Abstract
Drastic seasonal changes in higher latitudes and altitudes impact the phenology of plant growth forms differently and thus diet of ungulates feeding on them. We examined how fecal nitrogen (N), an indicator of diet quality, varied with season against the background variation in forage biomass and N in habitats of two sympatric ungulates in subtropical Himalaya. We conducted this study in Kyongnosla Alpine Sanctuary, Sikkim, where the Himalayan goral Naemorhedus goral occurred from 3000 to 3600 m and Himalayan musk deer Moschus chrysogaster from 3300 to 4200 m. We measured biomass and N content of forbs and graminoids and browse in their habitats and proportions of monocots and dicots and N content in their fecal pellets. Seasonal variation in biomass, primarily determined by forbs, was similar in goral and musk deer habitats. Goral had a graminoid-dominated diet switching to dicots in autumn and winter. Musk deer had a dicot-dominated diet in all seasons. Fecal N in both the ungulates was higher than forage N in all seasons except spring when the latter was greater. Forage and fecal N declined sharply from spring as seasons progressed. Fecal N in goral was considerably lower than in musk deer in all seasons, probably below maintenance levels in autumn and winter. As evident from peaks and duration of high diet quality, goral is likely a capital breeder and musk deer an income breeder. Results suggest that linkages between diet and reproductive seasonality in ungulates will have important implications in face of climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ungulates living in higher latitudes and altitudes face drastic seasonal changes in the quality and biomass of forage, with peak quality in spring and peak biomass in summer (Post 2003; English et al. 2012; Stoner et al. 2016). They need to accumulate enough reserves during the growing season in order to carry through costly gestation starting from late autumn or early winter, when both forage biomass and quality are the lowest (Barboza and Parker 2008; McArt et al. 2009; Parker et al. 2009; Thompson and Barboza 2017). Some ungulates track the “green wave” due to altitudinal and latitudinal differences in the onset of plant growth following winter through migration, thus effectively prolonging the short growing season (Bischof et al. 2012; Searle et al. 2015; Merkle et al. 2016; Middleton et al. 2018). Most ungulates in the boreal and arctic ecosystems switch their diets from growing season in spring and summer to lean season in winter. Wood bison Bison bison switch from a mixed diet to lichens (Larter and Gates 1991); woodland caribou Rangifer tarandus from graminoids to lichens (Thompson et al. 2015); mountain goat Oreamnos americanus from sedges and forbs to conifers, shrubs, and lichens (Fox and Smith 1988); and mule deer Odocoileus hemionus from forbs to shrubs and wapiti Cervus canadensis roosevelti from shrubs to tree foliage (Hosten et al. 2007). Such shifts between growth forms, therefore, seem crucial for these ungulates to optimize a combination of forage biomass and quality.
Such dietary switches are followed by a drastic dip in dietary nitrogen (N) in winter due to low N content and digestibility of the forage and high snow cover, which further restricts access to forage (Klein 1990; Barboza et al. 2018). N is the most limiting nutrient for terrestrial vegetation (Vitousek and Howarth 1991; Elser et al. 2007) as well as the wild ungulates consuming it (Robbins 1993; White 1993). Five to 9% of crude protein (CP) content (N × 6.25) is needed to maintain minimum protein balance in wild adult ruminants (Robbins 1993). Ungulates reported to face such near or below maintenance levels of protein during winter include red deer Cervus elaphus (Maloiy et al. 1970), white-tailed deer Odocoileus virginianus (Asleson et al. 1996), and wapiti (McCullough 1969) in north-temperate regions and bharal Pseudois nayaur in subtropical mountains (Suryawanshi et al. 2010). Moreover, an increase in biomass in later phenological stages results in increased structural tissues indicated by carbon (C) content in plants. The CN ratio, therefore, gives a combined estimate of the overall plant quality. An increase in CN ratio resulting from the dilution of leaf N with increased amounts of structural tissues (Walsh et al. 1997), therefore, indicates a reduction in plant nutritional quality (Leingartner et al. 2014). Fecal N has a relatively consistent relationship with dietary N except on occasions when consumption of woody plant species containing secondary metabolites might inflate fecal N concentrations (Robbins 1993; Palo & Robbins 1991; Hola et al. 2016).
