Abstract
Water stress causes major agricultural loss throughout the world as survival of the crops remained under stress and loss in yield. Plants respond to drought stress by means of different adaptive mechanisms such as accumulation of osmoprotectants to counteract the water stress. Amino acid proline is known to occur widely in higher plants and normally accumulates in large quantities as an osmolyte in response to environmental stresses. Biochemical estimation of proline was done in the drought-affected wheat genotypes by spectrophotometric method. Proline promoted a positive effect as root/shoot ratio was enhanced in wheat germplasm under drought stress. SSR primer pairs (45) were tested for polymorphism among selected wheat genotypes. The dendrogram results have shown the wheat genotype association with the levels of proline during induced drought stress. The relationship between pattern of drought responsive biochemical attributes and DNA markers in the selected wheat genotypes was recognized to select drought tolerant genotypes for sowing in drought affected areas of the country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat is the most widely cultivated cereal crop, and due to its different varieties and nutritive value, it is a staple food for more than one third of the world’s population [1]. It shares the largest cereal market due to its global production at more than 651.4 million metric tons per annum [2]. Wheat production can be enhanced through the development of improved cultivars with wider genetic base capable of producing better yield under various agro-climatic conditions and stresses [3].
In wheat, an assessment of the effects of drought stress on proline accumulation in a drought-tolerant and a drought-sensitive cultivar revealed that the rate of proline accumulation and utilization was significantly higher in the drought-tolerant cultivar [4]. Proline is a highly water soluble amino acid and is accumulated in leaves of many halophytic higher plants grown in saline environments [5, 6]. Proline protects membranes and proteins against the adverse effects of high concentration of inorganic ions and temperature extremes. It is also functional as a protein-compatible hydrotope and as a hydroxyl radical scavenger [7].
Osmotic adjustment provides the means to avoid cellular dehydration, which is essential for maintaining cellular activity [8]. Initially, it was thought that osmotic adjustment occurred only in plants subjected to high salinities, but later studies on plants grown on dry soils showed that this response is also common in conditions of water stress [9, 10]. In addition to its role as an osmolyte for osmotic adjustment, proline contributes to stabilizing sub-cellular structures (e.g., membranes and proteins), scavenging free radicals, and buffering cellular redox potential under stress conditions. The primary function of these osmolytes is to maintain osmotic balance within the cell, and even their high concentrations may not impair the normal physiological function of the cell [11]. Osmotic adjustment is normally facilitated by these organic osmolytes which are presumed to be osmoprotectants or plant life savers even in the deserts’ harsh environment [12].
Pakistan is facing a problem of wheat shortage due to drought situation and had to import wheat to fulfill the need of the country. Plant productivity is hampered by environmental stresses [13], and water shortage definitely limits plant growth and productivity even more than any other environment factor [14]. The basic biotechnology tools can be employed to manage stress tolerance and hence improving yield stability, whereas different genetic markers are identified as linked to different traits of interest [11]. We have exploited SSR markers linked with drought tolerance as genetic markers are important determinants for the study of polymorphism. Our main focus was on DNA fingerprinting of wheat (Triticum aestivum L.) germplasm of different groups like local genotype, mapping population, synthetic, and the National Institute for Biotechnology and Genetic Engineering (NIBGE) varieties on the basis of polymorphism. Biochemical estimation of proline was determined [15], and values were compared within genotype groups to study association of proline linked with drought stress on the bases of DNA fingerprinting. Researchers have an essential task to improve wheat crop production to meet with the shortage situation of this valuable crop of Pakistan.
Materials and Methods
The study was conducted on four different genotypes of wheat samples viz local genotype (LG), mapping population (MP), synthetic (Syn), and NIBGE cultivars subjected to drought stress. Wheat samples were collected from Plant Genomic Molecular Biology lab at NIBGE. Estimation of proline was done partly at the Molecular Biochemistry Lab., and Central Hi-Tech Lab., University of Agriculture, Faisalabad.
