Abstract
Context
Theory predicts that movement limitation due to landscape fragmentation can reduce population viability. Understanding how landscape heterogeneity influences movement is thus critical for testing theory and developing conservation strategies. Consequently, studies are needed that link movement data with landscape features influencing dispersal.
Objectives
We used experimental translocations to test whether forest fragmentation constrains movements of two fragmentation-sensitive bird species. We also tested for evidence of multiple behavioral movement phases (i.e., exploring, homing) and evaluated whether fragmentation effects varied between them.
Methods
Over two breeding seasons we translocated territorial Wood Thrushes (Hylocichla mustelina; n = 36) and Ovenbirds (Seiurus aurocapilla; n = 19) 1–1.2 km across landscapes spanning a fragmentation gradient and recorded spatial (movement path) and temporal (return time) homing data using VHF transmitters and receivers.
Results
Ninety-one percent of individuals returned home, taking up to 72.2 h. Movements of 98% of returning birds indicated distinct exploring (i.e., short, undirected movements and course reversals) and homing (i.e., large, fast steps towards home) movement phases. Both species chose steps minimizing gap exposure in both phases. However, landscape fragmentation had no negative effect on homing times or path straightness.
Conclusions
Our results suggest movement limitation does not drive fragmentation sensitivity in these species. Discrepancy between step- and path-level analyses either indicate that fine-scale movement data do not reflect landscape connectivity, or that artificially motivated animals respond unnaturally to behavioral barriers. Given evidence for dichotomous movement behavior, future studies linking these behaviors to life stages will elucidate when and how landscape features influence movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation are some of the greatest threats to biodiversity worldwide (Rands et al. 2010; Haddad et al. 2015). These processes alter habitat structure, resource availability, and interactions among species (Ries et al. 2004; Fletcher et al. 2007), they create smaller habitat patches with more extinction-prone populations (Hanski 1998; Fahrig 2003), and they hinder patch colonization by reducing the availability of proximal dispersers (Fahrig 2003, 2013). Yet demographically viable populations can be supported in fragmented landscapes when animals can move among patches (i.e., when the landscape is functionally connected; Fahrig and Merriam 1985; Beier and Noss 1998). Thus, understanding how landscape heterogeneity influences animal movement is one of the greatest challenges facing ecologists (Taylor et al. 1993; Bélisle 2005).
Functional connectivity is defined as ‘the degree to which a landscape facilitates or impedes movement’ (Taylor et al. 1993). While there is disagreement over the term’s use (e.g., Tischendorf and Fahrig 2000; Moilanen and Hanski 2001; Tischendorf and Fahrig 2001), here we adopt the perspective that functional connectivity is a landscape-scale metric (Tischendorf and Fahrig 2000, 2001) resulting from intrinsic characteristics of mobile organisms (i.e., motion and navigation capacity; Nathan et al. 2008) interacting with spatial and environmental constraints imposed by the landscape (Taylor et al. 1993; Tischendorf and Fahrig 2000; 2001; Harris and Reed 2002; Bélisle 2005; Baguette and Van Dyck 2007; Vasudev et al. 2015). Because of this complex relationship, understanding functional connectivity requires knowledge of how animals assess and move through their environments (Tischendorf and Fahrig 2000; Bélisle 2005; Baguette and Van Dyck 2007; Vasudev et al. 2015). However, such information is rarely incorporated into connectivity modeling (Zeller et al. 2012; Vasudev et al. 2015). For example, ecologists often describe connectivity among patches solely in terms of inter-patch distance ignoring complexities in the structure of real landscapes (Tischendorf and Fahrig 2000; Ricketts 2001; Tischendorf and Fahrig 2001; Castellón and Sieving 2006; Kennedy and Marra 2010). When more complicated resistance surfaces are considered, these tend to be informed by habitat preferences rather than actual movement data (Tischendorf and Fahrig 2000; Zeller et al. 2012; Vasudev et al. 2015) which can result in biased connectivity assessments (e.g., Vasudev and Fletcher 2015). Further, both approaches usually ignore species and individual differences in mobility or navigational capacity which are fundamental components of how animals move through a landscape (Lima and Zollner 1996; Nathan et al. 2008). Thus, there is a need to transcend a connectivity framework focused on where organisms might disperse, towards one that uses actual movement data to identify dispersal limitations (Tischendorf and Fahrig 2000; Bélisle 2005; Baguette and Van Dyck 2007; Vasudev et al. 2015).
One major challenge to quantifying functional connectivity is accounting for an individual’s internal motivation, which can be influenced by past experiences, or localized predator densities, food availability, and habitat quality (Bélisle 2005; Betts et al. 2015). Thus, experimental manipulations that standardize motivation among individuals likely provide the most meaningful assessments of functional connectivity (Desrochers et al. 1999; Bélisle 2005). One such approach involves translocating territorial individuals to provide motivation to move towards a specific destination (i.e., home). This technique has been used for evaluating connectivity for diverse taxonomic groups, including mammals (e.g., Smith et al. 2011), birds (e.g., Bélisle et al. 2001), reptiles (e.g., Butler et al. 2005), fish (e.g., Turgeon et al. 2010), and insects (e.g., Fletcher et al. 2013).
