Abstract
Urban areas not only provide wildlife with new ecological niches, in terms of food availability, human protection and den sites but also they increase the possibility of conflict with man. Despite being a protected species in Italy, the crested porcupine is considered as an agricultural pest, with a tasty meat, thus widely poached. We studied food selection and other ecological factors shaping the ranging behaviour of crested porcupines in a suburban area, where a high poaching pressure could be expected. We monitored radio-tagged adult, paired crested porcupines throughout 1 year. Over 70% of individually marked porcupines were poached. Despite the local absence of predators, but in presence of poaching pressure, porcupines avoided clear moonlight nights and daylight activity, establishing dens in thorny thickets. Deciduous woodlands and shrubwood were positively selected for feeding throughout the year, while farmlands and fallows were underused. Although the crested porcupine has been confirmed as a “generalist” species in terms of food selection, with adaptations to dig underground storage organs, a strong preference for fruits and epigeal parts of plants was detected in our study. Porcupines evolved in Asia and Africa with a number of competing grazing herbivores, as well as in presence of a heavy predation risk leading to development of quills. This might have confined them to exploit roots and rhizomes as food, as well as scrub habitats for protection. Our results suggest that porcupines can revert to the use of optimal food resources, when local selective forces allow it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization has been widely reported as one of the main threats to biodiversity, representing the major determinant of habitat loss for wild species (Antrop 2004; Garden et al. 2006; McKinney 2006). In spite of habitat degradation, urban areas offer a range of habitat niches and may host a high species diversity (Niemelä 1999; Collins et al. 2000; Chace and Walsh 2004; Magle et al. 2012). Wild species adapt to live in these new conditions, expanding or modifying their natural ecological niches (Luniak 2004; Russo and Ancillotto 2014; Mori and Bertolino 2015). Native (Harris 1986; Beringer et al. 2002; DeStefano and DeGraaf 2003; Parker and Nilon 2008; Herr et al. 2009) and nonnative (Bertolino et al. 2004; Clergeau and Vergnes 2011; Walther et al. 2011) species may benefit from urbanization, in terms of food availability, human protection and shelter sites. Nevertheless, urbanization increases interactions between animals and humans, enhancing opportunities for conflict (Conover et al. 1995; Heimlich and Anderson 2001; DeStefano and DeGraaf 2003).
Attitude towards urban/suburban wildlife is mainly positive (Coluccy et al. 2001; König 2008; Menchetti and Mori 2014), although it may range from “wildlife as a resource” to “wildlife as pests” (Conover et al. 1995). Wildlife influences human activities through (1) transmission of zoonoses (König 2008; Mackenstedt et al. 2015); (2) predation on livestock, poultry and scavenging of urban waste (Doncaster et al. 1990; Prange et al. 2003; Abay et al. 2011); (3) attacks to humans (Timm et al. 2004; Abay et al. 2011; Bateman and Fleming 2012); (4) religion and popular beliefs (Daigle et al. 2002) and (5) crop damages (Connelly et al. 1987; Conover et al. 1995). Human-induced stress may also alter animal behaviour in urban ecosystems (Ditchkoff et al. 2006). As a consequence, the urbanization of wildlife may pose a challenge to resource managers, emphasizing the importance to analyse the behaviour of problematic species in urban environments.
The crested porcupine Hystrix cristata is a large monogamous rodent of African origin, probably introduced to Italy in the early medieval times (Bertolino et al. 2015). The members of a pair share the same home range and show overlapping activity rhythms, but, during reproductive periods, partners alternate in the den with newborns (Mori et al. 2016). This species recently underwent a substantial range expansion in Italy, reaching areas where it was historically absent (Mori et al. 2013). The crested porcupine is listed in the Berne Convention (All. II) and in the Habitat Directive (All. IV). It has been protected by the Italian law since 1977 (National Law 968/1977) and listed among the “especially protected species” in the National Law 157/1992. Therefore, killing porcupines in Europe is severely banned. Despite this level of legal protection, the species is still extensively poached (Amori et al. 2008; our own data, for Southern Tuscany, 23.07%, N = 60 radio-tagged porcupines). Porcupines are sensitive to disturbance, e.g. in Western Terai (Nepal), this rodent tended to be active when its main predator, the common leopard Panthera pardus, was not (Fattorini and Pokheral 2012). It is unknown whether poaching influences ranging behaviour of porcupines in areas free of natural predators.
Throughout the world, porcupines are considered as agricultural pests (Hystrix indica: Alkon and Saltz 1985a; Khan et al. 2000; Hystrix africaeaustralis: Gaigher and Currie 1979; Hystrix brachyura: Chuan 1969; Greaves and Khan 1978; Linkie et al. 2007). As to the crested porcupine, crop damages lamented by farmers are actually negligible in Central Italy (Laurenzi et al. 2016). Major damage seems to occur in private vegetable gardens in the surroundings of human settlements (Laurenzi et al. 2016), thus increasing the conflict with humans. Intolerance against this rodent is locally increased because of wounds provoked to hounds during hunting trips targeting other den-living species, e.g. the red fox Vulpes vulpes (Mori et al. 2014a), as well as because of damage to riverbanks through burrow digging.
