Abstract
Understanding wildlife movements and habitat selection are critical to drafting conservation and management plans. We studied a population of eastern Hermann’s tortoise (Testudo hermanni boettgeri) in a traditionally managed rural landscape in Romania, near the northern edge of the species geographic distribution. We used telemetry to radio-track 24 individuals between 2005 and 2008 and performed a Euclidian distance-based habitat selection analysis to investigate habitats preferred by tortoises at both landscapes (second-order order selection) and individual (third-order selection) home range scales. The home range size for tortoises in our study area was 3.79 ± 0.62 ha and did not differ by gender or season (pre- and post-nesting seasons). Their movement ecology was characterized by short-distance movements (daily mean = 31.18 ± 1.59 m), apparently unaffected by habitat type. In contrast to other studies, movements of males and females were of similar magnitude. At the landscape (population home range) scale, grasslands and shrubs were preferred, but tortoises also showed affinity to forest edges. At the individual home range scale, tortoises selected grassland and shrub habitats, avoided forests, and used forest edges randomly. Creeks were avoided at both spatial scales. Our results suggest that tortoise home ranges contain well-defined associations of habitats despite a higher selection for grasslands. As such, avoiding land conversion to other uses and maintaining habitat heterogeneity through traditional practices (e.g., manual mowing of grasslands, livestock grazing) are critical for the persistence of tortoise populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The eastern Hermann’s tortoise (Testudo hermanni subsp. boettgeri) is a Palearctic land tortoise restricted to southeastern Europe (Cheylan 2001). One of its northernmost populations occupies an area of approximately 700 km2 in southwestern Romania (Rozylowicz and Dobre 2010). Despite the relatively optimistic assessments regarding its European conservation status at species level (Cox and Temple 2009), Hermann’s tortoise populations are declining across its entire range, mainly due to habitat loss and degradation and illegal collecting for pet trade (Bertolero et al. 2011). Along with these threats, future climate change may act synergistically to increase the extinction risk for this species (Fernández-Chacón et al. 2011). The eastern subspecies was assessed as having an endangered status in Romania (Rozylowicz and Dobre 2010). Hermann’s tortoise benefits of European-level protection status under the European Union Habitats Directive (2006/105/EC 2006; 92/43/EEC 1992).
As a long-lived, low-turnover species, with limited dispersal abilities, tortoises are particularly vulnerable to habitat loss and fragmentation (Pough 2004). The geographic distribution of Hermann’s tortoise in Romania was found to be strongly affected by conversions of land cover from traditional-use semi-natural habitats to intensively managed agricultural lands (Rozylowicz and Dobre 2010). Such effects are prevalent at the eastern distribution limit of Hermann’s tortoise in Romania, where bioclimatic envelope models suggest that suitable climate space extends farther east from the current area of occurrence (Rozylowicz 2008).
A thorough understanding of the home range behaviors and habitat selection is critical for drafting effective management plans and conservation strategies aimed at population recovery (Börger et al. 2008). Habitat selection studies analyze resource use by individuals with respect to their availability (Manly 2002). Habitat selection is a hierarchical process occurring across multiple spatial scales, from the individual home range to the geographical range of the species (Johnson 1980; Aebischer et al. 1993). Resource selection functions and habitat use are inextricably linked to individual movements (Manly 2002). For Hermann’s tortoise, the extent of distances traveled depends on the configuration and composition of habitats, as well as seasonal behavioral patterns (e.g., nesting, searching for mates, aestivation, hibernation (Luiselli and Rugiero 2006; Mazzotti et al. 2002)). As such, movement data can provide managers realistic information regarding the ecology of species (Kernohan et al. 2001).
One of the conceptual tools used for analyzing habitat selection is represented by the Euclidian distances from animal locations or home ranges to habitat (Conner and Plowman 2001; Conner et al. 2003). Distance-based approaches integrate areal and linear features (Conner et al. 2005), which are known to determine habitat selection in Hermann’s tortoises (Calzolai and Chelazzi 1991; Longepierre et al. 2001). Linear or near-linear habitats such as forest edges or relatively small patches of shrubs are critical to tortoise thermoregulation behavior (Cheylan 2001).
This study investigates habitat selection by the eastern Hermann’s tortoise in a managed landscape in Romania using a distance-based approach. This study has high biological relevance given that ecological studies of northern populations (north of the River Danube) are completely lacking, and little is known about the status of these populations. In addition, Hermann’s tortoise represents the conservation focus of several Romanian Natura 2000 protected areas (Ioja et al. 2010), and this study has the potential to offer clear habitat management recommendations. The study objectives are: (a) to investigate habitat selection at two local scales (landscape scale—home range location within the landscape and home range scale—individuals’ locations within their annual home ranges) and (b) to assess the tortoises’ home range and movements through forests and grassland patches.
