Abstract
Information on reproductive biology of the European hare (Lepus europaeus) in different environmental and landscape conditions comprises part of fundamental knowledge regarding species’ adaptive responses as well as many aspects of its biology. Most of the studies conducted on European hare reproduction are confined to midlatitude and northern populations, whereas no data exist on the indigenous southern populations. Here, we present information on reproductive characteristics of European hares inhabiting Mediterranean ecosystems on the island of Crete, Greece for two successive hunting seasons. Although the annual reproductive cycle of the species is well known, with an autumn sexual inactivity, the duration of this period is subjected to fluctuations in different years and for different areas. According to our data, hare populations of Crete present an autumn–early winter reproductive activity with high proportions of pregnant females observed in all the months of the study. Furthermore, the estimated mean litter size (1.54 SE ± 0.07) while signed to the lowest values ever observed for European hares is similar to values obtained in continuous breeding species of the same genus, Lepus granatensis, Lepus corsicanus, Lepus (capensis) mediterraneus, and Lepus capensis also inhabiting warm climates. In conclusion, our results suggest that Cretan European hare populations exhibit a reproductively active period during autumn–early winter where proportions of pregnant females and litter size give a strong indication of a continuous reproduction throughout the year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproduction consists one of the most important demographic factors of population growth that is relatively easy to measure (Keith 1981). Although several environmental variables (daylength, temperature, rainfall, food availability) are presently known to affect animal’s sexual activity, their impact mainly depends on the biotope. Moreover, animals living in their natural habitat are never subjected to a single factor; there is always a covariation of all such environmental factors (Pèvet 1987). Thereby, formulating general rules on reproductive cycles of different mammalian species is extremely difficult since sexual activity is being affected by multiple factors that vary in different areas and for different species. Sometimes, differences can be seen even between distinct populations of the same species inhabiting areas with different characteristics. The observed variation between regions and years in the onset and termination of breeding for Snowshoe hares (Lepus americanus Keith 1981) constitutes such an example. The European hare (Lepus europaeus Pallas 1778) could be placed among these cases since it has a wide distribution range inhabiting a variety of divergent terrestrial ecosystems (Mitchell-Jones et al. 1999).
Several studies have been carried out on the reproductive biology of L. europaeus, in its natural (Europe: Kolosov 1941 in Raczyński 1964; Raczyński 1964; Lincoln 1974; Hewson and Taylor 1975; Pielowski 1976; Frylestam 1980; Broekhuizen and Maaskamp 1981; Kovács 1983; Pépin 1987; Hansen 1992; Bensinger et al. 2000; Hackländer et al. 2001; Marboutin et al. 2003) and introduced range (Argentina: Bonino and Montenegro 1997; Canada: Reynolds and Stinson 1959; New Zealand: Flux 1967 and Parkes 1989; and Australia: Stott and Harris 2006). Most of the studies on indigenous populations have been confined to midlatitude and northern regions, while information on reproductive characteristics of southern populations is still lacking. Although the annual reproductive cycle of this species is well known, with a sexual inactivity occurring in autumn according to the above documentation, the duration of this period is subjected to fluctuations in different years. Usually, the reproductive season begins in January and lasts till September (exceptionally October). In areas where winter is harsh (i.e., Lithuania: Likevičiené 1962 in Raczynki 1964; Russia: Korneev 1960 in Raczynki 1964), the reproduction may not start until March where the weather conditions will be ameliorated, while during mild winters, reproductively active females can be observed exceptionally early in the breeding season (e.g., December Hay 1953 in Raczyński 1964; November Korneev 1960 in Raczynki 1964; all year round Likevičiené 1962 in Raczyński 1964). Contrary to this reproductive pattern, the introduced populations of European hare in Australia seem to breed all over the year in warmer climates (Stott et al. 2008). Thus, available information strongly suggests that regional climates affect the European hare’s breeding season and that local conditions contribute significantly to annual variations in its onset and termination and hence, in breeding season length (Flux 1967; Hewson and Taylor 1975; Cary and Keith 1979).
The time at which breeding usually begins seems highly to affect the average number of litters produced (e.g., Snowshoe hare), which largely reflects the length of the breeding season (see Keith 1981 and examples therein). Differences observed in breeding season length have many implications in the reproductive biology of the species that may lead to different reproductive strategies of populations within the species geographic range. Some of those differences have proven to be indicators of a more profound diversity in the genetic level (i.e., differences in litter size of populations of L. americanus in different latitudes reflect populations genetic diversity, Keith et al. 1966).