Despite widespread use of experimental translocations, little is known about how the method itself influences movement behavior (Betts et al. 2015). Theory posits that variation in the spatial and temporal patterning of movement behavior (i.e., movement phases) should reflect variable movement goals (e.g., foraging, acquiring territory, or avoiding predation; Getz and Saltz 2008; Nathan et al. 2008). Further, these behaviors can profoundly affect connectivity assessments (Morales and Ellner 2002). Yet, while behavioral variability has been noted in translocated animals (e.g., Reinert and Rupert 1999; Heidinger et al. 2009; Tsoar et al. 2011), its effect has largely been ignored. Translocated animals often initially exhibit exploratory movements associated with orientation (e.g., Reinert and Rupert 1999; Tsoar et al. 2011; Kesler et al. 2012) or may require long bouts of rest and foraging to recover from translocation stress (Betts et al. 2015). Such movements may heavily influence typical metrics recorded in translocation experiments such as homing time (e.g., Bélisle et al. 2001; Bélisle and St. Clair 2001; Gobeil and Villard 2002), travel distance (e.g., Hadley and Betts 2009), or path tortuosity (e.g., Hadley and Betts 2009; Biz et al. 2017), yet poorly reflect an individual’s ability to navigate landscape obstacles. Further, the effects of landscape features may differ among movement phases, providing opportunities to understand how functional connectivity varies with behavior.
Here we use experimental translocations to evaluate the effects of forest composition and configuration on movements of translocated Wood Thrushes (Hylocichla mustelina) and Ovenbirds (Seiurus aurocapilla). While it has been hypothesized that migratory birds are unlikely to be limited by movement barriers (Harris and Reed 2002), empirical studies suggest this is not always true (Bélisle et al. 2001; Bélisle and St. Clair 2001; Tremblay and St. Clair 2011), and evidence for the impact of fragmentation on functional connectivity for these two species is mixed. Both are known to disperse upwards of 80 km across complex landscapes (Hobson et al. 2004; Tittler et al. 2006) and exhibit an ability to cross forest gaps during routine movements (Bayne and Hobson 2001; MacIntosh et al. 2011). However, patch abundance (Lynch and Whigham 1984; Robbins et al. 1989) and occupancy rates (Villard et al. 1999; Valente and Betts 2019) for both species are negatively influenced by forest fragmentation (i.e., they exhibit fragmentation sensitivity), and mechanistic models show this pattern is at least partially driven by inter-patch distances (Villard et al. 1995). Further, Ovenbird homing abilities consistently decrease with decreasing forest cover (Bélisle et al. 2001; Gobeil and Villard 2002; Desrochers et al. 2011). These results imply fragmentation may negatively influence functional connectivity during some movement phases, but this has not been rigorously evaluated with fine-scale movement data.
Our study was designed to test two hypotheses. The first is that forest loss and fragmentation affect movement decisions and therefore will reduce functional connectivity for Wood Thrushes and Ovenbirds. If true, we expect individuals to choose fine-scale movement steps that minimize exposure to non-forested areas; at broader scales, these movement decisions should result in longer homing times and more tortuous paths. Secondly, we test whether translocated individuals exhibit dichotomous movement phases (i.e., exploring and homing), and evaluate: (1) if and how these behaviors influence connectivity assessments; and (2) whether the effects of fragmentation differ among movement phases. Results from this study will improve our understanding of when and how fragmentation influences movement decisions, as well as the information that can be gleaned from translocation experiments.
Methods
Study sites and translocations
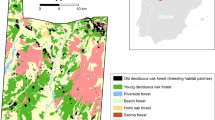
During the breeding seasons of 2015 and 2016 we conducted translocation experiments at two properties in southern Indiana, USA (Fig. 1). Naval Surface Warfare Center Crane is a Department of Defense property characterized by large, contiguous tracts of forest, whereas Glendale Fish and Wildlife Area is an Indiana Department of Natural Resources property dominated by small forest fragments interspersed with agricultural fields. Full details about the site selection and translocation process are provided in Online Resource 1. Briefly, we used stratified random sampling to choose 17 landscapes (4 km2) that spanned a gradient in forest cover (mean 68.20%, SD 17.96) and number of patches (mean 20.29, SD 13.48). Within each, we identified, male Wood Thrushes and Ovenbirds and confirmed territoriality using behavioral observations (i.e., presence of a nest or territorial singing on 3 consecutive days) to maximize the likelihood they would be motivated to return. We attempted to translocate two conspecifics (from the same patch, where possible) in each landscape; one individual was challenged by having to cross multiple gaps during homing, while the other had predominantly contiguous forest between the release site and its home territory (Fig. 1). We captured identified birds in mist nets, measured mass and tarsus length to assess body condition, ensured there was evidence of breeding (cloacal protuberance), attached a 0.7 g Pip Ag376 VHF transmitter (Biotrack Ltd., Wareham, UK) using a leg loop harness (Rappole and Tipton 1991), and translocated them to a release site 1–1.2 km away. Mean time between capture and release was 59.85 min (SD 13.25).
Locations of field sites (Naval Surface Warfare Center Crane and Glendale Fish and Wildlife Area) used for experimental translocations of Ovenbirds and Wood Thrushes in southern Indiana. Crane was dominated by large contiguous forest tracts, separated by small road gaps (a), while Glendale was a more heterogeneous mix of forest and agricultural fields (b). We chose multiple landscapes on each site and attempted to translocate two conspecifics from the same forest patch across local landscapes (ellipses) that varied in terms of the amount of forest and number of forest patches
Tracking
Post translocation, technicians immediately began tracking bird movements from dawn until dusk using handheld TRX-1000S telemetry receivers (Wildlife Materials, Inc., Murphysboro, IL). Observers recorded the birds’ locations every 20 min, or more frequently when birds were moving quickly (mean 13.32 min, SD 27.33). Location information consisted of a GPS point of the observer’s position, an estimated distance to the bird based on telemetry receiver strength (calibrated through extensive field testing), and a directional compass bearing. Observers attempted to stay within 50 m of the bird though maintained greater distances when their presence appeared to incite movements (i.e., flushing the bird; e.g., Hadley and Betts 2009; Volpe et al. 2014). Accuracy of location estimates recorded > 100 m from the bird were unreliable and discarded. Seventy percent of retained points were recorded within 50 m of the bird and 93% within 75 m. Monitoring continued continuously for 4 days, or until the bird returned home. Successful return was defined by a bird being located within 100 m of its capture location. We assumed two additional Wood Thrushes were home at 250 m from the capture site because their behaviors (e.g., feeding fledglings) indicated they were on or very close to their territory. Birds that did not return home within 4 days were located daily and deemed a homing failure on the 10th day. Logistical constraints prevented us from tracking birds between sundown and sunrise (both years), and between approximately 1200 h and 1600 h (2015 only). Though this resulted in the loss of some fine scale movement data, an observer was present for the homing event of every individual, resulting in unbiased homing times.
