Abstract
Drought is one of the most important environmental stresses that severely reduce plant growth and crop productivity. This study was carried out to investigate the difference in the response of six soybean cultivars (Giza 21, 22, 35, 82, 83 and 111) under water stress and the genetic difference between these cultivars using retroelements technique. The results showed that drought stress caused reduction in morphological criteria, photosynthetic pigments, starch, phospholipids, glycolipids, pectin, cellulose and lignin in shoots of all soybean cultivars except Giza 22 and Giza 83. On the other hand, there was a considerable increase in root length, soluble sugars, proline, glycine betaine, total lipids and hemicellulose contents in the shoots of the soybean cultivars in response to water stress. The soybean cultivars Giza 22 and 83 were more drought tolerant than the other cultivars while Giza 21 and Giza 111 were the most sensitive. Inter-primer binding sites (iPBS) and inter-retrotransposon amplified polymorphism (IRAP) techniques were used to fingerprint the six soybean cultivars using a set of eight primers. The techniques successfully tagged each cultivar with specific bands and detected molecular genetic markers related to drought tolerance in soybean.
Zusammenfassung
Trockenheit ist eine der wichtigsten Umweltbelastungen, die das Pflanzenwachstum und die Pflanzenproduktivität stark reduzieren. Diese Studie wurde durchgeführt, um den Unterschied in der Reaktion von sechs Sojasorten (Giza 21, 22, 35, 82, 83 und 111) unter Wasserstress und den genetischen Unterschied zwischen diesen Sorten mittels Retroelement-Technik zu untersuchen. Die Ergebnisse zeigten, dass Trockenstress zu einer Verringerung der morphologischen Kriterien, der photosynthetischen Pigmente sowie zu einer Reduktion von Stärke, Phospholipiden, Glycolipiden, Pektin, Cellulose und Lignin in den Sprossen aller Sojasorten außer Giza 22 und Giza 83 führte. Auf der anderen Seite kam es als Reaktion auf Wasserstress zu einer beträchtlichen Zunahme der Wurzellänge und des Gehalts an löslichen Zuckern, Prolin, Glycinbetain, Gesamtlipiden und Hemicellulose in den Sprossen der Sojabohnensorten. Die Sojasorten Giza 22 und 83 waren trockentoleranter als die anderen Sorten, während Giza 21 und Giza 111 am empfindlichsten waren. Die iPBS- (inter-primer binding site) und die IRAP-Technik (inter-retrotransposon amplified polymorphism) wurden verwendet, um einen genetischen Fingerabdruck der sechs Sojabohnensorten unter Verwendung eines Satzes von acht Primern zu erstellen. Mithilfe dieser Techniken konnten jeder Sorte erfolgreich spezifische Banden zugeordnet werden. Außerdem wurden molekulargenetische Marker entdeckt, die mit der Trockentoleranz von Sojabohnen in Zusammenhang stehen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water deficit is caused due to the absence of water or moisture which helps the plant in the growth, development and differentiation (Zhu 2002). In arid and semi-arid zones the inadequacy of water available for irrigation frequently exposes plants to drought (Wang et al. 2005). Water stress affects the activity of some enzymes and causes the accumulation of proteins and compatible solutes i. e., sugars, glycine betaine and amino acids (Gong et al. 2005). In addition, it causes inhibition of the crop productivity and alters the metabolism of lipids which help in the membrane synthesis (Ford and Barber 1983). Water stress also caused reduction in linolenic acid, glycolipid and phospholipid contents of leaf tissues (Wilson et al. 1987).
Plants can adapt or tolerate stress by the accumulation of osmoprotectant compounds such as trehalose, polyamines and proline which help the plant to cope abiotic and biotic stress (Mohamed and Akladious 2014; Ashry and Mohamed 2011). Genotypes differ in their response to environmental stresses in proportion with their difference in the genetic based antioxidant systems resulting in tolerant and sensitive genotypes in the same species (Sairam et al. 1998). Drought stress affects growth and yield of soybean cultivars depending on the sensitivity of cultivated varieties and duration of water deficit (Frederick et al. 2001).