Methods
Study site
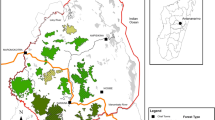
The study was implemented in Iron Gates Natural Park (44° N, 21° E), a protected area located in southwestern Romanian Carpathians (Fig. 1). The Iron Gates Natural Park is managed as a category V IUCN (protected landscape/seascape), which places emphasis on nature conservation and interactions with humans through traditional management practices (Pătroescu and Rozylowicz 2000; Phillips 2002). The climate is hot summer continental, with an average annual temperature of 11.6 °C. The warmest month of the year is July (average = 29.7 °C) and the coldest is January (average = 2.3 °C). Total annual rainfall is 690 mm, with May and June as the wettest months (≅ 80 mm per month) and August as the driest month (≅ 42 mm) (Rozylowicz 2008). The study period (2005–2008) did not deviate from the long-term normal in terms of annual precipitation and average and maximum summer temperatures (recorded at the Drobeta Turnu Severin weather station).
The area is dominated by thermophilous oak woodlands (Quercus cerris, Quercus frainetto, Quercus pubescens) at altitudes <200 m, sessile oak forests (Quercus petraea) at altitudes between 200 and 500 m, and beech forests at elevations between 500 and 1,000 m. A big percentage of the natural forests located at low elevations, in lowlands as well as the slopes adjacent to the Danube River, have been replaced by secondary thermophilous shrubs. This human-induced process started at least two to three centuries ago through small clearings and maintenance of open habitat for hayfield and livestock grazing (Matacă 2002). Land management is dominated by non-intensive, traditional land management, such as limited logging to meet local demands for heating, small-scale grazing, and manually harvested hayfields. Open habitats (i.e., pastures, hayfields) are maintained through mowing, regular clearing of shrubs and trees, and animal grazing (e.g., goats, cattle, and horses) (Matacă 2005).
Habitat delineation
We delineated the following habitat types based on vegetation structure and management regimes: (1) grassland—open areas managed as hayfield or pastures, (2) shrub—areas >10 m2, (3) forest—closed canopy areas >100 m2, (4) forest edge—as 10-m wide strips within a forest habitat, (5) creek—major channels of temporary streams and creeks, and (6) road—unpaved and logging roads. We defined shrubs as any habitat patch composed of woody shrubs and thickets up to 5 m in height and >10 m2 in area. By doing so, we excluded individual, non-contiguous shrubs, which could not be assigned an areal habitat representation. We defined forest habitats as a minimum of 100-m2 contiguous forest or woodland composed of tree species >5 m in height and canopy closure >40 %. Similarly, we excluded small patches of one to three individual trees which, despite offering potential shelter and resting habitat, are not functionally forest due to edge effects (Chen et al. 1999). All habitat types were digitized using ArcGIS 9x (ESRI, Redlands, CA, USA) from 2005 aerial imagery (0.5-m resolution) and were verified on the ground. From the vector data, we developed 5 × 5-m resolution grids for performing habitat selection analyses.
Data collection—radio-telemetry
We radio-tracked 24 adult tortoises (15 females and 9 males) between 2005 and 2008. In 2005, we tracked 7 tortoises, in 2006 12 tortoises, and in 2008 5 tortoises. Very-high-frequency glue-on transmitters R1930 (ATS Inc, Isanti, MN, USA) were fixed on the lateral-anterior part of the carapace using an epoxy-based glue. The average weight of the tortoises was 1,522 ± 69 g, with males weighting less than females (males, 1,108 ± 24 g; females, 1,776 ± 19 g). The weight of the transmitter was ~23 g, an average of 1.42 % (range = 1.15–2.02 %) of the tortoises weight. Each tortoise was located at irregular intervals (1–30 days between consecutive relocations) using radio-telemetry until visual contact between March and November of each year. Generally, it is acknowledged that Hermann’s tortoises are more active in the pre-nesting season compared to the post-nesting season (Huot-Daubremont and Grenot 1997; Loy and Cianfrani 2010; Luiselli et al. 2009; Willemsen 1991; Cruce and Răducan 1975). Thus, we defined two activity periods: the pre-nesting season (April–June) and post-nesting season (July–October). Nesting can still occur later in the season (i.e., July) but only among few individuals in the population (Cruce and Răducan 1975; Cruce and Raducan 1976).