So far, the only published study on the diet of the crested porcupine throughout the year has been carried out in a rural area, mainly composed by fallow and woodland (Bruno and Riccardi 1995). According to Bruno and Riccardi (1995) and Mohamed (2011), porcupines appear to be generalist rodents who feed on a variety of plant parts in relation to seasonal availability.
Porcupines have also been observed in suburban and urban areas (Ramat Aviv, Israel: Sever and Mendelssohn 1989; Siena, Italy: Lovari et al. 2013; Rome, Italy: Petrozzi et al. 2012; Grosseto, Italy: E. Mori, personal observation), but no data are available on their ecology, except for their ranging behaviour (Lovari et al. 2013) and activity rhythms (Corsini et al. 1995). In this work, we have aimed at a holistic approach (i.e. habitat selection, activity rhythms, food habits and selection) to the study of some ecological factors shaping up the ranging behaviour of crested porcupines in a suburban area, where the probability of encroaching human activities is the highest.
We predicted that (1) if crested porcupines are generalist herbivores, food selection would reflect local environmental availability of each food resource, including cultivated vegetables; (2) ranging movements would change according to the seasonal distribution of food resources and (3) in presence of diurnal human harassment, crested porcupines would develop more strictly nocturnal activity rhythms to avoid direct encounters with man.
Materials and methods
Study area
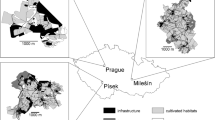
Our study area (about 120 ha, 270–345 m a.s.l., 43° 20′ N, 11° 20′ E) was included in a suburban area located in the northern outskirts of Siena, in the close surroundings of the New General Hospital “Le Scotte” in the suburb of San Miniato (Municipality of Siena). The area was heavily used for human activities, in particular small-scale agriculture and construction works. The climate was relatively mild, with average monthly temperatures of >0 °C in cold months (i.e. October 1990–March 1991, temperature range: 4.4–6.8 °C) and <30 °C in warm months (i.e. April–September 1991: 11.7–24.3 °C). Monthly average rainfall, concentrated in autumn and winter, was c. 63.8 ± 8.4 mm (Lovari et al. 2013). About 42% of the study site included farmlands (Fig. 1). Proportion of deciduous woodland (Quercus pubescens, Quercus cerris, Quercus ilex, Acer campestre, Ulmus minor, Castanea sativa and introduced Robinia pseudoacacia) was 14.8%, together with buildings and other human settlements (17.4%) as well as irrigation ditches (4.0%) and fallow (14.8%). Shrubwood (Cornus mas, Crataegus monogyna, Clematis vitalba, Rubus ulmifolius, Prunus spinosa, Spartium junceum) covered about 4.8% of the area, and the remaining 2.2% was characterized by coniferous woodland (Pinus pinea).
Captures and radio-tracking
Crested porcupines were caught in home-made metal box traps (40 × 50 × 140 cm), baited with fruits and vegetables (plums, apples, carrots, potatoes) and kept active for at least seven nights a month (Lovari et al. 2013; Mori et al. 2014b). Trapped porcupines were sedated according to the protocol in Massolo et al. (2003). If adult (Van Aarde 1985; Mori and Lovari 2014), they were equipped with a radio collar (VHF radio collars: AVM P2, Livermore, CA).
Each crested porcupine was radio-tracked for one full night every 4 days and one full day (24 h) every 2 weeks. Porcupines were trapped all at the same time of the year (late May–early June) and, thus, all monitored for 14–20 h/week/individual for 1 year. Only biologically independent fixes (Lair 1987) were used for our analysis.
The mean location error was determined by positioning radio-collars in 100 known locations, at ground level, and by calculating the difference in metres between actual and estimated locations. Radio-tracking procedures included a mixture of distance (1 fix/70 min, mean location error = 32 m) and homing-in locations (1 fix/15 min, mean location error = 15 m), with a mean value of 41 fixes/month/individual. Seasonal home range (hereafter, HR) sizes were estimated through the 95% minimum convex polygon (MCP 95%) and the 95% fixed kernel (ker 95%: Lovari et al. 2013), by using the statistical software R 3.1.3, packages ade4 (Dray and Dufour 2007) and adehabitat (Calenge 2006). The software RANGES V (Kenward and Hodder 1992) was used to assess HR overlap (ker 95%) between members of the same pair.
Habitat selection
All the fix locations were ascribed to a category of habitat type. The Ivlev’s electivity index (Ivlev 1961) was computed to assess the selection of crested porcupine for habitat types, through the statistical software R 3.1.1, package Gplots (Warnes et al. 2014). This index ranges from −1 (total avoidance) to +1 (total preference): selection/avoidance occurs at values >+0.3 or <−0.3, respectively (Laurenzi et al. 2016).
Activity rhythms
Activity rhythms were assessed seasonally (Corsini et al. 1995) to evaluate the temporal behaviour of crested porcupines in relation to moon phases and round-the-clock. The activity of crested porcupines was determined through the variation of signal intensity within 60 s (Garshelis and Pelton 1980; Corsini et al. 1995). Porcupines were considered as being “inactive” when in the den: thus, duration of activity was measured as the difference between the times of onset and termination of surface activity (Corsini et al. 1995).