Long terminal repeat-retrotransposons (LTR-RTs) are the most considerable genomic components in plant genomes (Du et al. 2010). In the last few years, the most of the plant LTR-RTs have been amplified (Xiang et al. 2016). Soybean (Glycine max L. Merr.) is the most important crop in the world and belongs to leguminosae family. Plants of this family contain a large amount of protein and are used as food and animal feed worldwide (Graham and Vance 2003). Recently, soybean has been sequenced due to its economic value (Schmutz et al. 2010). Du et al. (2010) characterized LTR-RTs in the soybean genome, their work resulted in 950 Mb mapped to the 20 soybean chromosomes. The retrotransposon markers have been successfully applied to the analysis of genetic diversity in different species such as sunflower (Vukich et al. 2009), flax (Smýkal et al. 2011) and brassica (Nouroz et al. 2015).
Transposable elements (TEs) are DNA sequences that move around the genome. There are two classes of TEs: retrotransposons and DNA transposons (Wicker et al. 2007). Transposable elements have two properties: (1) its ability to move within a genome from one place to another, (2) the ability to double their copy via transposition. Under stress conditions, the expression and mobility of TEs are different (Grandbastien et al. 2005).
Molecular markers are essential in plant breeding and biodiversity applications, LTR-RTs are used as molecular markers. Inter-primer binding sites (iPBS) technique is based on the virtually universal presence of a tRNA complement as a reverse transcriptase primer binding site (PBS) in LTR-RTs (Kalendar et al. 2010). The inter-retrotransposon amplified polymorphism (IRAP) method displays insertion polymorphisms by amplifying the segments of DNA between two retrotransposons and need for prior sequence information to design suitable primers (Flavell et al. 2003). RE based techniques were previously used to detect molecular markers for soybean (Sandhu et al. 2017).
This study was aimed to test the effect of water stress on some morphological, physiological and biochemical parameters of the six Egyptian soybean cultivars, to study the genetic variation among soybean cultivars and to detect molecular genetic markers related to drought stress tolerance in soybean using retrotransposon-based molecular markers.
Materials and Methods
Plant Material and Growth Conditions
A pot experiment was conducted in a wire house at the Faculty of Education, Ain Shams University, Egypt during the period from 5 June to 30 July 2016. This experiment was conducted under following environmental conditions (day length 12–14 h, temperature 30–33 °C and humidity ~65%). Soybean seeds (Glycine max L. Giza 21, Giza 22, Giza 35 Giza 82, Giza 83 and Giza 111) were obtained from the Agricultural Research Centre, Egypt. Seeds of the same size and colour were selected and the surface sterilized in distilled water and left to dry at room temperature (25 ºC). Seven seeds/pot (30 cm diameter and 40 cm depth) were sown containing equal amounts of soil. The soil characteristics were: sandy loam in texture, sand 80%, silt 15.9%, clay 4.1%, pH 7.7, EC 0.4 dSm−1 and organic matter 0.60%. Rhizobial inoculants were applied as peat slurry containing 107Rhizobium/g. Seven seeds were sown per pot and were thinned to four after two weeks from planting. Five pots for each treatment were used as replicates. Soybean plants were grown with normal water supply until 25th day from sowing and then were divided into two groups: (i) the first group receives 80% maximum holding capacity (well-watered), (ii) the second group receives 25% of maximum holding capacity (drought). After they were 55 days old, five plants were randomly chosen from each treatment and used to determine morphological criteria. Shoots were used for the biochemical analyses.
Biochemical Analyses
Determination of Photosynthetic Pigments
Carotenoids and total pigments were determined in soybean leaves. The spectrophotometric method recommended by Vernon and Seely (1966) was used.
Determination of Soluble Sugars and Starch Content
Soluble sugars and starch content in shoots of soybean were determined according to Dubois et al. (1956) using phenol sulfuric acid reagent. The absorbance of spectrophotometer was determined at 485 nm.
Determination of Proline Content
Free proline in shoots of soybean plants was determined using the method of Bates et al. (1973) and then the absorbance was determined by Uv-visible spectrophotometer at 520 nm.
Determination of Glycine Betaine
Glycine betaine in shoots of soybean plants was determined according to Grieve and Grattan (1983). The absorbance was measured at 365 nm.