In higher latitudes and altitudes, plant growth forms are one of the major determinants of vegetative phenology and thus of the forage biomass and quality available to the ungulates. This is primarily due to their differential responses to seasonal changes in environmental conditions (Iversen et al. 2009). For instance, shrubs and other evergreens, with slow nutrient uptake as well as release, have slow and steady phenological changes. In contrast, grasses and forbs, which have relatively fast nutrient uptake in early spring, show fast phenological changes. Thus, plant growth form is a good predictor of seasonal changes in nutritive value and palatability of forage plants to ungulates (Iversen et al. 2014) depending upon their feeding habits (Post and Stenseth 1999). Such differences among plant growth forms in phenology and nutritive value can affect seasonal changes in diet quality of sympatric ungulates differently, depending upon their primary feeding adaptations. For example, in the same habitat, a grazer can be expected to face more drastic seasonal changes in forage quality and thus in diet quality, compared to a browser. This can also influence the scheduling of reproduction by grazers and browsers (Owen-Smith and Ogutu 2013) and the degree of reproductive synchrony (Srivastava et al. 2021). However, the relationships between seasonal variations in the nutritive value of different plant growth forms and diet quality of sympatric ungulates have been explored very little.
We examined how diet composition and quality of two sympatric ungulates with different feeding habits varied with season, against the background variation in biomass and quality of different growth forms (graminoids, forbs, and browse) in their habitats. The two species (the Himalayan goral, Naemorhedus goral, and the Himalayan musk deer, Moschus chrysogaster) are sympatric in several areas in Himalaya, although the former is a relatively low altitude species and the latter, a higher altitude species (Srivastava and Kumar 2018). We specifically asked how do the biomass and quality (measured as N, C, and CN ratio) of different growth forms varied with season in the altitudinal ranges of the two species. We also asked how do the seasonal switches between growth forms affect diet quality of the two ungulate species having different feeding habits. We expected both forage biomass and quality to decline drastically from spring to winter with different magnitudes for different growth forms. Moreover, goral having a graminoid-dominated diet (Mishra and Johnsingh 1996; Ilyas and Khan 2003), and musk deer being predominantly a concentrate feeder or browser (Green 1987; Syed and Ilyas 2015), we expected greater seasonal changes in diet quality in goral.
Materials and methods
Study area
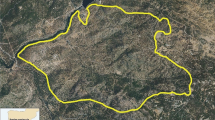
We conducted this study in Kyongnosla Alpine Sanctuary (31 km2) and the adjoining forests in the state of Sikkim (27°N 88°E) (Fig. 1a) in an altitudinal range from 3000 to 4200 m. We found musk deer mostly above 3300 m, goral below 3600 m, while serow Capricornis sumatraensis thar, the largest and rarest among the three species, probably migrated to lower altitudes in winter (Srivastava and Kumar 2018). During this study, from April 2013 to March 2014, the maximum temperature was 18.50 ± 0.70 °C in July below 3600 m and minimum temperature was − 8.27 ± 0.52 °C in January above 3600 m. Areas < 3600 m had snowfall from January to April, with 80% snow cover in January, while areas > 3600 m remained snow covered from November till May, with the snow cover between 50 and 100%. We grouped the months into four seasons: spring (April–June), summer (July–September), autumn (October–December), and winter (January–March).
Study area and sampling setup. a Location of Kyongnosla Alpine Sanctuary (KAS) in Sikkim. b Altitude zones and location of sampling trails in KAS. c Schematic of a sampling trail (600 m) with permanent plots (10 m × 10 m, P1 to P7), random subplots (1 m × 1 m) for fortnightly vegetation sampling, and subsub plots for collection of plant samples (0.5 m × 0.5 m) for aboveground biomass and CN ratio estimation
Areas < 3300 m had a tree cover of Betula utilis and Acer spp., while Abies densa and Sorbus microphylla formed the tree cover between 3300 and 3900 m along with several species of Rhododendron. The alpine zone (> 3900 m) had a much higher shrub cover with Rhododendron nivale, Rhododendron anthopogon, and Rhododendron aerogenosa.
Methods
We marked eight trails each of 600-m length at altitude intervals of 150 m from 3000 to 4200 m (Fig. 1b). These trails served as the basic sampling units for assessing forage biomass and quality as well as diet composition and quality.
Assessing forage biomass and C, N, and CN ratio
We marked permanent plots of 10 m × 10 m at every 100-m interval on alternate sides of the trails, resulting in 52 plots (Fig. 1c). We sampled ground vegetation (graminoids and forbs) from a randomly selected subplot of 1 m × 1 m within each of these permanent plots every fortnight between April and December 2013. From each subplot, we clipped aboveground plant biomass from one randomly selected 50 cm × 50 cm quadrat. We could not sample the ground vegetation in winter (January to March) as it was completely buried under deep snow especially in the higher altitude areas. However, at the end of our sampling in autumn (October–December), the ground vegetation had almost senesced to litter. Therefore, we assumed that the forage biomass and quality values in winter remained approximately the same as that in autumn, though the ground vegetation would have been unavailable to the ungulates due to snow cover.
We sampled tree browse biomass up to a height of 1.6 m from 10 m × 10 m permanent plots and shrub biomass from 5 m × 5 m plots within the same, once in every season. We sorted all the harvested samples into species and stored them in separate labeled paper envelopes. We weighed these samples after drying in a hot air oven at 60 °C for 48 h. The samples were ground in a Willey mill at 0.20-mm mesh size to measure total N and C using a CN analyzer (Leco Instruments, St Joseph, MI, USA).