Data analyses
In total, we translocated 36 Wood Thrushes and 19 Ovenbirds. However, we chose to use only data from the individuals that successfully homed (34 Wood Thrush and 15 Ovenbirds) in analyses because: (1) we could not distinguish exploratory and homing movements for non-returning birds; and (2) most unsuccessful individuals were predated (n = 2) or dropped their transmitters (n = 1). For the 49 remaining birds, data analysis consisted of three broad steps. First, we tested for evidence of two distinct movement phases within individuals using behavioral change point analysis (BCPA; Gurarie et al. 2009). Secondly, we tested for fragmentation effects on step choices, where a step is defined as the incremental movement made between two subsequent GPS points. Lastly, we tested for fragmentation effects on path-level movement data (i.e., homing time and path straightness), where the path was the entire sequence of steps from the time birds were released until they returned home. All steps were used in the BCPA and path-level analyses. For the step-level analyses, we eliminated steps where start and endpoints were recorded over 20 min apart, or that were less than 25 m in length. While, only 44% of all steps were included in the step-level analyses (Wood Thrush n = 945; Ovenbird n = 621), our approach ensures these steps represented relatively straight-line movements and were not dominated by telemetry error, respectively, and follows established precedent (e.g., Gillies et al. 2011; Volpe et al. 2014).
Behavioral change point analysis
BCPA is a robust method for objectively identifying changes in movement behavior that does not require equally spaced sampling times nor a priori assumptions about where and when behavioral changes occur (Gurarie et al. 2009). For each bird, we conducted a BCPA on the temporal series of persistence velocities (Vp), which measures the tendency and magnitude of movements to persist in the same direction (Gurarie et al. 2009). Each step a bird took (mean 79.33 steps per bird, SD 57.51) was characterized by the time it was recorded (t). Persistence velocity for the step was calculated as
where Vt is the speed of movement, and θt is the angular change in trajectory from the previous step. For each bird, which took a total of T steps, we iteratively split the time series at every Vpt, and fit an autocorrelated time series sub-model to each data subset:
Here, µ represents the mean persistence velocity, and ρ represents the autocorrelation between two observations, which decreases exponentially as a function of the time interval between them (τ). The subscript i = 1,2 represents the movement phase, and j = 1, 2, …, t when i = 1, and j = t + 1, t + 2, …, T when i = 2. For each iteration, we recorded the likelihood for the full model as the product of the likelihoods from the two sub-models; we chose the value of t* where this full likelihood was maximized as the most probable behavioral change point (BCP; Gurarie et al. 2009). We chose to split the data for each bird into only two periods (i.e., one change point) to objectively test our hypothesis that individuals would switch from exploring to homing.
This procedure identifies the most likely BCP, though a model assuming constant behavior could be more plausible. Thus, once we had identified t*, we fit a null model to the data that assumed all parameters (µ, ρ, and σ) were identical on both sides of the BCP, along with seven additional models that allowed one, two, or three of the parameters (μ, ρ, σ) to vary (Gurarie et al. 2009). We compared models using Akaike Information Criterion adjusted for small samples size (AICc) and concluded there was no behavioral change if the null model had the most support. Further, while the BCPA tells us when movement behavior changes, it does not identify how behaviors differ, or whether they were indicative of ‘exploring’ and ‘homing’ as hypothesized. Thus, we aggregated the steps from all individuals and compared their lengths, speeds, turning angles, and deviation angles (angular difference between step direction and a direct line path home) before and after the BCP. We compared log-transformed step length and speed using linear mixed effects models that included a random intercept for ‘individual.’ We compared the distribution of turning and deviation angles (which have circular distributions) among behavioral phases using Kolmogorov–Smirnov tests. All analyses were conducted in R (v. 3.3.3) using additional functions from the bcpa (Gurarie 2014) and nlme (Pinheiro et al. 2017) packages.
Step-level analyses
We used step selection functions (i.e., mixed conditional logistic regression) to model factors influencing the probability of an individual taking a step given available options (Fortin et al. 2005; Duchesne et al. 2010). For each step chosen by an individual, we generated 20 unused steps by: (1) calculating the percentage of steps that fell into 10 m (step length) and 0.2 rad (turning angle) bins for each individual; (2) averaging these percentages across all other individuals of the same species (to avoid circularity; Fortin et al. 2005); and (3) randomly drawing lengths and turning angles from this average distribution (Fortin et al. 2005; Gillies et al. 2011; Volpe et al. 2014). We modeled the effects of all explanatory variables with random, individual-specific regression coefficients. Models were fit using the gmnl() function in the gmnl R package (Sarrias and Daziano 2017), and all covariates were standardized.
For each species, we began with a baseline model that included 4 covariates. The first indicated whether the step endpoint landed in forest (1) or not (0; hereafter, ENDFOR). We included this covariate because 93% of used steps ended in forest, while only 86% of unused steps did, and we expected it to significantly influence choice. Though it is highly unlikely either species would land in an open area, we retained steps that did not end in forest because the resolution of our aerial imagery was likely insufficient to identify isolated trees or narrow fencerows acting as stepping stones (Online Resource 1). Second, we included a measure of the distance between the step endpoint and the capture location (hereafter, CAPDIST) to account for directional motivation. Additionally, we expected directional affinity to change between exploring and homing phases, so we included an interaction between CAPDIST and an indicator of whether the bird was exploring (0) or homing (1; hereafter, HOME). Lastly, we included a covariate representing distance between the step endpoint and the nearest riparian zone (hereafter, RIPDIST). While we were unsure if and how this covariate would affect step choice, we wanted to account for it because streams are known to both impede (Bélisle and St. Clair 2001; St. Clair 2003) and facilitate (Gillies and St. Clair 2008; Hadley and Betts 2009; Volpe et al. 2014) avian movement. We considered an interaction between RIPDIST and HOME, but this model had low support and the interaction was dropped.