Determination of Lipid Components
Lipids were extracted three times from air dried shoots of soybean plants according to Navari-Izzo et al. (1989). Glycolipids were determined by measuring monosaccharide content by the phenol sulfuric acid reagent (Hodge and Hofreiter 1962). Phospholipids were determined according to Woods and Mellon (1941).
Determination of Cell Wall Fraction
Cell wall fraction was determined according to Bishop et al. (1958) with modification by Dever et al. (1968).
Genetic Relationships Among Soybean Cultivars
IRAP and iPBS techniques were the molecular markers of choice in the present study to reveal differences among soybean cultivars and to detect molecular markers related to water stress. A number of primers were tested for suitable amplifications. Five IRAP and three iPBS primers designed based on LTR-sequences were informative, their sequence GC:AT ratios and suitable annealing temperature are listed in Table 1. Genomic DNA was isolated from leaves of the six soybean cultivars using CTAB methods (Kidwell and Osborn 1992). Amplification was carried out in 20 µl reaction mixture containing 5 ng Templet DNA, 2 µl 10x Dream Taq buffer, 0.4 µl dNTPs, 2 µl IRAP primer, 0.2 µl Dream Taq polymerase (5 U/µl). The amplification conditions were one initial denaturation at 95 °C for 3 min followed by 35 cycles each consists of 95 °C for 20 s, 60 ºC for 30 s, 72 ºC for 90 s, followed by a final extension at 72 ºC for five minutes. 10 μl IRAP-PCR products were loaded with 1% agarose gel in 1xTHE buffer, stained with ethidium bromide and fractionated at constant voltage of 40 V for 16 h. Both monomorphic and polymorphic bands were scored visually.
Statistical Analysis
The results were analyzed according to Gomez and Gomez (1984). The treatments were compared using Duncan Multiple Range Test (Duncan 1955) using MSTAT-c computer software package 1990.
Results
Changes on Morphological Criteria
The results in Table 2 show that the six soybean cultivars varied in their response to water stress. Water stress significantly decreased shoot and root lengths in all soybean cultivars except in Giza 22 and Giza 83 which showed insignificant effects in shoot length and significant increase in root length compared with well watered plants. In addition, fresh weights of shoots and roots were significantly decreased in stressed plants of all soybean cultivars except Giza 22 and Giza 83 which showed insignificant effects in shoots fresh weight and significant increase in roots fresh weight compared with well watered plants.
Moreover, no significant effect in shoots dry weight of stressed plants was observed in all soybean cultivars except Giza 21 and Giza 111 which showed a significant decrease compared with well watered plants. Roots dry weight was significantly decreased in all soybean cultivars but significantly increased in cultivars Giza 22 and Giza 83 when compared with control plants.
Changes in Biochemical Components
Data in Table 3 show that total photosynthetic pigments content was significantly decreased in some soybean cultivars (Giza 21, Giza 111, Giza 35, and Giza 82) but significant increased in the two genotypes Giza 22 and Giza 83 compared with control plants. In addition, water stress caused insignificant difference in carotenoids content in leaves of all soybean cultivars except in Giza 22 and Giza 83 cultivars which showed significant increases compared with well watered plants. Starch content in shoots of all soybean cultivars was significantly decreased compared to well watered plants. Compared to control plants, a drastic increase in total soluble sugars content was observed in shoots of all soybean cultivars. The most pronounced increase was recorded in soybean cultivars Giza 22 and Giza 83. Proline and glycine betaine content was significantly increased in shoots of all soybean cultivars compared to control plants. The most pronounced increase was recorded in cultivars Giza 22 and Giza 83.
Changes in Lipid Components
Total lipids content was significantly increased in shoots of all soybean plants compared to well watered plants. In addition, phospholipids and glycolipids content was significantly decreased in shoots of all soybean cultivars except in the two cultivars Giza 22 and Giza 83 which showed significant increases compared with control plants (Table 4).
Changes in Cell Wall Components
Drought stress caused a significant decrease in cell wall components (pectin, cellulose and lignin) of shoots of all stressed soybean cultivars except the two cultivars Giza 22 and Giza 83 which showed significant increases in lignin content compared to unstressed plants. Hemicellulose contents were significantly increased under water stress (Table 5).