Nocturnal fixes were divided according to four moon phases: phase 1, from new moon to ¼ (532 fixes collected in 28 nights); phase 2, from ¼ to ½ (483 fixes collected in 23 nights); phase 3, from ½ to ¾ (494 fixes collected in 32 nights); and phase 4, over ¾ (456 fixes collected in 27 nights). Variation in moonlight intensity has been reported also over the 29.5 days of lunar cycle (day 0, new moon; day 15, full moon: Mori et al. 2014c). The dependence of activity rhythms on moon phases was assessed through circular statistics for each season (Mori et al. 2014c). We computed the Rayleigh test for uniformity to the circular distributions to test for the concentration of fixes around the mean (Batschelet 1981; Mori et al. 2014c), through the statistical software R 3.1.1, package circular (Lund and Agostinelli 2009). The main statistics were calculated to test if inactive fixes were randomly distributed along a lunar cycle or not. The density curves indicating the concentration of inactive fixes along the lunar cycle were kernel smoothed and double plotted on Cartesian axes to better show circularity (Mori et al. 2014c).
Feeding ecology
Four seasons have been astronomically defined. Droppings were collected once a month throughout 1 year, along the routes used by our radio-tagged porcupines, identified through radio tracking in the preceding days. A total of 8–10 droppings per month were analysed, as this number was considered to be representative to assess the proportion of species included in the diet of this rodent (Bruno and Riccardi 1995). Droppings were analysed following the protocol by Bruno and Riccardi (1995). A solution of NaOH 0.059 M was used to dissolve the mucous film which covered the droppings (10–20 min at 40 °C: Zyznar and Urness 1969). Then, the sample was washed through a 1-mm square mesh sieve. Fragments from the washed sample were separated by hand according to the food categories they belonged to; then, they were identified either by eye or through a stereomicroscope (Wild M3C, Heerbrugg: 128–320×).
Food categories were (1) monocotyledonous herbs (leaves and stems), (2) dicotyledonous herbs (leaves and stems), (3) roots and rhizomes, (4) bulbs and tubers, (5) fruits and (6) cultivated vegetables. “Bark” was not included among the categories as debarking by porcupines was never observed in the course of this study. Each category was identified at the family to species levels through comparison with private reference collections and herbarium specimens. Volumes of each food category were measured by water displacement (Jackson 1980; Bruno and Riccardi 1995). Relative frequencies and volumes were then plotted in a graph (Kruuk 1989). The availability of trophic resources was measured through the point intercept method (Goodall 1953; Jonasson 1988). This method measures the relative cover of vegetal categories which builds up the diet of the crested porcupine (monocotyledonous herbs, dicotyledonous herbs, plants with bulbs and tubers, plants with rhizomes, fruit plants, vegetables). It is designed to sample within-area variation and quantify changes of plant species cover over time. A total of 90 linear transects of 10 m were placed randomly along the study site throughout the year, using a stratified sampling designs, and throughout all the identified habitat types. On each transect, 50 plant hits were carried out, one every 20 cm. Assessment points were accomplished with a 30-cm pin, which was sharpened to create a point and placed perpendicularly to the ground. Detected plants were then reported on a field tab together with the date, the number of the transect and the habitat type. The relative frequency of each category was calculated. Food selection by porcupines was then evaluated through the Ivlev’s electivity index, by using the R 3.1.1 package Gplots (Warnes et al. 2014). Selection/avoidance occurs at values >+0.3 or <−0.3, respectively (Laurenzi et al. 2016).
Results
We caught a total of 18 crested porcupines, 11 of which were adults and were equipped with radio collars. Among our research individuals, 44.4% were poached within the first 3 months from radio tagging (i.e. unequivocal signs of poaching activities were found: a severed radio collar and a used snare, often in combination with a number of bloodied quills) and 33.4% disappeared so that just 22.2% were radio tracked for at least one full year (two pairs: M1-F1 and M2-F2). We are aware of the snags related to our small sample size; on the other hand, with a much greater number of experimental individuals, a holistic approach (as the one we have used in our study) would have been particularly challenging. All poached/disappeared porcupines showed the tendency to range in the close neighbourhoods of human settlements (59.81% fixes, N = 1428), differently from the others.
Of our radio-tagged pairs, one had its den in a bramble thicket, whereas the other one changed three setts in 1 year, eventually locating the last one in a bramble thicket. Seasonal HRs are shown in Table 1; home ranges were larger in the warm months with respect to the cold ones (Wilcoxon matched pairs signed-rank test: W = 56; P = 0.01).
No significant differences in seasonal HR sizes occurred between sexes (Mann-Whitney U test: U = 27, P = 0.65), with extensive spatial overlap between HRs of members of the same pair (median = 78.85%, Q1 = 75.42%, Q3 = 87.43%, throughout the year: Table 2). HR overlap of different pairs was negligible (4.2–16.8%).
Temporal overlap was high between pair members, with a decrease in winter for pair M1-F1 and in autumn for pair M2-F2, when cubs were present (median = 80.55%, Q1 = 57.08%, Q3 = 89.18%, throughout the year: Table 2).
Temporal activity of radio-tagged porcupines (median value per night 7 h 40 min, Q1 = 7 h 32 min, Q3 = 8 h 6 min, throughout the year: Table 3) did not vary significantly between warm (spring–summer) and cold months (autumn–winter: Wilcoxon matched pairs signed-rank test W = 286, P = 0.19). Porcupines completely avoided daylight hours and were only active at night.