We then identified three variables to characterize steps in terms of exposure to non-forested habitat (i.e., fragmentation). These included the number of forest gaps (GAPS), the proportion contained in forest (FOR%), and the total distance of forest gaps (GAPDIST). Each fragmentation variable was added to the baseline model with and without an interaction with HOME. These models allowed us to test whether the fragmentation variables influenced step choice (while accounting for baseline covariates), and whether these effects differed between movement phases. We did not include multiple fragmentation variables in any models simultaneously because they represent similar metrics (i.e., ratio of forest to non-forest) and thus were highly correlated with one another (Table S1, Online Resource 2). However, we tested models incorporating all three separately because we had no a priori reason to suspect one would be more important than the others. We compared the resulting 7 models (one baseline, 6 fragmentation) using AICc.
Path-level analyses
At the path level, we identified the local landscape to which individuals were exposed by drawing an ellipse using the capture and release locations as foci (Selonen et al. 2010). The minor axis was 500 m wide and the major axis was 1.4 times the distance between the capture and release sites (Fig. 1). Local landscapes encompassed, on average, 91.79% (SD = 14.78) of all points recorded per individuals. Within each ellipse, we quantified landscape composition as the proportion covered by forest (hereafter, PROPFOR), and configuration (i.e., fragmentation) by the number of forest patches (hereafter, PATCHES). PROPFOR ranged from 0.52 to 0.96 with a mean of 0.77 (SD = 0.14). PATCHES ranged from 1 to 25 with a mean of 9.08 (SD = 5.75). The Pearson’s correlation between PROPFOR and PATCHES was − 0.67.
We evaluated the effects of PROPFOR and PATCHES on log-transformed homing time and path straightness for the entire path, and in each of the behavioral phases separately using general linear models. We calculated straightness by dividing the length of the straight-line path between start and endpoints by the total distance traveled. Start and endpoints for total straightness were the release points and the last point recorded on the bird, respectively. The endpoint for the exploring phase and the starting point for the homing phase was the spatial location where the bird’s behavior changed.
If birds captured in more fragmented landscapes have lower physiological condition compared to those in contiguous areas, effects of fragmentation on homing could be confounded with condition (Bélisle 2005; Betts et al. 2015). Thus, for each response variable, we assembled a baseline model that included a ratio of body size to tarsus length as a measure of condition (see Online Resource 2, Table S2 for comparison with other condition metrics). The baseline model for all straightness responses also included a covariate for number of steps because distance traveled would almost certainly increase as the number of recorded locations increases. We then constructed four additional models containing linear predictors: 1) PATCHES, 2) PROPFOR, 3) PATCHES + PROPFOR, and 4) PATCHES + PROPFOR + PATCHES*PROPFOR. Evidence suggests there may be thresholds in habitat cover below which fragmentation has a stronger effect on species distributions (e.g., Andrén 1994; Pardini et al. 2010), which could imply fragmentation has a greater negative effect on dispersal movements when habitat cover is low. Thus, though PATCHES and PROPFOR were correlated (Table S1, Online Resource 2), we included them in the same models to test for such potential thresholds in movement limitation. Again, all covariates were standardized (see Online Resource 2, Table S1 for Pearson’s correlation), and we compared the resulting 5 models (one baseline, 4 composition/configuration) for each response variable using AICc.
Results
Of the 35 Wood Thrushes with known fates (one lost transmitter), 34 (97%) returned home. The one that failed did not cross a ~ 500 m road gap between the capture and release points and was last located ~ 6 km from its territory. Of the 19 translocated Ovenbirds, 15 (79%) returned home. Two were predated, one did not cross a ~ 500 m road gap, and the last made no substantial movements toward home over 10 days (despite having no gaps to cross). Among birds that returned, homing times ranged between 3.1 and 72.2 h, and Wood Thrushes (mean 17.8 h, SD 14.6) returned quicker than Ovenbirds (mean 40.5 h, SD 22.1; Fig. 2).
Behavioral change point analysis
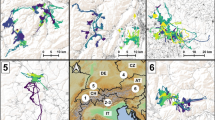
The BCPA identified statistically distinct exploring and homing movement phases for 48 of 49 birds that returned successfully (see Online Resource 2, Table S3 for full model results). The one Wood Thrush that did not exhibit dichotomous behavior immediately moved towards its capture site after release and had the fastest return time (3.1 h) of any bird. Thus, all of this bird’s movements were considered part of the ‘homing’ phase. During exploring, movement steps were significantly shorter and movement speed was significantly slower than during homing for both species (Table 1). Additionally, exploratory movements were more random with respect to the capture site and consisted of a large amount of course reversal, whereas homing movements were straighter and oriented towards the capture site (Fig. 3). Consequently, the distributions of turning (Wood Thrush, D = 0.11, p < 0.01; Ovenbird, D = 0.09, p = 0.01) and deviation angles (Wood Thrush, D = 0.10, p < 0.01; Ovenbird, D = 0.08, p = 0.03) also significantly differed between phases.