Assessing diet composition and fecal C, N, and CN ratio
We used fecal samples to assess diet composition and diet quality with fecal C, N, and CN ratio as indicators of quality. Once every fortnight we collected fresh pellet samples (10–12 pellets from a pellet group) of the study species along each trail. We oven-dried these samples at 60 °C for 48 h and stored for further laboratory analysis.
For assessing diet composition, we used microhistological analysis following Morrison (2008). We examined 12 samples/season for musk deer (total 48 samples) and 13 samples/season for goral except in summer (12) (total 51 samples). We prepared three slides of plant fragments from each fecal sample, which we viewed under an Olympus MVX10 Stereo microscope at 250 × magnification. We used diagnostic epidermal features from the reference slides made from common plants for identification of the fragments in the pellet slides into those of dicots (forb and browse) and monocots (grass and sedge). Identification of fragments up to species was found not feasible. In the pellet slides, we selected 10 non-overlapping fragments on a randomly selected transect line on each of the three slides (Sparks and Malechek 1968), resulting in 30 fragments for each pellet sample. For measuring diet quality, we ground these fecal samples in a Willey mill at 0.20-mm mesh size and measured total N and C using a CN analyzer (Leco Instruments, St Joseph, MI, USA).
Data analysis
We averaged biomass, C, N, and CN ratio for different growth forms across all plots lay within each fortnight sampling session, which was then averaged across all sessions within each season as described above. The averaging was restricted to the altitudinal range in which the species was found—3000–3600 m for goral and 3300–4200 m for musk deer. Percentage of fragments of different growth forms and the C, N, and CN ratio values for fecal pellets were also averaged across all pellet groups for each season. We used one-way and two-way ANOVA for comparisons among seasons and growth forms, followed by Tukey’s test for pair-wise comparisons only if the differences were significant at p < 0.05. All the analyses were done with statistical software R 3.0.1 (R Development Core Team 2013).
Results
Our results showed that the C content in forage growth forms as well as diet of both the ungulates did not vary much with seasons (Fig. 3). Therefore, the seasonal variation in CN ratio was mostly a function of N content and we used the same to describe the variations in forage and diet quality of the ungulates in the following results.
Forage biomass and N content
We sampled biomass in each of the 52 plots a total of 16 times for ground vegetation and 3 times for browse in a year. We obtained 259 species in three growth forms, forbs being the most species-rich (194) followed by graminoids (37) and browse (28). The seasonal variations in biomass of different growth forms were similar in the habitats of goral and musk deer (Fig. 2a, b). Forbs formed > 70% of the biomass in summer, with a drastic increase from spring to summer and a decline in autumn. However, the seasonal changes in forbs biomass were sharper in the habitat of musk deer, with a greater biomass in summer than that in the goral habitat. Biomass of graminoids also increased from spring to summer but did not show a decrease through autumn. Browse biomass did not show much variation among seasons in both habitats.
A drastic reduction in the N content of all the growth forms was evident from spring to autumn in both goral and musk deer habitats (Fig. 3a, b). In goral’s habitat, the N fell by 38.6% in forbs, 42.8% in graminoids, and 36.5% in browse, with a similar decline between spring and summer, and summer and autumn. In the habitat of musk deer in the higher altitude, the fall in N content was 50.7% in forbs, 48.2% in graminoids, and 35.3% in browse.
Diet composition and fecal N
For estimating diet composition, we examined 1440 and 1530 plant fragments from 48 musk deer and 51 goral pellets, respectively. We used 82 musk deer and 78 goral pellets for estimating fecal C, N, and CN ratio. Monocots dominated the diet of goral in spring, summer, and autumn, with dicots also forming an almost equal part of the diet in winter (Fig. 4a). Musk deer, on the other hand, had a dicot-dominated diet with forbs and browse contributing to > 70% of its diet in all seasons (Fig. 4b). Only in summer, monocots also formed a large percentage of its diet. A two-way ANOVA showed that fecal N was significantly different between the two ungulate species (goral having lower fecal N than musk deer; F = 87.42, p < 0.001) and among seasons (F = 37.91, p < 0.001; fecal N in spring and summer being significantly different from that of autumn and winter, Tukey’s test, p < 0.002), with the interaction between species and seasons not being significant (two-way ANOVA, F = 1.40, p = 0.246) (Fig. 3a, b).