According to these results we may consider soybean cultivars Giza 22 and Giza 83 as tolerant cultivars and Giza 21 and Giza 111 as sensitive cultivars when exposed to water stress.
Molecular Tagging of Soybean Cultivars Using Retrotransposon-Based Markers
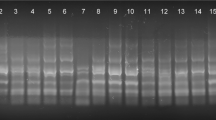
Five IRAP and three iPBS primers were applied to six soybean cultivars (Giza 21, Giza 22, Giza 35, Giza 82, Giza 83 and Giza 111) to determine the genetic difference. A summary of the accumulated results over the eight tested primers is tabulated in Table 6 and Fig. 1. A total number of 127 bands were produced; amongst, 19 were monomorphic and the remaining 108 were polymorphic. Primer IRAP-4377 gave the highest number of polymorphic bands (20 bands) while primers IRAP-4368 showed the lowest number (9 bands). The highest percentage of polymorphic bands was produced by primer IRAP4361 (100%) and the lowest was produced by both primers IRAP-4368 and IRAP-4364 (65% each). In addition, the eight primers produced 35 unique bands that specifically identified their respective genotypes; amongst 21 were positive and 14 were negative bands; primer IRAP-4364 gave the highest number of unique bands/primer (8 bands).
Developing Molecular Markers Related to Water Stress Tolerance in Soybean Cultivars
The reported data showed that the two cultivars Giza 22 and Giza 83 are the most tolerant cultivars and Giza 21 and Giza 111 are the most sensitive ones. The electropherograms of the tolerant cultivars vs. sensitive ones were used to detect molecular markers. Electrophoretic banding patterns resulted from tested IRAP and iPBS primers were examined for markers related to drought tolerance in soybean. Nine bands co-segregated with either tolerant or sensitive cultivars and marked them, these bands resulted from iPBS-2389 and IRAP-4341, IRAP-4368 and IRAP-4377, the resulted band size of different markers are presented in Table 7. Primers IRAP-4361 and IRAP-4377 gave rise to three negative markers for drought tolerance, whereas iPBS-2399, IRAP-4341 and IRAP-4368 revealed one positive marker each that correlated to tolerant cultivars.
Discussion
Water stress caused a significant decrease in plant growth criteria of soybean plants which is similar to results reported by Abass and Mohamed (2011). In a previous study on Egyptian soybean cultivars, Mohamed and Akladious (2014) found that all growth parameters of soybean plants cv. Giza 22 and Giza 111 were significantly decreased under drought stress conditions and the cultivar Giza 22 was found to be more tolerant to drought stress than Giza 111. Also, Mohamed and Latif (2017) reported that drought stress caused reduction in the morphological criteria of soybean cultivars Giza 22 and Giza 35. Drought stress affects cell growth due to the reduction in turgor pressure and the soil water potential (Munns 2002). Root growth is less affected than shoot growth (Hsiao 1973) because the osmotic adjustment in the roots is more efficient than in the shoots (Ober and Sharp 2007). In addition, Jaleel et al. (2008) found that root growth in Catharanthus roseus increased under water stress. Shoot growth decreasing under drought stress might be due to decrease in cell elongation, cell turgor, cell volume and cell growth (Banon et al. 2006).
Total photosynthetic pigments decreased under water stress. These results are similar to Mohamed and Akladious (2014) who found that chlorophyll contents in soybean plants cv. Giza 22 and Giza 111 were significantly decreased under drought stress. The reduction of chlorophyll may be due to the degradation of chlorophyll enzymes (Sabater and Rodriguez 1978), the inhibition in the synthesis of photosynthetic pigments (Murkute et al., 2006), and the inhibition in the uptake of Mg (Sheng et al. 2008). Total photosynthetic pigments increased under water stress in the two soybean cultivars Giza 22 and Giza 83, these increases may be due to increase in leaf area (Benjamin and Nielsen 2006) which may help in the reduction of water loss and the increase in photosynthesis. Also, water stress caused the accumulation in carotenoids content in the leaves of soybean cultivars Giza 22 and Giza 83. Carotenoids are responsible for scavenging of singlet oxygen (Knox and Dodge 1985) and the decrease in carotenoids under water stress might also have contributed to the increased ROS, which further oxidized the photosynthetic pigments.