Home range analyses
The population home range (i.e., study area for landscape scale analysis) was calculated using the minimum convex polygon with 100 % from annual telemetry locations (100 % MCP). Individual home ranges were plotted as density kernels with a smoothing factor scaled so as to generate an area equal with that recorded at 100 % MCP (Row and Blouin-Demers 2006; Downs and Horner 2008; Edge et al. 2010). In addition, we added a 50-m buffer around the population home range (i.e., MCP) in order to completely include the limits of individual kernel-based home ranges within the population MCP. Thus, we accounted for potential consecutive-day movements that were not captured by our irregular telemetry intervals. We calculated the home ranges using software Biotas2a (Ecological Software Solutions LLC). We partitioned data by season (pre-nesting and post-nesting) and gender and tested for seasonal and gender-specific differences in home range size using non-parametric Mann–Whitney and Kruskal–Wallis tests.
Habitat selection analyses
We investigated habitat selection using a distance-based analysis (Conner and Plowman 2001; Conner et al. 2003) at two spatial scales: selection of the population home range within the entire study area (second-order—landscape scale) and selection of habitat features within individual home ranges (third-order—home range scale) (Johnson 1980).
We first simulated 3,023 points in a 25-m uniform pattern from a uniform random distribution within the population minimum convex polygon (i.e., random points at landscape scale (Conner and Plowman 2001)). The median number of random points within individual home ranges was 323 (range = 32–897). Following Conner and Plowman (2001), we measured the straight-line distance from each random point to the nearest representative of each habitat type and calculated a habitat availability vector (r i ) as the average distance between random points and each habitat type within the population home range. We then measured the straight-line distance between the known locations of each individual (i.e., telemetry points) and calculated a habitat use vector (u i ) as the average distance between telemetry points and each habitat type within that respective home range. We created a vector of ratios for each tortoise by dividing each element in the vector of habitat use by the corresponding vector of habitat availability (d i = u i /r i ). Lastly, we calculated the average of vector ratios (mean vector = ρ), which we used for the landscape scale analysis (second-order habitat selection).
To test whether tortoises exhibited second-order (landscape scale) habitat selection, we performed a multivariate analysis of variance (MANOVA) under the null hypothesis that the mean ratios vector ρ did not differ from a vector of 1 s (i.e., habitat selection did not occur). The alternative hypothesis was that the mean vector ρ differed from a vector of 1 s (i.e., habitats were preferred non-randomly and habitat selection occurred). If the MANOVA test rejected the null hypothesis, we then used a two-tailed t-test for pairwise comparisons of mean vector ratios for each habitat type and obtained a ranking matrix for the six types of habitats in our study area. If the mean vector for a certain habitat (ρ habitat) was <1, we can conclude that the corresponding habitat was used more than expected. If ρ habitat >1, the corresponding habitat was avoided more than expected (Conner and Plowman 2001). This pattern can be interpreted as selected (ρ habitat <1) or avoided (ρ habitat >1) habitats (Conner et al. 2003).
We repeated the same procedure (MANOVA followed by t-tests for pairwise comparisons among habitat types) to investigate third-order (home range scale) habitat selection for each tortoise. For each individual analysis, we only used random points that fell within each individual home range and telemetry points for the respective tortoise to build the vectors of availability and habitat use, respectively. To comply with the assumptions for parametric methods, we based the MANOVA and t-tests on 999 randomizations of the data (Manly 1997).
All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). We tested the normality using Shapiro–Wilk test (Zar 2010). Alpha level was 0.05 for all tests.
Movement analyses
For the analysis of movement, we measured the distance between radiolocations from consecutive days (i.e., step lengths in meters) using the Movements tool from software Biotas2a. Thus, we obtained a daily movement metric (m/day), which we further separated into movements between different habitat types and intra-grassland and intra-forests movements (i.e., movements within the respective habitats that did not intersect other habitat types). We tested for seasonal and gender-specific differences in daily distance traveled, intra-grassland, and intra-forest movements using t-test (for Gaussian-distributed data) or non-parametric Mann–Whitney and Kruskal–Wallis tests (for non-normal data). In addition, we investigated the movement patterns of both males and females on a monthly basis to identify high- versus low-activity periods. We performed non-parametric multiple comparisons among months using Dunn’s test (Zar 2010).