Comparison of forage and diet N
In spring, fecal N in goral was lower than the N content of all growth forms except browse in its habitat (one-way ANOVA, F = 16.06, p < 0.001 followed by Tukey’s test, p = 0.005), and in summer, it was higher than N content of graminoids and browse (one-way ANOVA, F = 26.41, p < 0.001 followed by Tukey’s test, p < 0.002). However, in autumn, fecal N was significantly higher than the N content of all the growth forms (one-way ANOVA, F = 56.72, p < 0.001; Tukey’s test, p < 0.002 for all growth forms) and in winter higher from all except forbs (one-way ANOVA, F = 50.86, p < 0.001; Tukey’s test, p < 0.002) (Fig. 3c). During spring, the fecal N in musk deer was significantly higher only than the N content of browse in its habitat (one-way ANOVA, F = 23.43, p < 0.001; Tukey’s test, p < 0.001). In all other seasons, the N content in its feces was higher than that of all the growth forms in its habitat (one way ANOVA, summer-F = 176.7, p < 0.001; autumn—F = 61.06, p < 0.001; winter—F = 108.9, p < 0.001; Tukey’s test, p < 0.001, for pairwise differences between fecal N and N content of each growth form in all seasons) (Fig. 3b).
Discussion
The seasonal variations in biomass and quality were clearly different among the growth forms in the habitats of the goral and musk deer. Only forbs showed a sharp increase in biomass from spring to summer, forming as much as 70% of the biomass of all growth forms, and an equally dramatic decrease from summer to autumn. Graminoids increased in biomass from spring to summer but did not show a decline in autumn. Browse biomass did not show any seasonal variation. This phenological pattern in biomass from browse being the slowest in phenological changes, followed by graminoids and forbs being the fastest, is similar to that in high altitude alpine ranges where growth form was the best predictor of phenology (Iversen et al. 2009). In contrast to biomass peaking in summer, nutritive value as indicated by N content, declined sharply from spring to autumn in all growth forms. It is very likely that this decline would have continued into winter in all growth forms in lower as well as in higher altitude where the ground vegetation (forbs, graminoids, and ferns) had completely dried up in autumn followed by heavy snow cover, due to which we did not have forage samples in winter. Forbs had the highest N in all three seasons followed by graminoids and browse. Among these three growth forms, seasonal decline was the highest in graminoids and least in browse. Among the dicots, forbs had a much higher N than browse, which included woody shrubs and low-hanging branches of trees. In general, dicots have higher N than monocots owing to the latter’s higher fiber and cellulose content (Holechek 1984). Moreover, as they attain maturity much earlier in the growing season, their nutritive value declines faster than other growth forms (Iversen et al. 2014). The decline in forage N was sharper in all growth forms in musk deer habitat, as expected from higher altitudes (Kudo 1991).
Seasonal variation in the composition of goral diet indicates that it is a mixed feeder, as Van Soest (1982) had classified. Although graminoids formed a high proportion of goral’s diet in all seasons, the proportions of dicots in autumn and winter were substantial (up to 49.67%). This was despite no reduction in grass biomass from summer to autumn and a drastic reduction in the biomass of forbs. However, grass N decreased from about 17.5% to nearly 10%, probably close to maintenance levels of 9% (Robbins 1993). Graminoids show much faster phenological changes than dicots (Iversen et al. 2009), which in this case included a decline in N and perhaps an increase in lignification also indicated by C (though not much here), which decreases their digestibility (Hofmann 1989). Thus, at the altitudes that we studied, goral remains a grazer only in spring and summer when N as well as digestibility in graminoids is high. Such dietary shifts from one growth form to another are characteristic of mixed feeders in response to changes in forage biomass or quality or due to competition with livestock (Mishra et al. 2004; Morgia and Bassano 2009; Suryawanshi et al. 2010). Hofmann (1989) attributed the diet switches by mixed feeders to lignification in forage which they cannot digest. The Himalayan goral is a non-migrant within most of its distributional range (Mishra and Johnsingh 1996; Srivastava and Kumar 2018), and such a flexible diet involving switches between growth forms clearly highlights the significance of varied seasonal abundance and quality of different growth forms present in its habitats, thus enabling its survival through the harsh winter by feeding on browse while enabling the exploitation of abundant high-quality grass in spring and summer.
Diet of musk deer was composed mostly of dicots which could have included both forbs and browse in all seasons. Forbs had the highest biomass as well as quality in all seasons. Therefore, the dicot-dominated diet of musk deer indicates its preference for high-quality forage as reported previously (Green 1987). Moreover, the inclusion of graminoids in its diet during summer when its quality was comparable to forbs further shows its preference for high-quality forage. We did not find evidence for a major switch from dicot to monocot in response to seasonal fluctuations in forage biomass and quality. However, there might have been a switch from forbs to browse during winter as reported previously from other areas (Bhattacharya et al. 2012) due to heavy snow cover during autumn and winter.
Both the ungulates in our study had fecal N higher than the forage N in almost all seasons except that in spring, thus showing selectivity for high-quality forage. In spite of a 50% decline in the N content of forbs from spring to autumn, the decline in fecal N of musk deer was only 29%. In goral, the diet quality peaked in summer and coincided with the peak in biomass and an intermediate quality of forage in its habitat. The decline in fecal N was far less than that in forage N.
