recent subtidal foraminifera along the Egyptian Red Sea shore between Mersa Alam and Ras Banas. Revista Espanola De Micropaleontologia, Espana, 30(3): 11–34
Piller WE and Pervesler P (1989) An actuoupalaeontological approach, the Northern Bay of Safaga, (Red Sea, Egypt). Beitr.Paläont. Österr.15: 103–147, Wien
Ramadan SE and Shata A (1993) Biogeochemical studies on the mollusk bivalve Anadara diluvii (Lamarck, 1805) (Pteriomorpha Arcidae). Bull. Nat. Inst. Ocn. & Fish., A.R.E. (19): 145–157
Rinkevich B and Loya Y (1977) Harmful effects of chronic oil pollution on a Red Sea scleractinian coral population. Proc. 3rd Int. Coral Reef Symp., 585–591
Rinkevich B, Loya Y (1979) Laboratory experiments on the effects of crude oil on the Red Sea coral Stylophra pistillata. Mar Pollut Bull 10:328–330
Saraswat R, Sujata RK, Mazumder A, Nigam R (2004) Foraminifera as indicators of marine pollution: a culture experiment with Rosalina leei. Mar Pollut Bull 48:91–96
Schelske CL, Conley DJ, Warwick WF (1985) Historical relationships between phosphorus loading and biogenic silica accumulation in bay of Quinte sediments. Can J Fish Aquat Sci 42:1401–1409
Shata A, Hassan A (2000) Geochemical and environmental studies on some bivalves inhabiting the intertidal zone surrounding the Eastern Harbour of Alexandria. Egypt J Aquat Biol & Fish 4(/3):147–171
Swan EF (1952) The growth of the clam Mya arenaria as affected by the substratum. Ecology 33:530–834
Thomposn G, Livingston HD (1970) Strontium and uranium concentrations in aragonite precipitated by some modern corals. Earth and Planet Sci Letters 8:439–442
Wainwright SA (1969) Stress and design in bivalve mollusc shell. Nature 244:777–779
Wye KR (1989) The mitchell beaxley pocket guide to shells of the world, london, 192
Yanko V, Kronfeld J, Flexer A (1994) Response of benthic foraminifera to various pollution sources: implications for pollution monitoring. J Foraminifer Res 24(1):1e17
Yanko V, Ahmad M, Kaminski M (1998) Morphological deformities of benthic foraminiferal test in response to pollution by heavy metals: implications for pollution monitoring. J Foraminifer Res 28:177e200
Yumun ZU (2017) The effect of heavy metal pollution on foraminifera in the Western Marmara sea (Turkey). J Afr Earth Sci Elsevier 129(2017):346–365
Ziko A, El-Sorogy AS, Aly MM, Nour HE (2001) Sea shells as pollution indicators Red Sea coast. Egypt Egypt Jour Paleontol 1:97–113
Acknowledgments
The authors, therefore, acknowledge with thanks the DSR technical and financial support.
Funding
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (22/363/1434).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alharbi, W.R., El-Taher, A. Environmental geochemistry and mineralogy of molluscan shells as related to their ecology. Arab J Geosci 10, 533 (2017). https://doi.org/10.1007/s12517-017-3273-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-3273-9