Results
Population and individual home ranges
The number of locations for each individual ranged between 25 and 40 (median = 36). The size of the population home range (62.18 ha) reached an asymptote after including 71 % from all radio-tracking points.
The average size (± 1 SE) of annual individual home ranges was 3.79 ± 0.62 ha (range = 0.52–10.84), with no significant differences between males and females (Mann–Whitney U = 41, p = 0.12) (Table 1). Moreover, there were no significant differences between home range sizes during study years (Kruskal–Wallis H = 1.54, df = 2, p = 0.46), during pre- and post-nesting seasons (Mann–Whitney U = 149, p = 0.09), and between the seasonal home range sizes by gender (pre-nesting, Mann–Whitney U = 21, p = 0.13; post-nesting, Mann–Whitney U = 47, p = 0.23). There was a negative correlation between body weight and home range size for males (Spearman rho = −0.6, p < 0.0001); no correlation was found for females (Spearman rho = −0.22, p = 0.22).
Habitat selection
Hermann’s tortoises showed strong habitat selection at the landscape scale (second-order). The distances from random locations within individual home ranges to the nearest habitats were different from the distances from random locations within population home ranges to the nearest habitats (F 6,18 = 19.38, p < 0.001). Tortoises showed habitat selection (ρ<1; Table 2) towards grasslands (t = −8.31, p < 0.001), shrubs (t = −4.30, p = 0.0003), and forest edges (t = −3.44, p = 0.002). The only habitat feature with a ratio greater than 1 (i.e., avoided) was represented by creeks (t = 5.44, p < 0.001). The forest habitats (t = 0.29, p = 0.77) and roads were selected at random (t = −1.21, p = 0.24). The pairwise comparison of distance ratios associated with each habitat indicated that individual home ranges were found to be significantly closer to grasslands than to forests, shrubs, and creeks (Table 3).
Distance-based habitat selection analyses at the individual home range scale (third-order) indicated that tortoise locations differed from random locations (F 6,18 = 23.04, p < 0.001). Grasslands (t = −11.47, p < 0.001), shrubs (t = −11.3, p < 0.001), and roads (t = −2.84, p = 0.009) were selected (ρ<1; Table 2), therefore closer to tortoise locations than expected. The only habitat selected randomly was forest edge (t = −0.91, p = 0.37). Tortoises were located at a greater distance than expected from forests (t = 6.5, p < 0. 001) and creeks (t = 4.07, p = 0.0005). The pairwise comparison of distance ratio associated with each habitat indicates that forests were most avoided and grasslands were preferred in comparison to other habitat features (Table 3).
Using pre- and post-nesting telemetry points separately, habitat selection was random at the landscape scale, and there were no differences between the pre- and post-nesting seasons (F 6,30 = 0.02, p = 0.99).
Daily, monthly, and seasonal movements
We recorded a total of 816 locations for all tortoises during the study period. The average distance (± standard error) between consecutive relocations was 41.10 ± 2.16 m (range = 0–558.15 m). From all tortoise relocations, 424 were day-to-day movements, with an average of 31.18 ± 1.59 m (range = 0–190.73 m) daily distance traveled (Fig. 2). There were no differences (t = 0.6, p = 0.95) in the average daily distance traveled by females (31.11 ± 2.14 m; range = 0–191 m) and by males (31.31 ± 2.31 m; range = 0–171 m).
As expected, tortoise movements varied by gender on a monthly basis (males, Kruskal–Wallis H = 19.66, df = 6, p = 0.003; females, Kruskal–Wallis H = 26.70, df = 6, p < 0.001) with significantly longer daily movements by males in August (average = 40.58 m/day) and by females in July (average = 45.27 m/day). When compared with data in July (a month with large movements for both sexes), males moved significantly less at the beginning of the spring season (April: average = 10.19 m/day; Dunn’s test z = 2.83, p = 0.005) as well as the beginning of fall (September: average = 22.58 m/day, z = 2.87, p = 0.006). Females moved significantly less in fall (September: average = 21.49 m/day; z = 4.82, p < 0.001) (Fig. 3).
We detected 261 intra-grassland movements, with an average of 30.01 ± 33.69 m (range = 0–191 m) in distance moved. No gender-specific differences were recorded in the mean daily distance traveled (t = −1.56, p = 0.12) or the mean daily distance traveled intra-grassland by season (t = −1.14, p = 0.26) (Table 1). We recorded 55 consecutive intra-forest movements, with an average of 38.83 ± 5.02 m (range = 0.85–112 m) of distance traveled. Tortoises were recorded only accidentally in closed-canopy forested habitats at distances >100 m from the edge (maximum distance = 112.7 m; median = 27.97 m).