One factor which can potentially obfuscate the intra- and inter-specific comparison of diet quality based on fecal N is the interference of tannin in protein digestion and excretion (see Leslie et al. 2008 for a review). High levels of tannin, which are more common in browse than in forbs and graminoids (Oehler et al. 2003), can elevate fecal N levels by reducing protein availability by as much as 46% in the moose Alces alces (McArt et al. 2009). In case of goral, this could have happened in autumn and winter when their diet contained more dicots, probably browse. This could only mean that the diet quality in goral during these two seasons was even lower than that indicated by fecal N. In case of musk deer, it is most likely that its diet consisted of more browse in autumn and winter when biomass of forbs had drastically declined and access to them was very limited due to high snow cover. It is unlikely that higher consumption of browse would have elevated fecal N since browsers typically avoid plants with very high tannin content (Cooper and Owen-Smith 1985), besides having adaptations to minimize the effect of ingested tannins. In fact, experimental studies with formulations similar to natural diets (Hodgman et al. 1996), and of free ranging ungulates (Oehler et al. 2003), have not found evidence that tannin has influence on fecal N. Therefore, the seasonal variation in fecal N in musk deer is unlikely to have been influenced by variation in tannin content in its forage. There is thus a direct correlation between forage N and fecal N in both the species, as reported in other ungulates (e.g., Leslie and Starkey1985; Kamler and Homolka 2005; Ueno et al. 2007), and between fecal N and diet quality. The differences between goral and musk deer in fecal N also reflect differences in their diet quality, the former having a comparatively poorer diet than the latter.
The primary criteria for selection of forage by ungulates may be high protein and low fiber content (Mysterud et al. 2001). A simple indicator of high preference for a forage type could be its abundance. For instance, selection of plant groups in red deer in alpine habitats was associated with high crude protein and low fiber and was negatively correlated with abundance (Zweifel-Schielly et al. 2012). However, this does not seem to be the case in the habitats of goral and musk deer, since we did not find any difference between the N content of forbs that formed 75% of the biomass and those that formed 25% (unpublished data). It is likely that N content in forage depends on the plant parts or their phenological stage (Klein 1990). A higher fecal N than the available forage N does not mean that N is the only criterion for selection of forage, and that the forage with the highest N is always selected. Nonetheless, our study clearly shows that forage selection in both the ungulates leads to a higher N in the diet compared to available background N.
Conclusion
The seasonal variations in diet quality have important implications for the scheduling of different reproductive stages consisting of estrous, gestation, parturition, and weaning in ungulates (Parker et al. 2009). Quality of diet in goral, primarily a grazer, peaked in spring and summer prior to sharply declining in autumn and winter, perhaps below the maintenance level as reported in reindeer (Barboza and Parker 2008). Therefore, it has to schedule the nutrient demanding late gestation and lactation during spring and summer and also build up enough body reserves to survive the next winter and carry on early gestation. Diet quality during spring–summer and the duration of the growing season are major determinants of female body mass, fecundity, and juvenile survival in many mountain ungulates (Cote and Festa-Bianchet 2001; Corlatti et al. 2018; Douhard et al. 2018; Lovari et al. 2020). Therefore, goral is likely to be a capital breeder (Jönsson 1997), showing high reproductive synchrony. In contrast, diet quality in musk deer remained relatively high even in autumn and winter, despite being in a higher altitude, due to its diet consisting primarily of dicot. Therefore, musk deer is likely to be an income breeder, with low synchrony in estrous cycles and parturition. Our data on estrous cycles, gestation, and parturition, based on fecal hormonal metabolites, support this conclusion (Srivastava et al. 2021). The linkage between diet and the above two breeding strategies has important implications when both resource availability and seasonality change, for example, due to climate change. For example, the reproductive performance of caribou (Rangifer tarandus), an income breeder, was more immediately affected by a trophic mismatch due to changes in plant phenology compared to muskoxen (Ovibos moschatus), a capital breeder, which was more affected by changes in plant phenology in the previous years (Kerby and Post 2013). Therefore, a better understanding of the linkages between diet and reproductive seasonality of ungulates in the Himalayan Mountains, the richest assemblage in the world with 20 species, is necessary to predict the distribution and abundance of their populations in the face of climate change (e.g., Lovari et al. 2020).
Availability of data and material
All data and materials support our published claims and comply with field standards.