The highest peaks of inactivity were recorded always around the full moon (winter: day 13.6 ± 2.6, Rayleigh test Z = 7.37, P < 0.001; autumn: day 13.9 ± 2.8, Rayleigh test Z = 7.37, P = 0.003; spring: day 14.8 ± 1.4, Rayleigh test Z = 31.43, P < 0.001; summer: day 15.8 ± 0.9, Rayleigh test: Z = 46.03, P < 0.001: Fig. 2).
Irrigation ditches, human settlement and coniferous woodland were avoided throughout the year; fallow in winter, spring and summer; and shrubwoods only in autumn. Deciduous woodland was positively selected throughout the year and shrubwoods in winter, spring and summer. Farmlands were used proportionally to their local availability (Table 4).
A total of 113 faecal samples collected throughout the year (winter, N = 31; spring, N = 19; summer, N = 34; autumn, N = 29) were analysed. Fruits were the staple of the diet mainly in autumn and winter, while roots and rhizomes prevailed in spring and dicotyledons, followed by fruits (especially figs Ficus carica: 33.3% relative frequency and 68.2% relative volume on the total of fruits) in summer (Fig. 3).
Volume of main food categories in total diet of crested porcupines, i.e. volume of each dietary component versus its relative frequency of occurrence (autumn, N = 29 droppings; winter, N = 31 droppings; spring, N = 19 droppings; summer, N = 34 droppings). 1, dicotyledons; 2, monocotyledons; 3, roots and rhizomes; 4, bulbs and tubers; 5, fruits; 6, vegetables
All the food categories were used proportionally to their local availability, with few exceptions. Roots and rhizomes were selected in winter and spring (especially Rumex crispus: 93.3% relative frequency and 95.7% relative volume, on the total of roots and rhizomes); bulbs and tubers (especially Cyclamen hederifolium: 78.0% relative frequency and 53.8% relative volume, on the total of bulbs and tubers) were preferred in autumn and underused in winter and spring. Fruits were positively selected in autumn. Cultivated vegetables were underused in autumn and spring, used in proportion to their availability in summer and not available in winter (Table 5).
Discussion
Differently from other areas (Alkon and Saltz 1988; Corsini et al. 1995; Mori et al. 2014c), activity rhythms of crested porcupines were strictly nocturnal in our study, with a sharp moonlight avoidance (especially in summer, when nights are short and cloudless), comparable to what was observed in areas where potential predators occur (Negev desert: Alkon and Saltz 1988; Western Terai: Fattorini and Pokheral 2012). Despite this, in summer, Indian porcupines foraged also during bright nights, to fulfil their water requirements (Alkon and Saltz 1988). In the absence of natural predators, light avoidance is not so strict (Corsini et al. 1995; Mori et al. 2014c), but poaching pressure may act as a force vicariant that of natural predators to maintain this behaviour. In our study area, poaching was especially common and at least 72.7% of artificially marked porcupines (N = 11, including 64% of radio-tagged ones) were killed in 12 months. Whereas the pair M1-F1 remained in the same den throughout the whole year, as commonly observed in crested porcupines (Monetti et al. 2005; Lovari et al. 2013; Mori et al. 2015), the pair formed by M2 and F2 changed their den three times in 1 year, progressively using more closed and inaccessible habitats (from open woodland to a S. junceum shrubwood, to a bramble thicket). In fact, digging dens in thick thorny bushes (cf. Fig. 1) could be a ploy to avoid poaching.
A high HR overlap between members of the same pair has been confirmed (Mori et al. 2014b). Pair partners also showed a high overlap of activity rhythms (Mori et al. 2014c), which only decreased in winter for pair 1, with cubs in the den (Mori et al. 2016). In closely related species, the availability of food resources has been reported as the main determinant of habitat selection (H. indica: Sharma and Prasad 1992; H. africaeaustralis: De Villiers et al. 1994). In the Mediterranean area, summer provides porcupines with a high amount of food categories (i.e. epigeal plants, fruits, vegetables: Bruno and Riccardi 1995), but underground plant storage organs may be difficult to reach because of sun-baked soil, thus determining an expansion of HR sizes (Mori et al. 2014b). As to habitat selection in suburban areas, crested porcupines avoided coniferous woodlands, fallows and human settlements, as they do not provide rodents with enough food resources, are unsuitable for denning and/or expose porcupines to poaching. As to avoidance of irrigation ditches, most likely the presence of water or mud made them difficult habitats for porcupine movements. Differently from what has been observed in other studies (Fattorini and Pokheral 2012; Mori et al. 2014b), in our study area, grass in fallows was kept short, thus providing little cover to porcupines. A positive selection occurred for deciduous woodlands, which were overused throughout the year with respect to their local availability. Shrubwoods were also selected throughout the year but avoided in autumn, when a high availability/consumption of fruits (mainly chestnuts and acorns) occurred in woodland. Furthermore, all the porcupine den setts detected in this study were located in closed habitats, i.e. deciduous woodland and shrubwood. While Mori et al. (2014b) showed a positive selection of cultivations in summer, farmlands were used proportionally to their availability in our work, which may partly contradict their findings. In our study area, tin fences (50-cm high from the ground) were used as a prevention to crop damage in house gardens, thus removing a particularly attractive food source, e.g. flower bulbs, melons, courgettes and onions (Laurenzi et al. 2016).