A comparison of the distribution of turning and deviation angles between exploring and homing phases for translocated Wood Thrushes and Ovenbirds. Deviation angles were more concentrated around 0° for both species during homing, indicating directed movement towards capture locations. Turning angles were concentrated near 180° during exploring, indicating a large amount of course reversal. Bars represent the percentage of recorded angles, and each grid circle radiating out from the center represents a 5% increment
Both species spent a large proportion of their time in the exploring phase (Figs. S1, S2, Online Resource 2). The average Wood Thrush spent 8.78 h (SD 8.95) exploring, or nearly half (mean 48.72%, SD 26.37) of its total return time. The average Ovenbird spent nearly a full day (mean 23.44 h, SD 15.04) exploring, comprising 60.28% (SD 24.02) of its total return time. Further, a large percentage of the total distance traveled occurred during exploring (Wood Thrush 30.52%, SD 16.48; Ovenbirds 51.23%, SD 17.92), even though individuals made little progress towards their capture sites (Figs. S1, S2, Online Resource 2). On average, Wood Thrushes traveled 0.95 km (SD 0.82), before entering the homing phase, yet were only 3.73% (mean 43.40 m, SD 147.30) closer to home than when they were released. Similarly, Ovenbirds traveled 1.95 km (SD 1.20) while exploring yet were only 28.18% (mean 326.50 m, SD 278.90) closer.
Step-level analyses
We found strong evidence that both species choose steps dominated by forest. AICc results (Table 2) indicated that the baseline model had the least support for both species. Further, the effects of GAPS and GAPDIST were both negative, while the effects of FOR % were positive in every model they were included in (see Online Resource 2, Table S4 for parameter estimates). While these three variables were correlated with one another (|r| > 0.61), we found differences in the way each species responded to them. The two best Wood Thrush models had roughly equal support (AICc weights 0.52 and 0.48); the top model indicated an avoidance of steps with longer gap distances (β = − 0.64 ± 0.16, Z = − 4.01, p < 0.01; Fig. 4), while the second model indicated a stronger avoidance during homing (GAPDIST*HOME β = − 0.18 ± 0.34, Z = − 0.53, p = 0.59). Wood Thrush models containing variables for GAPS and FOR % had almost no support (Table 2). On the other hand, the top Ovenbird model (AICc weight 0.75) indicated a preference for steps with fewer gaps during exploring (β = − 0.42 ± 0.15, Z = − 2.70, p < 0.01) and that preference got stronger during homing (GAPS*HOME β = − 0.42 ± 0.25, Z = − 1.68, p = 0.09; Fig. 4). Ovenbird models containing GAPDIST or FOR % had a summed AICc weight of only 0.1 (Table 2).
Results from the top step-level models revealed that Wood Thrushes (a) preferred steps with shorter forest gap distances, and Ovenbirds (b) showed preference for steps with fewer gaps that differed between behavioral movement phases (exploring and homing). Based on these top models, this figure shows the probabilities of choosing a step over an otherwise equivalent step with no gaps. The largest gap in any optional Wood Thrush step was 628 m and the maximum number of gaps in any optional Ovenbird step was 4. Dashed lines represent 95% confidence intervals
Though an interaction between one of the fragmentation variables and movement phase was included in the top models for both species, neither term was statistically significant (i.e., p < 0.05). Note that because these coefficients were treated as random, they represent the average effect across all individuals, and there was evidence for significant heterogeneity among individual Wood Thrush (SD 0.56 ± 0.17, Z = 3.28, p < 0.01) and Ovenbirds (SD 0.45 ± 0.13, Z = − 1.68, p = 0.09). The fact that this interaction was included in the top models suggests that birds vary in terms of their response to fragmentation, but on average, they tend to avoid crossing gaps more during homing than exploring.
Path-level analyses
Conversely, at the broader path scale we found little evidence that either forest composition or fragmentation negatively influenced return time or path straightness in any behavioral mode (i.e., total, exploring, homing) for either species. The baseline model had the most support for 10 of the 12 model sets considered, with the exceptions being the time Wood Thrushes spent in the homing phase, and the time Ovenbirds spent exploring (Table 3). Interestingly, the top two models for Wood Thrush homing time revealed a positive effect of PROPFOR (β = 0.40 ± 0.22, t = 1.80, p = 0.08), and a negative effect of PATCHES (β = − 0.37 ± 0.23, t = − 1.64, p = 0.11); thus, once Wood Thrushes entered the homing phase, they returned more slowly in landscapes with high forest cover, and more quickly in more fragmented landscapes. Similarly, the top model for Ovenbird exploring time indicated they spent less time exploring in more fragmented landscapes (β = − 0.41 ± 0.16, t = − 2.16, p = 0.05). These patterns are opposite of those expected if forest loss and fragmentation reduce landscape functional connectivity. The correlation between PROPFOR and PATCHES had a relatively small effect on our statistical tests as the variance inflation factors for all parameters in all models was ≤ 3.1 (Table S5, Online Resource 2).
Discussion
In this study we used translocation experiments to evaluate the effects of habitat fragmentation on movement decisions of two migratory bird species known to be fragmentation sensitive. One plausible explanation for such sensitivity is that fragmentation reduces landscape functional connectivity by inhibiting movement. Our results demonstrate that although both species made fine-scale step choices that avoided forest gaps, their overall homing times and routes were not impacted by forest cover or fragmentation. Further, we found that nearly all individuals exhibited dichotomous movement phases, and top models suggested step preference differed among these movement behaviors. Our findings thus raise several important questions regarding how fine-scale movement decisions scale up to movement routes, the nature of translocation experiments, and what they reveal about natural behaviors.