References
Asleson MA, Hellgren EC, Varner LW (1996) Nitrogen requirements for antler growth and maintenance in white-tailed deer. J Wildl Manag 60:744–752. https://doi.org/10.2307/3802373
Barboza PS, Parker KL (2008) Allocating protein to reproduction in arctic reindeer and caribou. Physiol Biochem Zool 81:835–855. https://doi.org/10.1086/590414
Barboza PS, Van Someren LL, Gustine DD, Bret-Harte MS (2018) The nitrogen window for arctic herbivores: plant phenology and protein gain of migratory caribou (Rangifer tarandus). Ecosphere 9:1–17. https://doi.org/10.1002/ecs2.2073
Bhattacharya T, Kittur S, Sathyakumar S, Rawat GS (2012) Diet overlap between wild ungulates and domestic livestock in the Greater Himalaya: implications for management of grazing practices. Proc Zool Soc 65:11–21. https://doi.org/10.1007/s12595-012-0025-4
Bischof R, Loe LE, Meisingset EL, Zimmermann B, Van Moorter B, Mysterud A (2012) A migratory northern ungulate in the pursuit of spring: jumping or surfing the green wave? Am Nat 180:407–424. https://doi.org/10.1086/667590
Cooper SM, Owen-Smith N (1985) Condensed tannins deter feeding by browsing ruminants in a South African savannah. Oecologia 67:142–146
Corlatti L, Gugiatti A, Ferrari N, Formenti N, Trogu T, Pedrotti L (2018) The cooler the better? Indirect effect of spring–summer temperature on fecundity in a capital breeder. Ecosphere 9:e02326. https://doi.org/10.1002/ecs2.2326
Cote SD, Festa-Bianchet M (2001) Birthdate, mass and survival in mountain goat kids: effects of maternal characteristics and forage quality. Oecologia 127:230–238. https://doi.org/10.1007/s004420000584
Douhard M, Guillemette S, Festa-Bianchet M, Pelletier F (2018) Drivers and demographic consequences of seasonal mass changes in an alpine ungulate. Ecology 99:724–734. https://doi.org/10.1002/ecy.2141
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x
English AK, Chauvenet ALM, Safi K, Pettorelli N (2012) Reassessing the determinants of breeding synchrony in ungulates. PLoS One 7:0041444. https://doi.org/10.1371/journal.pone.0041444
Fox JL, Smith CA (1988) Winter mountain goat diets in Southeast Alaska. J Wildl Manag 52:362–365. https://doi.org/10.2307/3801249
Green MJB (1987) Diet composition and quality in Himalayan musk deer based on fecal analysis. J Wild Manag 51:880–892. https://doi.org/10.2307/3801755
Hodgman TP, Davitt BB, Nelson JR (1996) Monitoring mule deer diet quality and intake with fecal indices. J Range Manag 49:215–222
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view their digestive system. Oecologia 78:443–457
Hola M, Jezek M, Kusta T, Cerveny J (2016) Evaluation of winter food quality and its variability for red deer in forest environment: overwintering enclosures vs. free-ranging areas. Forestry Journal 62:139–145. https://doi.org/10.1515/forj-2016-0018
Holechek JL (1984) Comparative contribution of grasses, forbs and shrubs to the nutrition of range ungulates. Rangeland 6:261–263
Hosten PE, Whitridge H, Broyles M (2007) Diet overlap and social interactions among cattle, horses, deer and elk in the Cascade-Siskiyou National Monument, southwest Oregon. U.S. Department of the Interior, Bureau of land management, Medford district. http://soda.sou.edu/bioregion.html
Ilyas O, Khan JA (2003) Food habits of barking deer (Muntiacus muntjak) and goral (Naemorhedus goral) in Binsar Wildlife Sanctuary, India. Mammalia 67:521–532. https://doi.org/10.1515/mamm-2003-0406
Iversen M, Brathen KA, Yoccoz NG, Ims RA (2009) Predictors of plant phenology in a diverse high-latitude alpine landscape: growth forms and topography. J Veg Sci 20:903–915. https://doi.org/10.1111/j.1654-1103.2009.01088.x
Iversen M, Fauchald P, Langeland K, Ims RA, Yoccoz NG, Brathen KA (2014) Phenology and cover of plant growth forms predict herbivore habitat selection in a high latitude ecosystem. PLoS One 9:1–11. https://doi.org/10.1371/journal.pone.0100780
Jönsson KI (1997) Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78:57–66. https://doi.org/10.2307/3545800
Kamler J, Homolka M (2005) Faecal nitrogen: a potential indicator of red and roe deer diet quality in forest habitats. Folia Zool 54:89–98
Kerby J, Post E (2013) Reproductive phenology of large mammals. Pp 467–479 in Phenology: an integrative environmental science (M. D. Schwartz ed)., Second Edition. Springer, Dordrecht, Netherlands.