The exhibited plasticity in the reproductive cycle of the European hare in different regions indicates adaptation of populations to different conditions that may or may not correspond to a more profound diversity (i.e., at the genetic level). The high level of genetic similarities observed between central European populations (e.g., Suchentrunk et al. 2000; Ben Slimen et al. 2007), in contrast to the high molecular variance observed between populations of the same species distributed in refugial areas as Greece (mtDNA average nucleotide divergence = 6.6% Kasapidis et al. 2005; F ST values that reach 0.3; Antoniou 2008), bears out the assumption that in areas with different historic, climatic, and landscape characteristics, new factors might be revealed to be responsible in shaping the reproductive behavior of the species.
Field observations on the island of Crete (Greek island, with Mediterranean climate) suggest that European hare populations reproduce throughout the year and not only during the main breeding season, as most continental European hare populations do. However, up to now, there is no scientific research supporting this statement. In this study, we present for the first time data on the reproductive biology of autochthonous European hare populations inhabiting Mediterranean ecosystems.
Materials and methods
The study was conducted on the island of Crete located at the southernmost part of Greece, with an area of 8,265 km2 and a typically Mediterranean climate. It is characterized by an extremely mountainous terrain of several massifs, with the highest summits of similar altitude to the majority of those in the Greek mainland (maximum elevation reaches up to 2,200 m) where the climate is mountainous (Matzarakis et al. 2005).
The cold and rainy period occurs between October to March, with January and February being the coldest months (minimum air temperature 7–9°C in coastal areas and 4–6°C in the mainland). The warm and dry period of April to September is characterized by almost no precipitation and slightly hot temperatures. The warmest months of this period are July and August (mean maximum air temperature 28–32°C, sometimes reaches 34–40°C). September is considered as summer month in Crete. The mean annual rainfall decreases from west to east and from north to south (range from 500 to 1,200 mm) and increases with altitude (1,600 mm in Lefka Ori mountain range, west Crete; Matzarakis et al. 2005).
The dominant habitat type on the island is the Sarcopoterium spinosum phrygana (27.32%), followed by the agricultural habitats (23.69%) Olea and Ceratonia forests (20.69%), Dehesas (5.38%), Mediterranean pine forests (4.84%), Acero Cupression (4.02%), and Oro-mediterranean phrygana (3.52%); other habitat types exist as well on the island represented by lower percentages (Sarris et al. 2005).
In Greece, hares are protected by law from mid-January to mid-September; regulation introduced in order to ensure an undisturbed breeding season. The data were obtained during two consecutive hunting seasons (mid-September to mid-January of 2001–2002 and 2002–2003). All sampling areas comprise parts of the communal shooting range where each hunter collected the small game himself, with (or without) the help of dogs. Questionnaires were send to all hunting associations of Crete where hunters could record the exact date and locality of each hare shot, its approximate weight (due to different types of balances used and their accuracy levels), sex, female reproductive status (based on pregnancy and lactating characteristics visible by eye or dissection), and litter size. Furthermore, additional samples were obtained during February (month outside the hunting season) provided by hunting association guards responsible for the eradication of illegal hunting.
The total number of females collected during the two successive hunting seasons was 198. Of these, only animals presumptively capable of reproduction were selected for further analysis. This discrimination, although abrupt, was made according to their weight. In particular, individuals that weighted less than the lightest reproductively active female were not considered capable of reproduction due to their early stage of life. This convention left us with 184 females that were classified according to their reproductive characteristics as inactive, pregnant, and lactating. It is worth noticing that only females at a later stage of pregnancy could be detected since inspection was based on visual contact of the embryos or fetuses. The fact that hunters were the only responsible for the collection of the acquired information rendered the age determination of specimens namely by controlling the lateral bony protrusion on the distal ulna (Stroh 1931) or estimating dry eye lens weights (Suchentrunk et al. 1991) impossible.
Monthly values of all parameters were calculated by pooling data of the two successive hunting seasons (2001–2002, 2002–2003). One-way ANOVA was employed in order to determine the effect of the different months on the reproductive activity. Assumptions underlying the statistical tests used in this study, normality, and homogeneity were checked using Kolmogorov–Smirnoff and Bartlett’s tests, respectively. Statistical analyses were performed using SPSS v. 12.0 (SPSS Inc. Chicago, IL, USA) and STATGRAPHICS Plus5.0 (StatisticalGraphics Corp.).