The crested porcupine has been confirmed to be a “generalist” herbivore, which feeds on a high variety of plants and organs, i.e. roots, stems and fruits (Bruno and Riccardi 1995; Mohamed 2011), in relation to their local seasonal availability. Fruits built up the staple of the diet in autumn and winter, when chestnuts were abundant. The presence of fruit with hard, thick epicarp was greater in the cold months with respect to warm ones. These fruits are a long-lasting, rich reserve of carbohydrates and have a clumped distribution in woodlands (Pignatti 1982), confirming results of our analysis of habitat selection. In summer, as to fruits, figs appeared to be a particularly attractive food resource, although concentrated around human inhabited, underused areas. One may predict that animals will tend to simultaneously maximise net energy intake and minimise risk of predation, to maximise fitness (Dill 1987). This prediction may explain why the usage of a sought after, clumped food resource, such as figs, contrasted with the short time spent in human inhabited areas: porcupines must have traded safety for food intake, i.e. quickly feeding and going back to safer habitats to avoid being poached (cf. Cavallini and Lovari 1991, for V. vulpes; De Villiers et al. 1994, for H. africaeaustralis).
The positive selection of roots (especially R. crispus) in spring could depend on field ploughing, which enhanced availability of underground storage organs in arable lands in that season. A positive selection for bulbs and tubers occurred early in the autumn, when the tuber of the ivy-leaved cyclamen C. hederifolium was overused with respect to its local availability. This may be due to the fact that secondary metabolites accumulated in underground storage organs are eliminated when the plant is flowering, thus reducing their toxicity (Fiori 1969). Furthermore, the palatability of the cyclamen is reduced by the increased concentration of the toxic glycoside outside the flowering period (Fiori 1969).
Despite being described as a species whose diet is composed mainly by vegetal underground storage organs (Santini 1980; Pigozzi and Patterson 1990), our work revealed that porcupines preferred fruits and stems. The greater use of epigeal plant parts (about 71.1% of total frequency, in our work) with respect to roots may represent a strategy to reduce poaching risk because of the longer time and higher amount of energy requested to search, dig and extract underground storage organs (Alkon and Saltz 1985b; Downs et al. 2015). Furthermore, water content of ripe fruits may allow crested porcupines not to move in moonlight nights, differently from what observed in an arid environment by Alkon and Saltz (1988). The use of hypogeal parts of the plant has been confirmed, although they were mainly consumed when fruits were poorly available and when the plough brought up roots, bulbs and rhizomes. Consumption of cultivated vegetables was very low and occurred only in summer (mainly on courgettes and tomatoes), proportionally to their local availability. Vegetables represent a source of rich and clumped food for the crested porcupine. Their usage may have been limited by the tin fences previously described and, most likely, also by the availability of other food resources present in less risky environments (Laurenzi et al. 2016).
Although the genus Hystrix originated in Asia (Nowak 1991, but see van Weers 2005), the species H. cristata evolved in Africa (Mohr 1965; Trucchi and Sbordoni 2009; Trucchi et al. 2016), where most of its present range occurs. During the Pleistocene, in the open habitats of Asia and Africa, e.g. savanna, the herbivore guild was particularly rich in species, e.g. 32 species of bovids and 9 species of equids in Africa (Kappelman et al. 1997; Steele 2007; Bernor et al. 2010; Gentry 2010), as well as several tens of potential porcupine predators (Werdelin and Peignè 2010). The presence of the latter may have determined the evolution of quills, whereas the former may have provided superior competitors (e.g. ruminants) for above ground food resources. In fact, earlier porcupines (e.g. Hystrix refossa: van Weers 1994) showed a hypsodont pattern of dentition, adapted to chew grass. As a consequence of competitor and predator presence, porcupines may have adapted to suboptimal habitats on time (e.g. subdeserts: Gutterman 1982; Alkon and Saltz 1985b; Alkon and Olsvig-Whittaker 1989; Bragg et al. 2005) and/or to feed on underground plant organs, which requires time and energy to dig them out, but it exposes them to predation risk. Quills, preference for thick thorny cover and nocturnal habits could be the results of this adaptation. In Italy, in near absence of predators and in presence of very few potentially competing wild ruminants, one could expect porcupines to have expanded again their ecological niche to the food-rich open habitats, which might explain the unusually high use of epigeal wild plants we recorded.