Klein DR (1990) Variation in quality of caribou and reindeer forage plants associated with season, plant part, and phenology. Rangifer 3:123–130. https://doi.org/10.7557/2.10.3.841
Kudo G (1991) Effects of snow-free period on the phenology of alpine plants inhabiting snow patches. Arct Alp Res 23:436–443. https://doi.org/10.1080/00040851.1991.12002863
Larter NC, Gates CC (1991) Diet and habitat selection of wood bison in relation to seasonal changes in forage quantity and quality. Can J Zool 69:2677–2685. https://doi.org/10.1139/z91-376
Leingartner A, Hoiss B, Krauss J, Steffan-Dewenter I (2014) Combined effects of extreme climatic events and elevation on nutritional quality and herbivory of alpine plants. PLoS One 9:e93881. https://doi.org/10.1371/journal.pone.0093881
Leslie DM, Starkey EE (1985) Fecal indices to dietary quality of cervids in old-growth forests. J Wildl Manage 49:142–146. https://doi.org/10.2307/3801860
Leslie DM Jr, Bowyer RT, Jenks JA (2008) Facts from feces: nitrogen still measures up as a nutritional index for mammalian herbivores. J Wildl Manage 72:1420–1433. https://doi.org/10.2193/2007-404
Lovari S, Franceschi S, Chiatante G, Fattorini L, Ferretti F (2020) Climatic changes and the fate of mountain herbivores. Clim Change 162:2319–2337. https://doi.org/10.1007/s10584-020-02801-7
Maloiy GM, Kay RN, Goodall ED, Topps JH (1970) Digestion and nitrogen metabolism in sheep and red deer given large or small amounts of water and protein. Br J Nutr 24:843–855. https://doi.org/10.1079/bjn19700087
McArt SH, Spalinger DE, Collins WB, Schoen ER, Stevenson T, Bucho M (2009) Summer dietary nitrogen availability as a potential bottom-up constraint on moose in south-central Alaska. Ecology 90:1400–1411. https://doi.org/10.1890/08-1435.1
McCullough DR (1969) The Tule elk: its history, behavior and ecology. University of California Publications in Zoology 88:1–209
Merkle JA, Monteith KA, Eikens EO, Hayes M, Hersey KR, Middleton AD, Oates BA, Sawyer H, Scurlock BM, Kauffman M (2016) Large herbivores surf waves of green-up in spring. Proc of Royal Soc B: Biological Science 233:20160456. https://doi.org/10.1098/rspb.2016.0456
Middleton AD, Merkle JA, McWhirter DE, Cook JG, Cook RC, White PJ, Kauffman MJ (2018) Green-wave surfing increases fat gain in a migratory ungulate. Oikos 00:1–9. https://doi.org/10.1111/oik.05227
Mishra C, Johnsingh AJT (1996) On habitat selection by the goral Naemorhedus goral bedfordi (Bovidae, Artiodactyla). J Zool (london) 240:573–580. https://doi.org/10.1111/j.1469-7998.1996.tb05307.x
Mishra C, Van Wieren E, Ketner P, Heitkonig IMA, Prins HHT (2004) Competition between domestic livestock and wild bharal Pseudois nayaur in the Indian Trans-Himalaya. J Appl Ecol 41:344–354. https://doi.org/10.1111/j.0021-8901.2004.00885.x
Morgia VL, Bassano B (2009) Feeding habits, forage selection and diet overlap in Alpine chamois (Rupricapra rupricapra L.) and domestic sheep. Ecol Res 24:1043–1050. https://doi.org/10.1007/s11284-008-0581-2
Morrison JI (2008) Using microhistological techniques to predict botanical composition of horse diets on cool-season grass pasture. Master of Science, thesis, University of Kentucky, USA.