Given that we found no negative effects of forest composition or configuration on path responses, our results suggest that landscape functional connectivity was not negatively influenced by fragmentation. These results were contrary to our expectations, and to results from other studies of forest-dependent birds (e.g., Bélisle and St. Clair 2001; Bélisle et al. 2001; Hadley and Betts 2009; Tremblay and St. Clair 2011). Indeed, numerous translocation experiments with Ovenbirds have demonstrated that reductions in forest cover have strong negative effects on homing time and success (Bélisle et al. 2001; Gobeil and Villard 2002; Desrochers et al. 2011). In most of these studies, translocation distances were longer (2–25 km) than in ours. Thus, it is possible that forest cover and fragmentation have a greater effect on movement at these broader spatial scales. We note, however, that the distances used in our study are similar to both the post-breeding and juvenile dispersal distances reported for these species (Anders et al. 1998; Vega Rivera et al. 1998, 1999; Vitz and Rodewald 2010) and our chosen scale was likely reasonable for assessing how functional connectivity affects movement. On the other hand, numerous studies have demonstrated that forest birds are willing to cross relatively small gaps (< 50 m) even in the presence of forested alternatives (Desrochers and Hannon 1997; St. Clair et al. 1998; Bélisle and Desrochers 2002), and it is possible that the gaps crossed in our landscapes (mean 39.47 m, SD 49.36) were not wide enough to inhibit movement. As noted, two of the birds that failed to home successfully did not attempt to cross a 500 m road gap, though other successful birds crossed gaps of > 450 m. Thus, we cannot determine whether these two individuals were deterred or simply unmotivated to return (we did not find nests at either capture site). Nonetheless, forest cover and fragmentation did not alter functional connectivity over a 1–1.2 km distance in our landscapes. Fragmentation sensitivity for these species may thus be better explained by habitat preference or quality owing to altered biophysical properties in fragmented patches (e.g., Ries et al. 2004).
That we found such starkly different results between our step- and path-level analyses is intriguing. Intuitively, continually making choices that avoid gaps should scale up to result in longer homing times and more tortuous routes in fragmented landscapes. However, on several occasions we observed birds moving along forest gap edges for multiple steps before crossing out of necessity. Indeed, this may help explain the large amount of course reversal noted during both movement phases (though inaccuracies in telemetry locations may play a role as well; Fig. 3). Regardless, this behavior likely resulted in multiple steps where the birds chose not to cross a gap, and only a few where they did, yet these additional movements were apparently insufficient to significantly influence overall homing time or path straightness.
Two non-mutually exclusive potential conclusions could be drawn from these findings. The first is that results from fine-scale movement analyses may not reliably scale up to describe landscape-level connectivity (Morales and Ellner 2002; Gillies et al. 2011; Volpe et al. 2014; Vasudev and Fletcher 2015). Other studies have similarly demonstrated that forest specialist birds are reluctant, though willing to cross gaps (e.g., Castellón and Sieving 2006; Gillies et al. 2011), and such hesitancy may not be indicative of where these species will disperse when sufficiently motivated. Alternatively, our results may reflect artificially high motivation to cross gaps in translocated birds, given that failure to home would result in loss of a partner or territory, and potentially reduced fitness. For many birds in our study, it was not possible to completely avoid gaps and return home successfully, and motivation may have ultimately trumped preference for traveling in contiguous forest. Therefore, it is possible that given these step-level preferences, fragmentation could create behavioral barriers (i.e., barriers they are physically capable of crossing but choose not to; Harris and Reed 2002) for naturally dispersing individuals in the absence of artificial motivation. We are aware of only one study comparing movement of translocated and non-translocated birds (Volpe et al. 2014), which did show similarities. However, there is a need for similar comparisons in other species to fully understand what translocation experiments can reveal about functional connectivity (Betts et al. 2015).
This recommendation is particularly critical given that the translocation procedure induced dichotomous movement behavior in our study, and that gap-avoidance differed among these movement phases. Linking these behaviors with natural movement process may help elucidate when and how landscape features affect movement. Immediately after release, translocated birds exhibited exploratory behavior, characterized by short, undirected movements and frequent returns to their release sites (Table 1, Fig. 3). Similar movement patterns have been demonstrated by other species translocated to novel environments (e.g., Reinert and Rupert 1999; Tsoar et al. 2011; Kesler et al. 2012), and in recently fledged juveniles (Vega Rivera et al. 1998; Kesler et al. 2012). Thus, these exploratory movements may resemble behaviors associated with dispersal or habitat prospecting in the post-breeding period (Nocera et al. 2006; Betts et al. 2008). On the other hand, birds eventually exhibited homing behavior in which they appeared to recognize their location and take larger, faster steps oriented towards home (Table 1, Fig. 3). These behaviors may more closely reflect decisions made by adults moving in known areas (Gillies et al. 2011), seeking out a quality territory, or engaging in larger-scale movements such as migration. Further, because both species avoided gaps in both behavioral modes, our results imply that fragmentation has potential to influence distributions in multiple ways; gap-avoidance during exploring could prevent birds from investigating novel environments in smaller more isolated fragments, while avoidance during homing (when birds were purportedly aware of their surroundings) may imply they are capable of exploring fragments but prefer to settle in contiguous patches (e.g., Groom and Grubb 2006).
Lastly, our results highlight the need to carefully consider the impact of exploratory behaviors on path-level measurements in translocation studies. Nearly half of the total homing times and path distances traveled by each species in this study were comprised of exploratory movements. Studies that do find landscape effects may need to consider whether those variables actually impact functional connectivity (i.e., homing movements) or rather create disorientation (i.e., exploring movements). Ultimately, we found no evidence that forest composition or configuration negatively affected path-level responses of either species overall or in either movement phase. Nonetheless, these results demonstrate need for a better understanding of the information experimental translocations reveal about movement behaviors and landscape connectivity.
Data availability
All data collected and analyzed during this study are included in Online Resources 3–6.