References
Abay GY, Bauer H, Gebrihiwot K, Deckers J (2011) Peri-urban spotted hyena (Crocuta crocuta) in northern Ethiopia: diet, economic impact and abundance. Eur J Widl Res 57:759–765
Alkon PU, Saltz D (1985a) Patterns of crested porcupine (Hystrix indica) damage to cultivated potatoes. Agric Ecos Environm 14:171–183
Alkon PU, Saltz D (1985b) Potatoes and the nutritional ecology of crested porcupine in a desert biome. J Appl Ecol 22:727–737
Alkon PU, Saltz D (1988) Influence of season and moonlight on temporal activity patterns of Indian crested porcupines (Hystrix indica). J Mammal 69:71–80
Alkon PU, Olsvig-Whittaker L (1989) Crested porcupine digs in the Negev desert highlands: patterns of density, size, and longevity. J Arid Environm 17:83–95
Amori G, Contoli L, Nappi A (2008) Mammalia II: Erinaceomorpha, Soricomorpha, Lagomorpha, Rodentia. Il Sole 24 Ore. Edagricole, Calderini Edizioni, Bologna, Italy
Antrop M (2004) Landscape change and the urbanization process in Europe. Lands Urb Plan 67:9–26
Batschelet E (1981) Circular statistics in biology. Academic Press, London
Bateman PW, Fleming PA (2012) Big city life: carnivores in urban environments. J Zool (Lond) 287:1–23
Beringer J, Hansen LP, Demand JA, Sartwell J, Wallendorf M (2002) Efficacy of translocation to control urban deer in Missouri: costs, efficiency, and outcome. Wildl Soc Bull 30:767–774
Bernor RL, Armour-Chelu MJ, Gilbert H, Kaiser TM, Schultz E (2010) Equidae. In: Werdelin L, Sanders WJ (eds) Cenozoic mammals of Africa. California University Press, Berkeley, California, pp. 685–721
Bertolino S, Mazzoglio PJ, Vaiana M, Currado I (2004) Activity budget and foraging behavior of introduced Callosciurus finlaysonii (Rodentia, Sciuridae) in Italy. J Mammal 85:254–259
Bertolino S, Colangelo P, Mori E, Capizzi D (2015) Good for management, not for conservation: an overview of research, conservation and management of Italian small mammals. Hystrix 26:25–35
Bragg CJ, Donaldson JS, Ryan PG (2005) Density of Cape porcupines in a semi-arid environment and their impact on soil turnover and related ecosystem processes. J Arid Environm 61:261–275
Bruno E, Riccardi C (1995) The diet of the crested porcupine Hystrix cristata L., 1758 in a Mediterranean rural area. Z Säugetierk 60:226–236
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Cavallini P, Lovari S (1991) Environmental factors influencing the use of habitat in red foxes, Vulpes vulpes (L., 1758). J Zool (Lond) 223:323–339
Chace JF, Walsh JJ (2004) Urban effects on native avifauna: a review. Landsc Urb Plann 74:46–69
Chuan CH (1969) Porcupines and grasshoppers as pests of the oil palm. Progress in Oil Palm, Incorporated Society of Planters, Kuala Lumpur, Malaysia, pp. 155–161
Clergeau P, Vergnes A (2011) Bird feeders may sustain feral rose-ringed parakeets Psittacula krameri in temperate Europe. Wildl Biol 17:248–252
Collins JP, Kinzing A, Grimm NB, Fagan WF, Hope D, Wu J, Borer ET (2000) A new urban ecology. Modeling human communities as integral parts of ecosystems poses special problems for the development and testing of ecological theory. Am Sci 88:416–424
Coluccy JM, Drobney RD, Graber DA, Sheriff SL, Witter DJ (2001) Attitudes of central Missouri residents toward local giant Canada geese and management alternatives. Wildl Soc Bull 29:116–123
Connelly NA, Decker DJ, Wear S (1987) Public tolerance of deer in a suburban environment: implications for management and control. Third East Wildl Damage Control Conf 8
Conover MR, Pitt WC, Kessler KK, DuBow TJ, Sanborn WA (1995) Review of human injuries, illnesses, and economic losses caused by wildlife in the United States. Wildl Soc Bull 23:407–414
Corsini MT, Sonnino S, Lovari S (1995) Temporal activity patterns of crested porcupines Hystrix cristata. J Zool (Lond) 223:43–54
Daigle JJ, Hrubes D, Ajzen I (2002) A comparative study of beliefs, attitudes, and values among hunters, wildlife viewers, and other outdoor recreationists. Hum Dim Wildl 7:1–19
DeStefano S, DeGraaf RM (2003) Exploring the ecology of suburban wildlife. Front Ecol Environm: 95–101
De Villiers MS, Van Aarde RJ, Dott HM (1994) Habitat utilization by the Cape porcupine Hystrix africeaustralis in a savanna ecosystem. J Zool (Lond) 232:539–549
Dill LM (1987) Animal decision-making and its ecological consequences: the future of aquatic ecology and behavior. Can J Zool 65:803–811
Ditchkoff SS, Saalfeld ST, Gibson CJ (2006) Animal behavior in urban ecosystems: modifications due to human-induced stress. Urb Ecosyst 9:5–12
Doncaster CP, Dickman CR, Macdonald DW (1990) Feeding ecology of red foxes (Vulpes vulpes) in the city of Oxford, England. J Mammal 71:188–194
Downs CT, Pillay KR, Wilson AL, Ramesh T (2015) Digestive parameters and energy assimilation of Cape porcupine on economically important crops. Afr Zool 50:307–312
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
Fattorini N, Pokheral CP (2012) Activity and habitat selection of the Indian crested porcupine. Ethol Ecol Evol 24:377–387