Furthermore, there were no gender-specific differences in the mean daily distance traveled intra-forest (Mann–Whitney U = 106, p = 0.78) or the mean daily distance traveled intra-forest by season (Mann–Whitney U = 87, p = 0.62).
We recorded 84 movements between forest and grassland habitat types (37 for males and 47 for females). The average distance moved (42.85 ± 3.83 m) was similar to the intra-grassland and intra-forest average movements.
Discussion
We found biologically significant habitat selection by eastern Hermann’s tortoises at both the landscape scale and home range scale. At the landscape scale, the habitat selection process was dominated by higher selection for grasslands over other habitat types, which suggests that grasslands are a critical habitat for tortoises. Forest edges and shrubs were also selected at the landscape scale, while forests, and roads are randomly selected in relation to each other. This selection pattern confirms the cumulative value of the three types of habitats—grasslands, shrubs, and forest edges—and suggests that tortoises base their home ranges on an association of habitats and not on a particular habitat type (e.g., grasslands). In our study, forest edges were an important habitat type for tortoises, and its importance has been demonstrated for other land tortoises, such as Testudo graeca (Anadon et al. 2007).
At the individual home range scale, the habitat selection process was more prominent compared to the landscape scale. Grasslands were the preferred habitat at the individual home range scale, followed by shrubs and forest edges. In Iron Gates Natural Parks, grassland habitats are managed primarily as hayfields (Matacă 2005) and are a critical habitat for feeding, basking, and egg laying (Rozylowicz 2008). Grassland patches also provide shelter during the pre-mowing season (which occurs in late summer) when the herbaceous vegetation can reach >0.5 m in height (Matacă 2002). At our study sites, shrubs and forests edges were mostly used for thermoregulation and overwintering, and such use is supported by data from behavioral studies of other reptile species (e.g., Blouin-Demers and Weatherhead 2001, 2002). Overwintering locations for the monitored tortoises were forest edges with deep leaf litter and understory dominated by Rubus ssp. or Carpinus orientalis (personal observation; see Fig. 1). The partial inconsistencies in habitat selection between the two scales of analysis could be attributed to locations of individual home ranges with respect to particular habitat features. For example, at the individual home range scale, tortoises showed a higher affinity for roads, which in our study area are represented by unpaved forestry roads with very low levels of traffic that do not pose a high mortality risk (Iosif 2012). Therefore, this result is likely an outcome of roads potentially acting as a low-resistance habitat that facilitate movements (Iosif 2012), as well as due to individual home ranges being located in proximity to valley bottoms with less steep topography, where roads also tend to occur.
The average individual annual home range size recorded in this study (3.79 ± 0.62 ha) is in agreement with those of other studies of both the eastern and western Hermann’s tortoise subspecies (Cheylan 2001; Longepierre et al. 2001; Loy and Cianfrani 2010; Luiselli et al. 2009; Hailey 1989). Nonetheless, there was high variability among individuals (differences up to 20 times), which is characteristic for herpetofauna (Pough 2004). Such differences may be a function of tortoise gender and age (Pough 2004) and the quality of the habitat inhabited (Del Vecchio et al. 2011b). In contrast to other studies (e.g., Mazzotti et al. (2002); Luiselli et al. (2009); Longepierre et al. (2001)), we found no significant gender-specific difference in home range size. This finding is potentially related to the landscape heterogeneity which allows for equal access to critical habitat resources (i.e., grasslands, shrubs, and forest edges) by all animals (Cheylan 2001). Pre- and post-nesting home range sizes were also similar, the seasonal variation—important in the study of ectothermic species—being smaller than for western Hermann’s tortoise species (e.g., Luiselli et al. 2009). This is likely another consequence of landscape heterogeneity in our study area, suggesting that tortoises have access to the same amount and types of habitat resources throughout the year.