References
Anders AD, Faaborg J, Thompson FR (1998) Postfledging dispersal, habitat use, and home-range size of juvenile Wood Thrushes. Auk 115:349–358
Andrén H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355–366
Baguette M, Van Dyck H (2007) Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landscape Ecol 22:1117–1129
Bayne EM, Hobson KA (2001) Movement patterns of adult male ovenbirds during the post-fledging period in fragmented and forested boreal landscapes. Condor 103:343–351
Beier P, Noss RF (1998) Do habitat corridors provide connectivity? Conserv Biol 12:1241–1252
Bélisle M (2005) Measuring landscape connectivity: the challenge of behavioral landscape ecology. Ecology 86:1988–1995
Bélisle M, Desrochers A (2002) Gap-crossing decisions by forest birds: an empirical basis for parameterizing spatially-explicit, individual-based models. Landscape Ecol 17:219–231
Bélisle M, Desrochers A, Fortin M-J (2001) Influence of forest cover on the movements of forest birds: a homing experiment. Ecology 82:1893–1904
Bélisle M, St. Clair CC (2001) Cumulative effects of barriers on movements of forest birds. Conserv Ecol 5:9
Betts MG, Gutzwiller KJ, Smith MJ, Robinson WD, Hadley AS (2015) Improving inferences about functional connectivity from animal translocation experiments. Landscape Ecol 30:585–593
Betts MG, Hadley AS, Rodenhouse N, Nocera JJ (2008) Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc R Soc B 275:2257–2263
Biz M, Cornelius C, Metzger JPW (2017) Matrix type affects movement behavior of a Neotropical understory bird. Perspect Ecol Conserv 15:10–17
Butler H, Malone B, Clemann N (2005) The effects of translocation on the spatial ecology of tiger snakes (Notechis scutatus) in a suburban Landscape. Wildl Res 32:165–171
Castellón TD, Sieving KE (2006) An experimental test of matrix permeability and corridor use by an endemic understory bird. Conserv Biol 20:135–145
Desrochers A, Bélisle M, Morand-Ferron J, Bourque J (2011) Integrating GIS and homing experiments to study avian movement costs. Landscape Ecol 26:47–58
Desrochers A, Hannon SJ (1997) Gap crossing decisions by forest songbirds during the post-fledging period. Conserv Biol 11:1204–1210
Desrochers A, Hannon SJ, Bélisle M, St. Clair CC (1999) Movement of songbirds in fragmented forests: can we ‘scale up’ from behavior to explain occupancy patterns in the landscape? In: Adams NJ, Slotow RH (eds) Proceedings of the 22nd International Ornithological Congress, Durban, pp 2447–2464
Duchesne T, Fortin D, Courbin N (2010) Mixed conditional logistic regression for habitat selection studies. J Anim Ecol 79:548–555
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L (2013) Rething patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Fahrig L, Merriam G (1985) Habitat patch connectivity and population survival. Ecology 66:1762–1768
Fletcher RJ, Maxwell CW, Andrews JE, Helmey-Hartman WL (2013) Signal detection theory clarifies the concept of perceptual range and its relevance to landscape connectivity. Landscape Ecol 28:57–67
Fletcher RJ, Ries L, Battin J, Chalfoun AD (2007) The role of habitat area and edge in fragmented landscapes: definitievely distinct or inevitably intertwined? Can J Zool 85:1017–1030
Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS (2005) Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86:1320–1330
Getz WM, Saltz D (2008) A framework for generating and analyzing movement paths on ecological landscapes. Proc Natl Acad Sci 105:19066–19071
Gillies CS, Beyer HL, St. Clair CC (2011) Fine-scale movement decisions of tropical forest birds in a fragmented landscape. Ecol Appl 21:944–954
Gillies CS, St. Clair CC (2008) Riparian corridors enhance movement of a forest specialist bird in fragmented tropical forest. Proc Natl Acad Sci 105:19774–19779
Gobeil J-F, Villard M-A (2002) Permeability of three boreal forest landscape types to bird movements as determined from experimental translocations. Oikos 98:447–458
Groom JD, Grubb TC (2006) Patch colonization dynamics in Carolina Chickadees (Poecile carolinensis) in a fragmented landscape: a manipulative study. Auk 123:1149–1160
Gurarie E (2014) bcpa: Behavioral change point analysis of animal movement. R package version 1.1. https://CRAN.R-project.org/package=bcpa
Gurarie E, Andrews RD, Laidre KL (2009) A novel method for identifying behavioural changes in animal movement data. Ecol Lett 12:395–408
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margueles CR, Melbourne BA, Nicholls AO, Orrock JL, Song D-X, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052
Hadley AS, Betts MG (2009) Tropical deforestation alters hummingbird movement patterns. Biol Lett 5:207–210
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Harris RJ, Reed JM (2002) Behavioral barriers to non-migratory movements of birds. Annu Zool Fenn 39:275–290
Heidinger IMM, Poethke H-J, Bonte D, Hein S (2009) The effect of translocation on movement behavior—a test of the assumptions of behavioural studies. Behav Process 82:12–17
Hobson KA, Wassenaar LI, Bayne E (2004) Using isotopic variance to detect long-distance dispersal and philopatry in birds: an example with Ovenbirds and American Redstarts. Condor 106:732–743
Kennedy CM, Marra PP (2010) Matrix mediates avian movements in tropical forested landscapes: Inference from experimental translocations. Biol Conserv 143:2136–2145
Kesler DC, Cox AS, Albar G, Gouni A, Mejeur J, Plassé C (2012) Translocation of Tuamotu Kingfishers, postrelease exploratory behavior, and harvest effects on the donor population. Pac Sci 66:467–480
Lima SL, Zollner PA (1996) Towards a behavioral ecology of ecological landscapes. Trends Ecol Evol 11:131–135
Lynch JF, Whigham DF (1984) Effects of forest fragmentation on breeding bird communities in Maryland, USA. Biol Conserv 28:287–324
MacIntosh T, Stutchbury BJM, Evans ML (2011) Gap-crossing by Wood Thrushes (Hylocichla mustelina) in a fragmented landscape. Can J Zool 89:1091–1097