Drought stress stimulated soluble sugars and decreased starch content in shoots of all soybean cultivars. The increment in soluble sugars under drought stress was also documented by Abass and Mohamed (2011) in commen bean plants. The increase in sugar concentration may be a result from the degradation of starch (Fischer and Höll 1991). Starch may play an important role in accumulation of soluble sugars in cells. Mohammadkhani and Heidari (2008) found that drought stress decreased starch content in maize shoots and roots. Under drought stress, the hydroxyl groups of sugars may replace for water to protect the interactions between membranes and proteins. Proteins and membranes of cells interact with hydrogen bond in sugars and prevent the denaturation of protein (Leopold et al. 1994).
Proline and glycine betaine accumulated under water stress in all soybean cultivars but the most pronounced increase was reported in the two cultivars Giza 22 and Giza 83. These differences may be due to the regulation of proline dehydrogenase (PDH) which is considered as proline degrading enzyme under a variety of stress conditions (Szekely et al. 2008). The increase of proline in plants may be due to an increase in the synthesis of proline enzymes and a decrease in the degradation of proline (Delauney et al. 1993). Proline not only acts as an osmolyte but also helps in the stability of membranes and proteins and in scavenging free radicals under stress conditions (Iqbal 2009).
Glycine betaine is an osmoprotectant which plays an important role in the osmotic adjustment (Munns 2002), protecting the proteins from degradation (Bohnert and Jensen 1996), protecting the structure of membrane (Crowe et al. 1992), protecting the photosynthetic mechanism (Sakamoto and Murata 2002) and scavenging free radicals (Smirnoff and Cumbes 1989). Several reports found that glycine betaine was accumulated under drought stress in drought tolerant species as drought sensitive (Rhodes et al. 1987).
Water stress caused variable changes in lipid fractions of soybean plants. These results are similar to Al-Hakimi (2006) who found that drought stress caused a significant increase in the total lipid and glycolipid contents of shoots and roots of soybean plants but the phospholipids content was significantly decreased. The phospholipids content increased in the tolerant cultivars of soybean (Giza 22 and Giza 83). The increase in the plant growth in these cultivars is attributed to increase in phospholipids content (Xue et al. 2007). On the other hand, Al-Hakimi (2003) reported that phospholipid and glycolipid contents in sunflower plants decreased with decreasing soil moisture content. Glycolipids are the most important compounds of photosynthesis and play an important role in the electron transport system and in the stabilization of thylakoids (Murphy and Woodrow 1983). The decrease in glycolipid contents may be due to the thinning of the chloroplast membranes and the reduction in the electron transport (Navari-Izzo et al. 1993).
Cell wall metabolism is an important component in cell division, elongation and plant growth (Al-Hakimi 2008). Al-Hakimi (2006) found that drought stress caused a significant decrease in the contents of cell wall components of shoots and roots of stressed soybean plants but hemicellulose contents of either shoots and roots were increased under drought stress.
Genetic diversity and relation between or within different individuals, species and populations has become an initial goal that might help breeders in constructing their crossing scheme. Several DNA techniques have been developed to determine genetic variability in different plants (Nybom et al. 2014). In the present investigation, retrotransposon-based techniques were used for tagging soybean cultivars. The technique successfully identified each cultivar with a specific banding pattern when tested against eight IRAP or iPBS primers. Different DNA-based marker technologies have been developed to study polymorphism using subsets of total genomic DNA (Kalendar and Schulman 2006). IRAP method has been exploited to study biodiversity in many plant genera and to determine genomic polymorphism (Kalendar et al. 2010, 2011).