In order to estimate the maximum bimonthly potential production of young, we calculated mean litter size and frequency of pregnant females by pooling the data of two consecutive months (i.e., September–October, November–December, January–February). Assuming that, at best circumstances, the average birth interval is similar to the length of gestation (41 to 42 days, Martinet et al. 1970), a healthy adult female might have 1.46 litters within a 2-month period at its maximum endeavor (Pépin 1989). Hence, we estimate the maximum bimonthly potential production of young per female by multiplying the bimonthly frequency of pregnant females with the mean bimonthly litter size and with the maximum number of litters within a 2-month period (1.46).
Results
The lightest (presumably the youngest) lactating female weighted 1.3 kg, while the lightest pregnant female weighted 1.5 kg. The approximate weight of the analyzed females ranged between 1.3 and 3.2 kg.
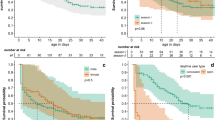
Pregnant females were found throughout the studied period (mid-September to mid-January), with monthly proportions ranging from 18% in October to 45% in January (Fig. 1a). The proportion of lactating females varies from 0% to 3.8% (Fig. 1a). The variation of the estimated monthly proportion of reproductively inactive and active (pregnant and lactating) females in respect to sample sizes is presented in Fig. 1b. The highest values were observed during months September (0.06) and February (0.03) due to small sample sizes.
a Variation in the proportion of inactive, pregnant, and lactating females of European hare populations of Crete (pooled data of two hunting seasons 2001–02 and 2002–03). b Variation in the proportion of inactive and active (pregnant and lactating) females of European hare populations and variance of the estimates in respect to sample size (s p 2 = pq/(n − 1); Zar 1996)
Changes in the percentage of pregnant females were not significant between the studied months (F (5,6) = 0.8, p = 0.588). Similarly, there were no differences in the proportion of lactating and inactive females (F (5,6) = 2.0, p = 0.212 and F (5,6) = 3.418, p = 0.083, respectively).
Mean litter size peaked in January (1.89) followed by October (1.83), while the lowest value was observed during September (1; Table 1). Changes observed in mean litter size were not significant between months (F (5,6) = 2.9, p = 0.116). The estimated overall mean litter size was 1.54 SE ± 0.07 (n = 54), with one and two being the most frequently observed litter sizes (26 and 27 cases, respectively; Table 1). The largest litter consisted of three embryos/fetuses and was observed only once.
The lowest value of the maximum bimonthly potential production of young estimated from pooled data was obtained in September–October (0.51) and the highest in January–February (1.06), while during November–December, the estimated value equals to 0.69.
Discussion
According to our data, hare populations of Crete presented an autumn–early winter reproductive activity during the two successive hunting seasons 2001–2003. Although inactive females represent a high proportion of female population, pregnant females were observed in all studied months. The observed variance in weights of the reproductively active females legitimates the assumption that pregnant juveniles/sub-adults have also been incorporated in the sample and that age-dependence of reproduction (already documented for the species in New Zealand by Flux 1967) might also be implied. It is worth noticing that, in general, the proportion of pregnant females is above 18% and reaches its highest value in January (45%). Contradictory to our data, in some midlatitude and northern areas (i.e., in western Poland, in Britain, in Netherlands, and in Argentina), the observed proportion of pregnant females during November and December is zero (Table 1; Raczyński 1964; Lincoln 1974; Broekhuizen and Maaskamp 1981; Bonino and Montenegro 1997, correspondingly), whereas in Crete, during the same months, equals to 26% and 41%, respectively. Furthermore, in areas where pregnant females have been observed during those months, the percentages were always below the aforementioned values (Flux 1967; Hewson and Taylor 1975; Pépin et al. 1981; Stott and Harris 2006), and most of the times, cases of pregnancy were considered as exceptional (Hay 1953 in Raczyński 1964; Korneev 1960 in Raczynki 1964; Raczyński 1964; Hewson and Taylor 1975), which is attributed to mild winters (Likevičiené 1962 in Raczynski 1964; Table 1). Unfortunately, the fact that in most of the studies different methodologies are being used renders comparisons between them very difficult.
From the above, it becomes evident that even though in populations with well-defined breeding season, reproductively active females have been observed during autumn and the first months of winter, though in significantly lower proportions than those observed in the present study, and were attributed to exceptional climate conditions.