Distances traveled by tortoises are influenced by changes in the availability and quality of food, thermal habitat characteristics, the suitability of habitat for nesting, wintering, or aestivation, as well as the reproductive status (e.g., search for mates) (Del Vecchio et al. 2011a, b; Huot-Daubremont and Grenot 1997; Hailey 1989; Willemsen 1991). Tortoise movement ecology in our study area is characterized by short-distance movements, apparently unaffected by habitat type. The daily average distance traveled by an individual tortoise was 31.18 m, with a maximum distance of 190.73 m. These movements are considerably lower compared to those of European land tortoises from semi-arid areas (e.g., 50 m for T. graeca in Spain (Diaz-Paniagua et al. 1995) and 80 m for T. hermanni in Greece (Hailey 1989)). Daily movements recorded in our study (see Fig. 2) are likely a result of the heterogeneity of our study area, with small habitat patches (and associated small inter-patch distances) meeting food (i.e., grassland) and thermoregulation (i.e., shrub, forest edge) requirements. Female tortoises traveled the same distances as males, in contrast with the findings of Hailey (1989), Longepierre et al. (2001), and Loy and Cianfrani (2010). Hermann’s tortoises moved at a similar low step-length within and between grasslands and forests, but in our study we recorded more intra-grassland than intra-forests moves. Thus, tortoises tend to avoid entering a forest habitat, which is only used as a temporary refuge habitat. A plausible explanation for the similarity of within- and between-habitat movements is that our study landscape has a very heterogeneous composition and configuration (e.g., small distances among diverse critical habitat resources). In addition, tortoise density in our study area (42 individuals/ha; Rozylowicz 2008) was close to the maximum density recorded for T. hermanni (45 individuals/ha; Cheylan 2001). Potentially, these factors make long movements in search for mates (i.e., for males) or critical habitat resources (i.e., nesting or high-quality feeding habitat for females) unnecessary.
Furthermore, pre-nesting (April–June) and post-nesting (July–October) season movements were similar in contrast to the study of Cruce and Raducan (1976), which found that tortoises traveled across greater distances in the pre-nesting season in search of mates. However, the analysis of monthly movements revealed periods of high versus low daily movements, which is to be expected given the biology and ecology of tortoises. The daily movements steadily increased in magnitude from April (the spring month with lowest distances traveled by both males and females) until August (for males) or July (for females), which were the most active months (Fig. 3). One would expect male movements to peak before females’ as males travel longer distances during the mating season and females increase movement activity post-mating in search of nesting sites. Therefore, the reversal of this pattern deserves further discussion. A simple explanation is that males could still be actively looking for mates through late July–August, after the females laid eggs. In addition, there is evidence of sperm storage from one year to another in some Hermann’s tortoise populations (Loy and Cianfrani 2010). If present in our study population, this process might determine longer female movements earlier in the season resulting from searches for nesting locations. Beginning in September, the activity is decreasing for both males and females.
In general, small changes in the availability of critical resources were found to influence site persistence in Hermann’s tortoises. For example, at a fine scale, Hermann’s tortoise occurrence was found to be highly dependent on particular plant species associated with patches of suitable habitat embedded within a matrix of unsuitable habitats (Del Vecchio et al. 2011a, b). At a coarser scale, Rozylowicz and Dobre (2010) found that populations from the eastern part of Hermann’s tortoise Romanian range (i.e., east of our study area) did not persist due to conversions of native forest and grassland habitat to croplands or intensively managed pastures despite suitable climate conditions (Rozylowicz 2008). Particularly to our study area, the availability of traditionally managed grasslands is likely a limiting factor for tortoise populations as the landscape has evolved over centuries of human inhabitation. Loss of such habitat through afforestation of abandoned grasslands or conversion to intensive management that creates homogenous landscapes (e.g., cropland) has the potential to lead to extirpation of local populations. From a management and conservation standpoint, we recommend that management efforts for Hermann’s tortoise should focus on maintaining traditional land uses, increasing landscape heterogeneity of preferred habitats (grasslands, shrubs, and forest edges), and avoiding both the abandonment and the intensive use of grasslands.