Fiori A (1969) Nuova flora analitica d’Italia. Vol. II. Edagricole, Bologna, Italy
Gaigher IG, Currie MH (1979) Preliminary studies on the ecology of the southern African porcupine, Hystrix africaeaustralis. Department of Nature and Environmental Conservation, Province Administration of the Cape of Good Hope, South Africa: 55–69
Garden J, Mcalpine C, Peterson A, Jones D, Possingham H (2006) Review of the ecology of Australian urban fauna: a focus on spatial explicit processes. Aust Ecol 31:126–148
Garshelis DL, Pelton MR (1980) Activity of black bears in the Great Smoky Mountains National Park. J Mammal 61:8–19
Gentry AW (2010) Bovidae. In: Werdelin L, Sanders WJ (eds) Cenozoic mammals of Africa. California University Press, Berkeley, California, pp. 747–822
Goodall DW (1953) Point-quadrat methods for the analysis of vegetation. Austr J Bot 1:456–461
Greaves JH, Khan AA (1978) The status and control of porcupines, genus Hystrix as forest pests. Commonw Forestry Rev 57:25–32
Gutterman Y (1982) Observations on the feeding habits of the Indian crested porcupine (Hystrix indica) and the distribution of some hemicryptophytes and geophytes in the Negev desert highlands. J Arid Environm 5:261–268
Harris S (1986) Urban foxes. Whitted Books Ltd, London
Heimlich R, Anderson WD (2001) Development at the urban fringe and beyond, impacts on agriculture and rural land. US Department of Agriculture Washington, DC, USA
Herr J, Schley L, Roper TJ (2009) Socio-spatial organization of urban stone martens. J Zool (Lond) 277:54–62
Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University Press, New Haven
Jackson J (1980) The annual diet of the roe deer (Capreolus capreolus) in the New Forest, Hampshire, as determined by rumen content analysis. J Zool (Lond) 192:71–83
Jonasson S (1988) Evaluation of the point intercept method for the estimation of plant biomass. Oikos 52:101–106
Kappelman J, Plummer T, Bishop L, Duncan A, Appleton S (1997) Bovids as indicators of Plio-Pleistocene paleoenvironments in East Africa. J Human Evol 32:229–256
Kenward RE, Hodder KH (1992) RANGES V. An analysis system for biological location data. Institute of Terrestrial Ecology, Furzebrook Research Station, Wareham, Dorset, UK
Khan AA, Ahmad S, Hussain I, Munir S (2000) Deterioration impact of Indian crested porcupine, Hystrix indica, on forestry and agricultural systems in Pakistan. Intern Biodet Biodegr 45:143–149
König A (2008) Fears, attitudes and opinions of suburban residents with regards to their urban foxes. A case study in the community of Grünwald—a suburb of Munich. Eur J Wildl Res 54:101–109
Kruuk H (1989) The social badger: ecology and behaviour of group-living carnivore (Meles meles). Oxford University Press, Oxford
Lair H (1987) Estimating the location of the focal centre in red squirrel home ranges. Ecol 68:1092–1101
Laurenzi A, Bodino N, Mori E (2016) Much ado about nothing: assessing the impact of a problematic rodent on agriculture and native trees. Mammal Res 61:65–72
Linkie M, Dinata Y, Nofrianto A, Leader-Williams N (2007) Patterns and perceptions of wildlife crop raiding in and around Kerinci Seblat National Park, Sumatra. Anim Cons 10:127–135
Lovari S, Sforzi A, Mori E (2013) Habitat richness affects home range size in a monogamous large rodent. Behav Proc 99:42–46
Lund U, Agostinelli C (2009) CircStats: circular statistics. In: Jammalamadaka RS, Sen Gupta A (Eds.) Topics in circular Statistics, World Scientific. URL http://cran.r-project.org/package=CircStats
Luniak M (2004) Synurbization—adaptation of animal wildlife to urban development. Proc 4th International Symposium on Urban Wildlife Conservation, Tucson: 50–55
Mackenstedt U, Jenkins D, Romig T (2015) The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int J Paras: Paras Wildl 4:71–79
Magle SB, Hunt VM, Vernon M, Crooks KR (2012) Urban wildlife research: past, present, and future. Biol Cons 155:23–32
Massolo A, Sforzi A, Lovari S (2003) Chemical immobilization of crested porcupine with tiletamine HCl and zolazepam HCl (Zoletil®) under field condition. J Wildl Dis 39:727–731
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Cons 127:247–260
Menchetti M, Mori E (2014) Worldwide impacts of alien parrots (Aves Psittaciformes) on native biodiversity and environment: a review. Ethol Ecol Evol 26:172–194
Mohamed WF (2011) The crested porcupine, Hystrix cristata (Linnaeus, 1758) in Misurata, Libya. Journal J Ecol Nat Environm 3:228–231
Mohr E (1965) Altweltliche Stachelschweine. A. Ziemsen Verlag, Westarp Wissenschaften (Eds.), Wittenburg Lutherstadt, Germany
Monetti L, Massolo A, Sforzi A, Lovari S (2005) Site selection and fidelity by crested porcupine for denning. Ethol Ecol Evol 17:149–159
Mori E, Lovari S (2014) Sexual size monomorphism in the crested porcupine (Hystrix cristata). Mamm Biol 79:157–160
Mori E, Bertolino S (2015) Feeding ecology of long-eared owls in winter: an urban perspective. Bird Study 62:257–261