It is worth noting that lactating females cannot be treated as an unbiased indicator since there is a great possibility that not all hunters were registering information concerning mammary glands, but most of them were registering pregnancy and number of embryos/fetuses present. This, coupled with the difficulty in visual identification of first few days’ pregnancies, renders underestimation of reproductively active females in this study highly probable. Nevertheless, our data give a strong indication of autumn–early winter reproduction in the European hare populations of Crete, further suggesting a continuous reproduction of the species in this Mediterranean ecosystem.
No significant changes were detected between months of the studied period for inactive, pregnant, and lactating females, indicating that the effect of the different months to the reproductive activity is not significant, further supporting the above hypothesis.
The mean litter size reported in this study (1.54 SE ± 0.07, n = 54) is signed on the lowest values ever observed not only for different populations of the same hare species but also for species of the same genus (Table 1). Considering that litter size is inversely related to the length of breeding season as illustrated by Flux (1981), the value observed in this study could be an indication of continuous breeding in the Cretan European hare populations. This hypothesis is supported by the fact that seasonal variation in the production of young in European hare is the result of changes in the percentage of pregnant females and litter size. Furthermore, it is in the middle of the breeding season where the maximum productivity is always observed (Raczyński 1964; Flux 1967; Hewson and Taylor 1975; Broekhuizen and Maaskamp 1981) and not at its onset or termination. Cases where similar values of mean litter sizes have been reported are in Lepus capensis (1.5) by Flux (1981) and in Lepus granatensis (1.6) by Alves et al. (2002), which are species that are characterized by a continuous breeding throughout the year. Moreover, preliminary studies on reproductive biology of Sardinian (Lepus (capensis) mediterraneus) and Italian (Lepus corsicanus) hares reveal a mean litter size (detected through placental scars and/or fetuses) of 1.35 (±0.63 SD) (De Marinis and Trocchi 2007; De Marinis et al. 2007b) and 1.5 (±0.71; De Marinis et al. 2007a, b) respectively. This fact, coupled with observations of moderate to high proportions of pregnant females (in September–October and October–November, respectively), provide indications of a continuous reproduction for those species though with a summer regressive phase.
To our knowledge, the only case where a reproductive activity of European hares has been observed during autumn–early winter was reported recently in the non-indigenous populations of Australia in a semi-arid habitat (Stott et al. 2008). The reported percentages of pregnant females are equal to those of our study or slightly lower especially during November and December (Table 1). Kolosov (1941 in Raczynski 1964) also refers to an almost continuous period of reproduction for European hare populations inhabiting Caucasus, though with a seasonal and cyclic character with the minimum percentages of pregnant females observed in November, something that contradicts to present study observations (Table 1).
Although a number of factors already proven to influence hare’s reproduction are present on the island of Crete (i.e., mild weather conditions, moderate to high food availability, low stress ought to predator pressure) and consent to the prolongation of the breeding season, its variation could also be associated to genetic characteristics. Moreover, according to De Marinis et al. (2007b), captive European hares of Italy, also inhabiting Mediterranean ecosystems, are not reproductively active throughout the year, a fact that further supports the above statement.
According to current genetic analyses, Cretan populations are differentiated from the remaining Greek and central European populations (Antoniou 2008), a fact that could be responsible for their discernible reproductive performance. This could also be true for hare populations of Cyprus, which likewise present a discernible genetic pool in comparison to other European and Greek populations (Antoniou 2008) and where indications of a continuous reproduction (through the presence of reproductively active females) also exist (person. com. with Game Fund Service, Cyprus Ministry of Interior). However, further detailed studies should be performed in order to confirm this hypothesis and to assess the relative weight of genetic versus environmental factors on the reproductive seasonality of Lepus species.
In conclusion, our data suggest that European hare populations of Crete do not exhibit an anoestrus period during autumn–early winter. On the contrary, proportions of pregnant females and litter size give a strong indication that these island populations reproduce all over the year. Despite the small sample sizes, the limited number of variables used and the short-term period of the present study, the observed proportion of pregnant females, and the low mean litter size are remarkable and quite similar to those observed in hare species with a continuous breeding season. Although the possibility of a reproductive pause during the warmer months of summer cannot be excluded, hunter’s field observations reject this case. Nevertheless, our data represent only an evidence of breeding activity during autumn–early winter for the Cretan populations, and future long-term studies are needed in order to reach a solid conclusion about the reproduction of the species on the island.