Mysterud A, Langvatn R, Yoccoz NG, Stenseth NC (2001) Plant phenology, migration and geographical variation in body weight of a large herbivore: the effect of a variable topography. J Anim Ecol 70:915–923. www.jstor.org/stable/2693495
Oehler M, Bowyer RT, Bleich VC (2003) Home ranges of female mountain sheep, Ovis canadensis nelson: effects of precipitation in a dessert ecosystem. Mammalia 67:385–402. https://doi.org/10.1515/mamm.2003.67.3.385
Owen-Smith N, Ogutu JO (2013) Controls over reproductive phenology among ungulates: allometry and tropical-temperate contrasts. Ecography 36:256–263. https://doi.org/10.1111/j.1600-0587.2012.00156.x
Palo RT, Robbins CT (eds) (1991) Plant defenses against mammalian herbivory. CRC Press. Inc., Boca Raton, Florida
Parker KL, Barboza PS, Gillingham MP (2009) Nutrition integrates environmental responses of ungulates. Nutritional Ecology 23:57–69. https://doi.org/10.1111/j.1365-2435.2009.01528.x
Post E (2003) Timing of reproduction in large mammals. In: Schwartz MD, eds. Phenology: an integrative environmental science. Tasks for Vegetation Science, vol 39. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0632-3_27
Post E, Stenseth NC (1999) Climatic variability, plant phenology, and northern ungulates. Ecology 80:1322–1339. https://doi.org/10.1890/0012-9658(1999)080[1322:CVPPAN]2.0.CO;2
R Development Core Team (2013) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Robbins CT (1993) Wildlife feeding and nutrition. Academic Press, New York
Searle KR, Rice MB, Anderson CR, Bishop C, Hobbs NT (2015) Asynchronous vegetation phenology enhances winter body condition of a large mobile herbivore. Oecologia 179:377–391. https://doi.org/10.1007/s00442-015-3348-9
Sparks DR, Malechek JC (1968) Estimating percentage dry weight in diets using a microscopic technique. J Range Manag 21:264–265
Srivastava T, Kumar A (2018) Seasonal habitat use in three species of wild ungulates in Sikkim Himalaya. Mamm Bio 88:100–106. https://doi.org/10.1016/j.mambio.2017.11.013
Srivastava T, Kumar A, Kumar V, Umapathy G (2021) Diet drives differences in reproductive synchrony in two sympatric mountain ungulates in the Himalaya. Front Ecol Evol 9:647465. https://doi.org/10.3389/fevo.2021.647465
Stoner DC, Sexton JO, Nagol J, Bernales HH, Edwards TC Jr (2016) Ungulate reproductive parameters track satellite observations of plant phenology across latitude and climatological regimes. PLoS One 112:e0148780. https://doi.org/10.1371/journal.pone.0148780
Suryawanshi KR, Bhatnagar YV, Mishra C (2010) Why should a grazer browse? Livestock impact on winter resource use by bharal Pseudois nayaur. Oecologia 162:453–462
Syed, Z, Ilyas O (2015) Habitat and feeding ecology of alpine musk deer (Moschus chrysogaster) in Kedarnath Wildlife Sanctuary, Uttarakhand, India. Anim Prod Sci. https://doi.org/10.1071/AN141028.
Thompson DP, Barboza PS (2017) Seasonal energy and protein requirements for Siberian reindeer (Rangifer tarandus). J Mammal 98:1558–1567. https://doi.org/10.1093/jmammal/gyx132
Thompson ID, Wiebe PA, Mallon E, Rodgers AR, Fryxell JM, Baker JA, Reid D (2015) Factors influencing the seasonal diet selection by woodland caribou (Rangifer tarandus tarandus) in boreal forests in Ontario. Canad J Zool 93:87–98. https://doi.org/10.1139/cjz-2014-0140
Ueno M, Nishimura C, Takahashi H, Kaji K, Saitoh T (2007) Fecal nitrogen as an index of dietary nitrogen in two sika deer Cervus nippon populations. Acta Theriol 52:119–128
Van Soest PJ (1982) Nutritional ecology of the ruminant: ruminant metabolism, nutritional strategies, the cellulytic fermentation and the chemistry of forages and plant fibres. O & B Books, Corvallis, USA
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea – how can it occur? Biogeochemistry 13:87–115. https://doi.org/10.1007/BF00002772
Walsh NE, McCabe TR, Welker JM, Parsons AN (1997) Experimental manipulations of snow-depth: effects on nutrient content of caribou forage. Glob Change Biol 3:158–164. https://doi.org/10.1111/j.1365-2486.1997.gcb142.x
White TCR (1993) The inadequate environment. Springer-Verlag, Berlin, Nitrogen and the abundance of animals
Zweifel-Schielly B, Leuenberger Y, Kreuzer M, Suter W (2012) A herbivore’s food landscape: seasonal dynamics and nutritional implications of diet selection by a red deer population in contrasting alpine habitats. J Zool 1:68–80. https://doi.org/10.1111/j.1469-7998.2011.00853.x
Acknowledgements
We thank the Department of Forests, Environment, and Wildlife Management, Government of Sikkim, India, for providing the research permits; Kalu Singh Rai, Sonam, and Biray Tamang for assistance infield data collection; Dr. G. S. Rawat for identification of most of the plants; Dr. Mahesh Sankaran for providing laboratory facilities; and Dr. H. Krishnamurthy for microscope facility. TS would like to thank her colleague Sunita Khatiwara for company in and off the field for nearly 5 years.
Funding
This work was supported by the Department of Biotechnology (DBT), Government of India (Ref. BT/001/NE/PS/NCBS/09 to AK).
Author information
Authors and Affiliations
Contributions
TS and AK conceived the idea and designed the study, TS conducted the fieldwork and laboratory analysis, TS and AK performed data analyses and interpreted the results, and TS and AK wrote and edited the manuscript. Both the authors contributed equally to the final submitted draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Srivastava, T., Kumar, A. Seasonal forage and diet quality in two subtropical ungulates in the Himalaya. Eur J Wildl Res 67, 77 (2021). https://doi.org/10.1007/s10344-021-01518-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-021-01518-x