Mori E, Sforzi A, Di Febbraro M (2013) From the Apennines to the Alps: recent range expansion of the crested porcupine Hystrix cristata L., 1758 (Mammalia: Rodentia: Hystricidae) in Italy. Ital J Zool 80:469–480
Mori E, Maggini I, Menchetti M (2014a) When quills kill: the defense strategy of the crested porcupine Hystrix cristata L., 1758. Mammalia 78:229–234
Mori E, Lovari S, Sforzi A, Romeo G, Pisani C, Massolo A, Fattorini L (2014b) Patterns of spatial overlap in a monogamous large rodent, the crested porcupine. Behav Proc 107:112–118
Mori E, Nourisson DH, Lovari S, Romeo G, Sforzi A (2014c) Self-defence may not be enough: moonlight avoidance in a large, spiny rodent. J Zool (Lond) 294:31–40
Mori E, Menchetti M, Balestrieri A (2015) Interspecific den sharing: a study on European badger setts using camera traps. Acta Ethol 28:121–126
Mori E, Menchetti M, Lucherini M, Sforzi A, Lovari S (2016) Timing of reproduction and paternal cares in the crested porcupine. Mamm Biol 294:31–40
Niemelä J (1999) Ecology and urban planning. Biodiv Conserv 8:119–131
Nowak R (1991) Walker’s mammals of the world. Johns Hopkins University Press, Baltimore
Parker TS, Nilon CH (2008) Gray squirrel density, habitat suitability, and behavior in urban parks. Urb Ecosyst 11:243–255
Petrozzi F, Angelici FM, Di Vittorio M (2012) Crested porcupine (Hystrix cristata) in the urban environment: habitat preferences and management strategies. Hystrix sup 2012:167
Pigozzi G, Patterson IJ (1990) Movements and diet of crested porcupines in the Maremma National Park, central Italy. Acta Theriol 35:173–180
Pignatti S (1982) Flora d’Italia, volume 1–2-3. Edagricole, Bologna
Prange S, Gehrt SD, Wiggers EP (2003) Demographic factors contributing to high raccoon densities in urban landscapes. J Wildl Manag 67:324–333
Russo D, Ancillotto L (2014) Sensitivity of bats to urbanization: a review. Mammal Biol 80:205–212
Santini L (1980) The habits and influence on the environment of the old world porcupine Hystrix cristata L. in the northernmost part of its range. Vertebrate Pest Conference Proceedings Collection: 149–153
Sever Z, Mendelssohn H (1989) Porcupines on the edge of town. Israel Land Nat 13:112–115
Sharma D, Prasad SN (1992) Tree debarking and habitat use by porcupine (Hystrix indica Kerr) in Sariska National Park in Western India. Mammalia 56:351–362
Steele TE (2007) Vertebrate records: late Pleistocene of Africa. In: Elias S (ed) Encyclopedia of quaternary science. Elsevier, Oxford, pp. 3139–3150
Timm RM, Baker RO, Bennett JR, Coolahan CC (2004) Coyote attacks: an increasing suburban problem. Proceedings of the 21st Vertebrate Pest Conference: 47–57
Trucchi E, Sbordoni V (2009) Unveiling an ancient biological invasion: molecular analysis of an old European alien, the crested porcupine (Hystrix cristata). BioMed Central Evol Biol 9:109
Trucchi E, Facon B, Gratton P, Mori E, Stenseth N, Jentoft S (2016) Long live the alien: is high genetic diversity a pivotal aspect of crested porcupine (Hystrix cristata) long-lasting and successful invasion? Mol Ecol 25:3527–3539
van Aarde RJ (1985) Age determination of Cape porcupines, Hystrix africaeaustralis. S Afr J Zool 20:232–236
van Weers DJ (1994) The porcupine Hystrix refossa Gervais, 1852 from the Pleistocene of Europe, with notes on other fossil and extant species of the genus Hystrix. Scripta Geol 106:35–52
van Weers DJ (2005) A taxonomic revision of the Pleistocene Hystrix (Hystricidae, Rodentia) from Eurasia with notes on the evolution of the family. Contrib Zool 74:301–312
Walther B, Lehmann M, Fuelling O (2011) Approaches to deal with the coypu (Myocastor coypus) in urban areas—an example of practice in Southern Brandenburg, Germany. Proceedings of the 8th European Vertebrate Pest Management Conference: 32–37
Warnes GR, Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, Venables B (2014) Gplots: various R programming tools for plotting data. Available online at: http://CRAN.R-project.org/package=gplots
Werdelin L, Peignè S (2010) Carnivora. In: Werdelin L, Sanders WJ (eds) Cenozoic mammals of Africa. California University Press, Berkeley, California, pp. 609–663
Zyznar E, Urness PJ (1969) Qualitative identification of forage remnants in deer faeces. J Wildl Manag 33:506–510
Acknowledgements
Mauro Lucherini and Andrea Sforzi helped, respectively, in field work and organization of database. Dawid Adam Iurino provided references on Pleistocenic wildlife of the African savanna. Francesco Ferretti and Paul Mazza kindly read a previous draft. Two anonymous reviewers and Danilo Russo improved our first draft with their comments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lovari, S., Corsini, M.T., Guazzini, B. et al. Suburban ecology of the crested porcupine in a heavily poached area: a global approach. Eur J Wildl Res 63, 10 (2017). https://doi.org/10.1007/s10344-016-1075-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-016-1075-0