Moilanen A, Hanski I (2001) On the use of connectivity measures in spatial ecology. Oikos 95:147–151
Morales JM, Ellner SP (2002) Scaling up animal movements in heterogeneous landscapes: the importance of behavior. Ecology 83:2240–2247
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE (2008) A movement ecology paradigm for unifying organismal Movement. Proc Natl Acad Sci 105:19052–19059
Nocera JJ, Forbes GJ, Giraldeau L-A (2006) Inadvertent social information in breeding site selection of natal dispersing birds. Proc R Soc B 273:349–355
Pardini R, Bueno ADA, Gardner TA, Prado PI, Metzger JP (2010) Beyond the fragmentation threshold hypothesis: regime shifts in biodiversity across fragmented landscapes. PLoS ONE 5:e13666
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2017) nlme: Linear and Nonlinear Mixed Models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme
Rands MRW, Adams WM, Bennun L, Butchart SHM, Clements A, Coomes D, Entwistle A, Hodge I, Kapos V, Scharlemann JPW, Sutherland WJ, Vira B (2010) Biodiversity conservation: challenges beyond 2010. Science 329:1298–1303
Rappole JH, Tipton AR (1991) New harness design for attachment of radio transmitters to small passerines. J Field Ornithol 62:335–337
Reinert HK, Rupert RR (1999) Impacts of translocation on behavior and survival of Timber Rattlesnakes, Crotalus horridus. J Herpetol 33:45–61
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Ries L, Fletcher RJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522
Robbins CS, Dawson DK, Dowell BA (1989) Habitat area requirements of breeding forest birds of the Middle Atlantic states. Wildl Monogr 103:3–34
Sarrias M, Daziano RA (2017) Multinomial logit models with continuous and discrete individual heterogeneity in R: the gmnl package. J Stat Softw. https://doi.org/10.18637/jss.v079.i02
Selonen V, Hanski IK, Desrochers A (2010) Measuring habitat availability for dispersing animals. Landscape Ecol 25:331–335
Smith MJ, Betts MG, Forbes GJ, Kehler DG, Bourgeois MC, Flemming SP (2011) Independent effects of connectivity predict homing success by northern flying squirrel in a forest mosaic. Landscape Ecol 26:709–721
St. Clair CC (2003) Comparative permeability of roads, rivers, and meadows to songbirds in Banff National Park. Conserv Biol 17:1151–1160
St. Clair CC, Bélisle M, Desrochers A, Hannon S (1998) Winter responses of forest birds to habitat corridors and gaps. Conserv Ecol 2:13
Taylor PD, Fahrig L, Henein K, Merriam G (1993) Connectivity is a vital element of landscape structure. Oikos 68:571–573
Tischendorf L, Fahrig L (2000) On the usage and measurement of landscape connectivity. Oikos 90:7–19
Tischendorf L, Fahrig L (2001) On the use of connectivity measures in spatial ecology. A reply. Oikos 95:152–155
Tittler R, Fahrig L, Villard M-A (2006) Evidence of large-scale source-sink dynamics and long-distance dispersal among Wood Thrush populations. Ecology 87:3029–3036
Tremblay MA, St. Clair CC (2011) Permeability of a heterogeneous urban landscape to the movements of forest songbirds. J Appl Ecol 48:679–688
Tsoar A, Nathan R, Bartan Y, Vyssotski A, Dell’Omo G, Ulanovsky N (2011) Large-scale navigational map in a mammal. Proc Natl Acad Sci 10:E718–E724
Turgeon K, Robillard A, Gregoire J, Duclos V, Kramer DL (2010) Functional connectivity from a reef fish perspective: behavioral tactics for moving in a fragmented landscape. Ecol 91:3332–3342
Valente JJ, Betts MG (2019) Response to fragmentation by avian communities is mediated by species traits. Divers Distrib 25:48–60
Vasudev D, Fletcher RJ (2015) Incorporating movement behavior into conservation prioritization in fragmented landscapes: an example of western hoolock gibbons in Garo Hills, India. Biol Conserv 181:124–132
Vasudev D, Fletcher RJ, Goswami VR, Krishnadas M (2015) From dispersal constraints to landscape connectivity: lessons from species distribution modeling. Ecography 38:967–978
Vega Rivera JH, McShea WJ, Rappole JH, Haas CA (1999) Postbreeding movements and habitat use of adult Wood Thrushes in northern Virginia. Auk 116:458–466
Vega Rivera JH, Rappole JH, McShea WJ, Haas CA (1998) Wood Thrush postfledging movements and habitat use in northern Virginia. Condor 100:69–78
Villard M-A, Merriam G (1995) Dynamics in subdivided populations of neotropical migratory birds in a fragmented temperate forest. Ecol 76:27–40
Villard M-A, Trzcinski MK, Merriam G (1999) Fragmentation effects on forest birds: relative influence of woodland cover and configuration on landscape occupancy. Conserv Biol 13:774–783
Vitz AC, Rodewald AD (2010) Movements of fledgling Ovenbirds (Seiurus aurocapilla) and Worm-eating Warblers (Helmitheros vermivorum) within and beyond the natal home range. Auk 127:364–371
Volpe NL, Hadley AS, Robinson WD, Betts MG (2014) Functional connectivity experiments reflect routine movement behavior of a tropical hummingbird species. Ecol Appl 24:2122–2131
Zeller KA, McGarigal K, Whiteley AR (2012) Estimating landscape resistance to movement: a review. Landscape Ecol 27:777–797
Acknowledgements
This research was funded by the U.S. Army Engineer Research and Development Center Environmental Quality and Installations 6270/896/04 (PE/Project/Task). We thank the Department of Defense Strategic Environmental Research and Development Program (grant RC-2121) for helping to facilitate this work. We also thank M. Bélisle and two anonymous reviewers for their constructive feedback which greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valente, J.J., Fischer, R.A., Ryder, T.B. et al. Forest fragmentation affects step choices, but not homing paths of fragmentation-sensitive birds in multiple behavioral states. Landscape Ecol 34, 373–388 (2019). https://doi.org/10.1007/s10980-019-00777-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00777-z