By conducting further studies on the reproductive characteristics of Cretan hare populations as well as on other mainland or insular populations of Greece where extensive genetic diversity has also been detected (Antoniou 2008), the formation of future guidelines regarding management and conservation practices will be elucidated based on combined knowledge and thus on solid foundations. Moreover, studying populations that are relatively recent, regarding their evolutionary history on the island, and well adapted to the warm climate of Crete will allow us to gain invaluable clues on the development, impact, and consequences of climate and anthropogenic challenges, with powerful implications for the future of species inhabiting northern climates.
References
Alves PC, Gonçalves H, Santos M, Rocha A (2002) Reproductive biology of the Iberian hare, Lepus granatensis, in Portugal. Mamm Biol 67:358–371
Antoniou A (2008) Population structure of the brown hare in Balkans. PhD thesis, University of Crete
Bensinger S, Kugelschafter K, Eskens U, Sobiraj A (2000) Untersuchungen zur jährlichen Reproduktionsleistung von weiblichen Feldhasen (Lepus europaeus Pallas, 1778) in Deutschland. Z Jagdwiss 46:73–83
Ben Slimen H, Suchentrunk F, Shahin AB, Ben Ammar Elgaaied A (2007) Phylogenetic analysis of mtCR-1 sequences of Tunisian and Egyptian hares (Lepus sp. or spp., Lagomorpha) with different coat colours. Mamm Biol 72(4):224–239
Bonino N, Montenegro A (1997) Reproduction of the European hare in Patagonia, Argentina. Acta Theriol 42:47–54
Broekhuizen S, Maaskamp F (1981) Annual production of young in European hares (Lepus europaeus) in the Netherlands. J Zool (London) 193:499–516
Cary J, Keith LB (1979) Reproductive change in the 10-year cycle of snowshoe hares. Can J Zool 57:375–390
De Marinis A, Trocchi V (2007) Reproductive biology of Sardinian hare Lepus (capensis) mediterraneus revealed by stained placental scars. Hystrix It. J. Mamm. (n.s.) Supp.:77
De Marinis A, Trocchi V, Mangiafico S (2007a) First data on reproductive biology of Italian hare Lepus corsicanus. Hystrix It. J. Mamm. (n.s.) Supp.:78
De Marinis A, Trocchi V, Mangiafico S, Fassò C, Mallia E (2007b) Strategie riproduttive in tre specie di Lepre (Lepus sp. pl.) in Italia. Conservazione di Lepus corsicanus De Winton, 1898 e stato delle conoscenze, de Filippo et al. (a cura di), 2007, IGF publ.:75–81
Flux JE (1967) Reproduction and body weights of the hare, Lepus europaeus Pallas, in New Zealand. New Zealand J Sci 10:357–401
Flux JE (1981) Reproductive strategies in the genus Lepus. In: Myers K, MacInnes CD (eds) Proceedings of the world lagomorph conference. Ontario, University of Guelph, pp 155–174
Frylestam B (1980) Reproduction in the European hare in southern Sweden. Holart Ecol 3:74–80
Hackländer K, Frisch C, Klansek E, Steineck T, Ruf T (2001) Die Fruchtbarkeit weiblicher Feldhasen (Lepus europaeus) aus Revieren mit unterschiedlicher Populationsdichte. Z Jagdwiss 47:100–110
Hansen K (1992) Reproduction in European hare in a Danish farmland. Acta Theriol 37:27–40
Hewson R, Taylor M (1975) Embryo counts and length of the breeding season in European hares in Scotland from 1960–1972. Acta Theriol 20:247–254
Kasapidis P, Suchentrunk F, Magoulas A, Kotoulas G (2005) The shaping of mitochondrial DNA phylogeographic patterns of the brown hare (Lepus europaeus) under the combined influence of Late Pleistocene climatic fluctuations and anthropogenic translocations. Mol Phyl Evol 34:55–66
Keith LB (1981) Population dynamics of hares. In: Myers K, MacInnes CD (eds) Proceedings of the world lagomorph conference. Ontario, University of Guelph, pp 395–440
Keith LB, Rongstad OJ, Meslow EC (1966) Regional differences in reproductive traits of the snowshoe hare. Can J Zool 44:953–961
Kovács G (1983) Variability of fecundity rates in a population of European hare. In: Spenik M, Hell P (eds) IUGB XVII, Strbske Pleso, pp 450–458
Lincoln GA (1974) Reproduction and March madness in the brown hare, Lepus europaeus. J Zool (London) 174:1–14