Molecular genetic markers became a corner stone in plant breeding programs, where they help in choosing parents and detecting specific traits throughout a breeding program. Developing molecular genetic markers related to economically valuable traits is fundamental. In this study, several markers related either to tolerance or sensitivity to water stress in soybean were detected. Stress was found to activate the expression of plant transposable elements (Grandbastien et al. 2005; Latif and Mohamed 2016). Retro-elements were suggested as an important creative force in the genome evolution; driving processes such as mutation, recombination, genome expansion and adaptation of an organism to changing environmental conditions (Gogvadze and Buzdin 2009). Under stress, increased levels of TE transposition occur (Oliver and Greene 2009), this might accelerate the rate of genome restructuring and promote potentially useful genetic variability, it is expected that some progeny inherit a favorable adaptation traits to enable survival in the face of biotic or environmental challenges.
Conclusion
The results conclude that the soybean cultivars Giza 22 and 83 are more drought tolerant than the other cultivars, while Giza 21 and Giza 111 were the most sensitives. iPBS and IRAP techniques were used to fingerprint the six soybean cultivars using a set of eight primers. The technique successfully tagged each cultivar with specific bands and also successfully detected molecular genetic markers related to drought tolerance in soybean. We suggest using the tolerant cultivars Giza 22 and Giza 83 in areas which suffer from drought stress.
References
Abass SM, Mohamed HI (2011) Alleviation of adverse effects of drought stress on common bean (Phaseolus vulgaris L.) by exogenous application of hydrogen peroxide. Bangladesh J Bot 41:75–83
Al-Hakimi AMA (2003) Physiological responses of sunflower—seedlings growing under drought stress. FSB 16:169–176
Al-Hakimi AMA (2006) Counteraction of drought stress on soybean plants by seed soaking in salicylic acid. Int J Bot 2(4):421–426
Al-Hakimi AMA (2008) Effect of salicylic acid on biochemical changes in wheat plants under khat leaves residues. Plant Soil Environ 54:288–293
Ashry NA, Mohamed HI (2011) Impact of secondary metabolites and related enzymes in flax resistance and/or susceptibility to powdery mildew. Afr J Biotechnol 11(5):1073–1077
Banon SJ, Ochoa J, Franco JA, Alarcon JJ, Sanchez-Blanco MJ (2006) Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ Exp Bot 56:36–43
Bates LS, Waldren RT, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Benjamin JG, Nielsen DC (2006) Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Res 97(2):248–253
Bishop CT, Bayley ST, Setterfield G (1958) Chemical comnposition of cell Wall of Aveia coleoptiles. Plant Physiol 33:283–289
Bohnert HJ, Jensen RG (1996) Metabolic engineering for increased salt tolerance-the next step. Aust J Plant Physiol 23:661–666
Crowe JH, Hoekstra FA, Crowe LM (1992) Anhydrobiosis. Annu Rev Physiol 54:579–599
Delauney AJ, Hu C‑AA, Kishor PBK, Verma DPS (1993) Cloning of ornithine δ‑aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coli and regulation of proline biosynthesis. J Biol Chem 268:18673–18678
Dever JE, Bandurski RS, Kivilaan A (1968) Partial chemical characterization of corn root cell walls. Plant Physiol 43:50–56
Du J, Tian Z, Hans CS, Laten HM, Cannon SB, Jackson SA, Shoemaker RC, Ma J (2010) Evolutionary conservation, diversity and specificity of LTR-retrotransposons in flowering plants: insights from genome-wide analysis and multi-specific comparison. Plant J 63(4):584–598
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Duncan BD (1955) Multiple ranges and multiple F. Test. Biometrics II:1–42
Fischer C, Höll W (1991) Food reserves in Scots pine (Pinus sylvestris L.). I. Seasonal changes in the carbohydrate and fat reserves of pine needles. Trees 5:187–195
Flavell AJ, Bolshakov VN, Booth A, Jing R, Russell J, Ellis TH, Isaac P (2003) A microarray-based high throughput molecular marker genotyping method: the tagged microarray marker (TAM) approach. Nucleic Acids Res 31:e115
Ford RC, Barber J (1983) Time-dependent decay and anisotropy of fluorescence from diphenylhexatriene embedded in the chloroplast thylakoid membrane. Biochim Biophys Acta 722:341–348
Frederick JR, Camp CR, Bauer PJ (2001) Drought stress effects on branches and main stem seed yield and yield components of determinate soybean. Crop Sci 41:759–763
Gogvadze E, Buzdin A (2009) Retroelements and their impact on genome evolutionand functioning. Cell Mol Life Sci 66:3727–3742
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. John Wiley & Sons Inc, Singapore, p 680
Gong Q, Li P, Ma S, Indu Rupassara S, Bohnert HJ (2005) Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. The. Plant J 44:826–839
Graham PH, Vance CP (2003) Legumes: importance and constraints to greater use. Plant Physiol 131:872–877
Grandbastien M‑A, Audeon C, Bonnivard E, Casacuberta JM, Chalhoub B, Costa APP, Le QH, Melayah D, Petit M, Poncet C, Tam SM, Van Sluys M‑A, Mhiri C (2005) Stress activation and genomic impact of Tnt1 retrotransposons in Solanaceae. Cytogenet Genome Res 110:229–241 (Special Issue on “Retrotransposable Elements and Genome Evolution”)
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Harrison JS, Trevelyan WP (1963) Phospholipid breakdown in baker’s yeast during drying. Nature 200:1189–1190
Hodge JE, Hofreiter BT (1962) Determination of reducing sugars and carbohydrates. In: Whistler RL, Wolfrom ML, Eds., Methods in Carbohydrate Chemistry, Academic Press, New York, 380–394.