References
2006/105/EC (2006) Council Directive 2006/105/EC of 20 November 2006 adapting Directives 73/239/EEC, 74/557/EEC and 2002/83/EC in the field of environment, by reason of the accession of Bulgaria and Romania. Official Journal L 363, 20/12/2006
92/43/EEC (1992) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora, vol 92/43/EEC. Official Journal, L 206, 22.7.1992
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74(5):1313–1325
Anadon JD, Gimenez A, Martinez M, Palazon JA, Esteve MA (2007) Assessing changes in habitat quality due to land use changes in the spur-thighed tortoise Testudo graeca using hierarchical predictive habitat models. Divers Distrib 13(3):324–331
Bertolero A, Cheylan M, Hailey A, Livoreil B, Willemsem RE (2011) Testudo hermanni (Gmelin 1789)—Hermann’s tortoise. In: Rhodin AGJ, Pritchard PCH, van Dick PP, Saumure RA, Buhlmann KA, Iverson JB, Mittermeier RA (eds) Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs, 5, 059.1–059.20
Blouin-Demers G, Weatherhead PJ (2001) Habitat use by black rat snakes (Elaphe obsoleta obsoleta) in fragmented forests. Ecology 82:2882–2896
Blouin-Demers G, Weatherhead PJ (2002) Habitat-specific behavioural thermoregulation by black rat snakes (Elaphe o. obsoleta). Oikos 97:59–68
Börger L, Dalziel BD, Fryxell JM (2008) Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol Lett 11(6):637–650
Calzolai R, Chelazzi G (1991) Habitat use in a Central Italy population of Testudo hermanni Gmelin (Reptilia-Testudinidae). Ethol Ecol Evol 3(2):153–166
Chen J, Saunders SC, Crow TR, Naiman RJ, Brosofske KD, Mroz GD, Brookshire BL, Franklin JF (1999) Microclimate in forest ecosystem and landscape ecology. Bioscience 49(4):288–297
Cheylan M (2001) Testudo hermanni Gmelin, 1789 – Griechische Landschildkröte. In: Fritz U (ed) Handbuch der Reptilien und Amphibien Europas. Band 3/IIIA: Schildkröten I. Aula-Verlag, Wiebelsheim, pp 179–289
Conner LM, Plowman BW (2001) Using Euclidean distances to assess non-random habitat use. In: Millspaugh JJ, Marzluff JM (eds) Radio tracking and animal populations. Academic, San Diego, pp 275–289
Conner LM, Smith MD, Burger LW (2003) A comparison of distance-based and classification-based analyses of habitat use. Ecology 84(2):526–531
Conner LM, Smith MD, Burger LW (2005) A comparison of distance-based and classification-based analyses of habitat use: reply. Ecology 86(11):3125–3129
Cox NA, Temple HJ (2009) European red list of reptiles. Office for Official Publications of the European Communities, Luxembourg
Cruce M, Răducan L (1975) Cycle d’activité chez la tortue terrestre (Testudo hermanni hermanni Gmel.). Rev Roum Biol (Biol Anim) 20:285–289
Cruce M, Raducan L (1976) Reproducerea la broasca ţestoasă de uscat (Testudo hermanni hermanni G.). Studii şi cercetări de Biologie. Seria Biologie Animală 28:175–180
Del Vecchio S, Burke RL, Rugiero L, Capula M, Luiselli L (2011a) Seasonal changes in the diet of Testudo hermanni hermanni in Central Italy. Herpetologica 67(3):236–249
Del Vecchio S, Burke RL, Rugiero L, Capula M, Luiselli L (2011b) The turtle is in the details: microhabitat choice by Testudo hermanni is based on microscale plant distribution. Anim Biol 61(3):249–261
Diaz-Paniagua C, Keller C, Andreu AC (1995) Annual variation of activity and daily distances moved in adult spur-thighed tortoises, Testudo graeca, in Southwestern Spain. Herpetologica 51(2):225–233
Downs JA, Horner MW (2008) Effects of point pattern shape on home-range estimates. J Wildlife Manage 72(8):1813–1818
Edge CB, Steinberg BD, Brooks RJ, Litzgus JD (2010) Habitat selection by Blanding's turtles (Emydoidea blandingii) in a relatively pristine landscape. Ecoscience 17(1):90–99
Fernández-Chacón A, Bertolero A, Amengual AD, Tavecchia G, Homar V, Oro D (2011) Spatial heterogeneity in the effects of climate change on the population dynamics of a Mediterranean tortoise. Global Change Biol: 17(10):3075–3088
Hailey A (1989) How far do animals move? Routine movements in a tortoise. Can J Zool 67(1):208–215
Huot-Daubremont C, Grenot C (1997) Activity rhythm of Hermann tortoise (Testudo hermanni hermanni) in semi-free state in the Massif des Maures (Var). La Terre et la Vie-Revue d’Ecologie 52(4):331–344
Ioja CI, Patroescu M, Rozylowicz L, Popescu VD, Verghelet M, Zotta ML, Felciuc M (2010) The efficacy of Romania's protected areas network in conserving biodiversity. Biol Conserv 143(11):2468–2476