Marboutin E, Bay Y, P, éroux R, Mauvy B, Lartiges A (2003) Population dynamics in European hare: breeding parameters and sustainable harvest rates. J Appl Ecol 40:580–591
Martinet L, Legouis JJ, Moret B (1970) Quelques observations sur la reproduction du lièvre européene (Lepus europaeus Pallas) en captivité. Annls Biol Anim Bioch Biophys 10:195–202
Matzarakis A, Karatarakis N, Sarantopoulos A (2005) Tourism climatology and tourism potential for Crete, Greece. Annalen der Meteorologie 41:616–619
Mitchell-Jones AJ, Amori G, Bogdanowicz W, Krystufek B, Reijnders P, Spitzenberger F, Stubbe M, Thissen J, Vohralik V, Zima J (1999) Atlas of European mammals. Academic, London, pp 166–168
Parkes JP (1989) Annual patterns in reproduction and perirenal fat of hares (Lepus europaeus) in sub-alpine Canterbury, New Zealand. J Zool (London) 217:9–21
Pépin D (1987) Dynamics of a heavily exploited population of brown hare in a large-scale farming area. J Appl Ecol 24:725–734
Pépin D (1989) Variation in survival of brown hare (Lepus europaeus) leverets from different farmland areas in the Paris basin. J Appl Ecol 26:13–23
Pépin D, Meunier M, Angibault J (1981) Etude de la reproduction du lièvre (Lepus europaeus) dans le Basin Parisien. Bull ONC Scientifique et Technique 11:3–26
Pèvet P (1987) Environmental control of the annual reproductive cycle in mammals.. In: Pèvet P (ed) Comparative physiology of environmental adaptations. vol. 3. ESCP Conference, Strasbourg, pp 82–100
Pielowski Z (1976) Number of young born and dynamics of the European hare population. In: Pielowski Z, Pucek Z (eds) Ecology and management of European hare populations. Polish Hunting Association, Warszawa, pp 75–78
Raczyński J (1964) Studies on the European hare V: reproduction. Acta Theriol 19:305–352
Reynolds JK, Stinson RH (1959) Reproduction in the European hare in southern Ontario. Can J Zool 37:627–631
Sarris A, Maniadakis M, Lazaridou O, Kalogrias V, Bariotakis M, Pirintsos S (2005) Studying land use patterns in Crete island, Greece, through a time sequence of landsat images and mapping vegetation patterns. WSEAS International Multiconference on Int Conf on Environment, Ecosystems and Development (EED05)
Stott P, Harris S (2006) Demographics of the European hare (Lepus europaeus) in the Mediterranean climate zone of Australia. Mamm Biol 71:214–226
Stott P, Harris S, Wight N (2008) Fertility and infertility in the European hare Lepus europaeus in Australia. In: Alves PC, Ferrand N, Hackländer K (eds) Lagomorph biology: evolution, ecology and conservation. Springer, Berlin, pp 225–240
Stroh G (1931) Zwei sichere Altersmerkmale beim Hasen Berl. Tierärztl Wschr 12:180–181
Suchentrunk F, Willing G, Hartl GB (1991) On eye lens weights and other age criteria of the Brown hare (Lepus europaeus Pallas, 1778). Z Säugetierkd 56:365–374
Suchentrunk F, Michailov C, Markov G, Haiden A (2000) Population genetics of Bulgarian brown hares Lepus europaeus: allozymic diversity at zoogeographical crossroads. Acta Theriol 45:1–12
Zar J (1996) Biostatistical analysis, 3rd edn. Prentice-Hall, New Jersey
Acknowledgments
We would like to thank all hunters and Hunting Associations of Crete and especially Mr. M. Fragiadoulakis (president of the 1st Hunting Association of Irakleio) for providing samples and sharing their experience. We thank K. Lika, M. Giannoulaki, N. Lampadariou, and A. Parmakelis for helping on the statistical analysis. We would also like to thank N. Poulakakis and A. Parmakelis for their suggestions on an earlier draft of this manuscript. Finally, we are indebted to the reviewer for very constructive comments. This study was performed in accordance with current Hellenic legislation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Antoniou, A., Kotoulas, G., Magoulas, A. et al. Evidence of autumn reproduction in female European hares (Lepus europaeus) from southern Europe. Eur J Wildl Res 54, 581–587 (2008). https://doi.org/10.1007/s10344-008-0182-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-008-0182-y