Hirsch CD, Springer NM (2017) Transposable element influences on gene expression in plants. Biochim Biophys Acta 1860(1):157–165
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570
Iqbal S (2009) Physiology of wheat (Triticum aestivum L.) accessions and the role of phytohormones under water stress. Ph.D. Thesis, Fac of Biological Sci, Quaid-i-azam Univ, Islamabad 83–154.
Jaleel CA, Manivannan P, Lakshmanan GMA, Gomathinayagam M, Panneerselvam R (2008) Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf B Biointerfaces 61:298–303
Kalendar R, Schulman AH (2006) IRAP and REMAP for retrotransposon-based genotyping and fingerprinting. Nat Protoc 1:2478–2484
Kalendar R, Antonius K, Smýkal P, Schulman AH (2010) iPBS: a universal method for DNA fingerprinting and retrotransposon isolation. Theor Appl Genet 121:1419–1430
Kalendar R, Flavell AJ, Ellis T, Sjakste T, Moisy C, Schulman AH (2011) Analysis of plant diversity with retrotransposon-based molecular markers. Heredity (Edinb) 106:520–530
Kidwell K, Osborn TC (1992) Simple plant DNA isolation procedures. In: Beckmann JS, Osborn TC (eds) Plant genomes: methods for genetic and physical mapping. Kluwer Academic Publishers, Netherlands, pp 1–14
Knox JP, Dodge AO (1985) Singlet oxygen and plants. Phytochemistry 24:889–896
Latif HH, Mohamed HI (2016) Exogenous applications of moringa leaf extract effect on retrotransposon, ultrastructural and biochemical contents of common bean plants under environmental stresses. S Afr J Bot 106:221–231
Leopold AC, Sun WQ, Bernal-Lugo L (1994) The glassy state in seeds: analysis and function. Seed Sci Res 4:267–274
Mohamed HI, Akladious SA (2014) Influence of garlic extract on enzymatic and non enzymatic antioxidants in soybean plants (Glycine max) grown under drought stress. Life Sci J 11(3):46–58
Mohamed HI, Latif HH (2017) Improving the tolerance of soybean genotypes to water stress by foliar application with methyl jasmonate. Physiol Mol Biol Plants 23(2):545–556
Mohammadkhani N, Heidari R (2008) Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl Sci J 3(3):448–453
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Murkute AA, Sharma S, Singh SK (2006) Studies on salt stress tolerance of citrus rootstock genotypes with arbuscular mycorrhizal fungi. Hortic Sci 33:70–76
Murphy DJ, Woodrow IE (1983) Lateral heterogeneity in the distribution of thylakoid membrane lipid and protein components and its implications for the molecular organisation of photosynthetic membranes. Biochim Biophys Acta 725:104–112
Navari-Izzo R, Quaracci MF, Izzo R (1989) Lipid changes in maize seedlings in response to field waterdeficits. J Exp Bot 40:675–680
Navari-Izzo R, Quatacci MF, Melfi D, Izzo R (1993) Lipid composition of plasma membranes isolated from sunflower seedlings grown under water-stress. Physiol Plant 87:508–514
Nouroz F, Noreen Sh, Heslop-Harrison JS (2015) Identification and evolutionary genomics of novel LTR retrotransposons in Brassica. Turk J Biol 39:740–757
Nybom H, Weising K, Rotter B (2014) DNA fingerprinting in botany: past, present, future. Investig Genet 5(1):1–35
Ober ES, Sharp RE (2007) Regulation of root growth responses to water deficit. In: Jenks PM, Hasegawa PM, Jain SM (eds) Advances in molecular breeding toward drought and salt tolerant crops. Springer, Dordrecht, pp 33–53
Oliver KR, Greene WK (2009) Transposable elements: powerful facilitators of evolution genes and genomes. Bioessays 31:703–714