Iosif R (2012) Evaluating the potential impacts of transportation infrastructure on Hermann’s tortoise (Testudo hemanni boettgeri) populations in Romania. MS thesis, University of Bucharest, Romania
Johnson DH (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Kernohan BJ, Gitzen RA, Millspaugh JJ (2001) Analysis of animal space use and movements. In: Millspaugh JJ, Marzluff JM (eds) Radio tracking and animal populations. Academic, San Diego, pp 125–166
Longepierre S, Hailey A, Grenot C (2001) Home range area in the tortoise Testudo hermanni in relation to habitat complexity: implications for conservation of biodiversity. Biodivers Conserv 10(7):1131–1140
Loy A, Cianfrani C (2010) The ecology of Eurotestudo h. hermanni in a mesic area of southern Italy: first evidence of sperm storage. Ethol Ecol Evol 22(1):1–16
Luiselli L, Rugiero L (2006) Ecological modelling of habitat use and the annual activity patterns in an urban population of the tortoise, Testudo hermanni. Ital J Zool 73(3):219–225
Luiselli L, Rugiero L, Celleti S, Papi R, Gracceva G, Stacchiotti M, Mancini F, Berretta G, Berretta L, Bombara G, Fiaschetti R, Lucioli E, Trionfetti MG, Ungaro A (2009) Autumnal home range in radio-tracked tortoises (Testudo hermanni) from a semi-arid mediterranean environment. La Terre et la Vie-Revue d’Ecologie 64(1):73–78
Manly BFJ (1997) Randomization, bootstrap and Monte Carlo methods in biology, 2nd edn. Texts in statistical science. Chapman and Hall, London
Manly BFJ (2002) Resource selection by animals: statistical design and analysis for field studies, 2nd edn. Kluwer Academic, Dordrecht
Matacă ŞS (2002) Vegetaţia forestieră şi arbustivă din Parcul Natural Porţile de Fier. Drobeta XI-XII:296–336
Matacă ŞS (2005) Parcul Natural Porţile de Fier. Floră, vegetaţie şi protecţia naturii. Editura Universitaria, Craiova
Mazzotti S, Pisapia A, Fasola M (2002) Activity and home range of Testudo hermanni in Northern Italy. Amphibia-Reptilia 23(3):305–312
Pătroescu M, Rozylowicz L (2000) Natural transborder parks: the direction of biodiversity preservation in Romania. In: Crabbé P, Holland A, Ryszkowski L, Westra L (eds) Implementing ecological integrity: restoring regional and global environmental and human health, vol 1. NATO Science series: IV: earth and environmental sciences, vol IV: earth and environmental series. Kluwer Academic, Dordrecht
Phillips A (2002) Management guidelines for IUCN Category V Protected Areas Protected Landscapes/Seascapes. World Commission on Protected Areas (WCPA), Best Practice Protected Area Guidelines Series No. 9. IUCN Gland, Switzerland and Cambridge, UK
Pough FH (2004) Herpetology, 3rd edn. Prentice Hall, Upper Saddle River
Row JR, Blouin-Demers G (2006) Kernels are not accurate estimators of home-range size for herpetofauna. Copeia 2006(4):797–802
Rozylowicz L (2008) Metode de analiză a distribuţiei areal-geografice a ţestoasei lui Hermann (Testudo hermanni Gmelin, 1789) în România. Studiu de caz: Parcul Natural Porţile de Fier. Editura Universităţii din Bucureşti, Bucureşti
Rozylowicz L, Dobre M (2010) Assessing the threatened status of Testudo hermanni boettgeri Mojsisovics, 1889 (Reptilia: Testudines: Testudinidae) population from Romania. North-West J Zool 6(2):190–202
Willemsen RE (1991) Differences in thermoregulation between Testudo hermanni and Testudo marginata and their ecological significance. Herpetol J 1(12):559–567
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson Prentice Hall, Upper Saddle River
Acknowledgments
This work was supported by a grant of the Romanian National Authority for Scientific Research, CNCS—UEFISCDI, project number PN-II-RU-TE-2011-3-0183. The study has been carried out with the logistic support of the Iron Gates Eşelniţa Field Center, Center for Environmental Research and Impact Studies. We are grateful to our colleagues who helped us with radio-tracking and study design: Maria Pătroescu, Mariana Dobre, Steluţa Manolache, Vasile Bagrinovschi, Georgeta Bagrinovschi, Iulian Niculae, Cristian Tetelea, Gabriel Vânău, and Mihai Răzvan Niţă. We would like to thank two anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Rozylowicz, L., Popescu, V.D. Habitat selection and movement ecology of eastern Hermann’s tortoises in a rural Romanian landscape. Eur J Wildl Res 59, 47–55 (2013). https://doi.org/10.1007/s10344-012-0646-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-012-0646-y