Rhodes D, Rich PJ, Myers AC, Rueter CC, Jamieson GC (1987) Determination of betaines by fast atom bombardment mass spectrometry: identification of glycine betaine deficient genotypes of Zea mays L. Plant Physiol 84:781–788
Sabater B, Rodriguez MT (1978) Control of chlorophyll degradation in detached leaves of barley and oat through effect of kinetin on chlorophyllase levels. Physiol Plant 43(3):274–276
Sairam R, Deshmukh P, Saxena DC (1998) Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol Plant 41:387–394
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Sandhu D, Ghosh J, Johnson C, Baumbach J, Baumert E, Cina T et al (2017) The endogenous transposable element Tgm9 is suitable for generating knockout mutants for functional analyses of soybean genes and genetic improvement in soybean. PLoS ONE 12(8):1–17
Schmutz J, Cannon SB, Schlueter J et al (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Sheng M, Tang M, Chan H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296
Smirnoff N, Cumbes OJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
Smýkal P, Bačová-Kerteszová N, Kalendar R, Corander J, Schulman AH, Pavelek M (2011) Genetic diversity of cultivated flax (Linum usitatissimum L)germplasm assessed by retrotransposon-based markers. Theor Appl Genet 122:1385–1397
Szekely G, Abraham E, Cselo A et al (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Vernon LP, Seely GR (1966) The chlorophylls. Academic Press, New York
Vukich M, Giordani T, Natali L, Cavallini A (2009) Copia and Gypsy retrotransposons activity in sunflower (Helianthus annuus L.). Bmc Plant Biol 9:150
Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA (2005) Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol 162:465–472
Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O et al (2007) A unified classification system for eukaryotic transposable elements. Nat Rev Genet 8:973–982
Wilson RF, Burke JJ, Quisenberry JE (1987) Plant morphological and biochemical responses to field water deficits. II. Responses of leaf glycerolipid composition in cotton. Plant Physiol 84:251–254
Woods JT, Mellon MG (1941) Chloro stannous reduced molybdophosphoric blue colour method, in sulfuric acid system. In: Jackson ML (ed) Soil Chemical Analysis. Printico-Hall International Inc, London
Xiang Lu, Qing-Tian L, Qing Xiong, Li W, Bi Y‑D, Lai Y‑C, Xin-Lei Liu, Wei-Qun M, Wan-Ke Zhang, Biao M, Shou-Yi Chen, Zhang J‑S (2016) The transcriptomic signature of developing soybean seeds reveals the genetic basis of seed trait adaptation during domestication. Plant J 86:530–544
Xue H, Chen X, Li G (2007) Involvement of phospholipid signaling in plant growth and hormone effects. Curr Opin Plant Biol 10:483–489
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgements
The molecular study in this work was supported in part by the ICI project, funded by the Finnish ministry of foreign affairs through the research project entitled with “Enhancing water use efficiency and production methods in faba bean and alfalfa under saline conditions in sinai”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. I. Mohamed, S. A. Akladious and N. A. Ashry declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mohamed, H.I., Akladious, S.A. & Ashry, N.A. Evaluation of Water Stress Tolerance of Soybean Using Physiological Parameters and Retrotransposon-Based Markers. Gesunde Pflanzen 70, 205–215 (2018). https://doi.org/10.1007/s10343-018-0432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-018-0432-1