Cultivation of Plants and Culture Conditions
Healthy seeds of 66 wheat genotypes were screened out and geminated in sand-filled plastic glasses. Hoagland solution was applied per pot first time. Later on, polyethylene glycol (PEG) was applied on alternate days to induce drought to the experimental pots up to 25–30 mL each pot. Control seedlings were fertilized with full strength Hoagland’s nutrient solution [16]. After the 9th day, thinning was done to select healthy plants for ongoing trail. Selection of the wheat germplasm was made on the basis of local genotype (LG = 20), mapping population (MP = 20), synthetic (Syn = 20), and NIBGE = 06 varieties. Root/shoot ratio was also recorded.
Proline Estimation
After 5 weeks experiment, the plants were collected in polyethylene bags and placed in liquid nitrogen for some time and stored in −80 °C freezer till further use. Proline was determined spectrophotometrically based on ninhydrin procedure [15]. The original method was modified in order to handle a number of samples, and absorbance was measured at 520 nm using toluene as reference on spectrophotometer (Perkin Elmer, λ 25, UK).
Isolation and Amplification of DNA
CTAB method was used to extract DNA from young plant leaves by following Doyle and Doyle [17] protocol briefly describes as follows. Four to five young leaves were cut, washed with distilled water, blot dried, and ground into a very fine powder in liquid nitrogen. The powder was transferred to a 50-mL falcon tube, and 15 mL of hot (65 ° C) 2X CTAB were added. Mixed gently by inverting the tube for several times and incubated at 65 ° C for half an hour. The sample was extracted twice with chloroform/isoamyl alcohol (24:1) and precipitated in 0.6 volumes of ice-cold isopropanol. The pellet was washed thrice with 70 % ethanol and resuspended in TE buffer or dH2O. RNA was removed by treating the samples with RNase for 1 h at 37 °C. Integrity of the isolated DNA was checked by ethidium bromide staining through agarose gel electrophoresis. Concentrations of the isolated DNA genotypes were determined on GeneQuantpro (Amersham). The SSR primer pairs of KSUM series were deigned from wheat genome database and obtained from Eurofins mwg/operon, Canada. Forty five primer pairs were selected and used in PCR amplification for DNA fingerprinting of selected wheat genotypes. The isolated samples were amplified by PCR run on agarose gel electrophoresis, and the results were recorded on gel documentation system [18].
Results and Discussions
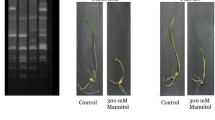
The study was conducted on 66 different genotypes of four wheat groups with or without PEG application up to 41 days under growth control chamber experiment. The root-shoot dry weight was recorded to find out root/shoot ratio for each genotype viz NIBGE, mapping population, local genotype, synthetic along with respective mean value with standard deviations as reflected in Table 1.
Considerable variations in root/shoot ratios were observed among the genotypes those were found better ratios under normal than under the moisture stress conditions, indicating suppression of variability under moisture stress conditions. The root/shoot length ratio was increased under osmotic stress as compared to the normal conditions. Correlation studies indicated that the osmotic membrane stability of the leaf segment was the most important trait, followed by root/shoot ratio and root length on the basis of their relationships with other traits.
Proline Estimation
Proline is an amino acid synthesized from glutamate or ornithine since both pathways contribute to proline synthesis under normal physiological conditions. Plants under environmental stress may present increased levels of certain compounds like Proline which accumulates in leaves as a response to such stress types, especially water stress [19].
The values of all LG genotypes showed high levels of proline in drought situation as compared to their respective controls indicating the high need of this osmolyte rise in case of oxidative stress. Genotypes LG 03 reflecting higher levels of proline with lower levels of control sample i.e., 7.8 μmol/g for drought and 2.8 μmol/g with just lower levels as shown in Fig. 1a. The proline is a vital osmolyte frequently used by cells to accommodate osmotic pressure within the effected cells to avoid cellular injury due oxidation phenomenon.
The values of all MP genotypes showed high levels of proline in drought situation as compared to their respective controls indicating the high need of this osmolyte rise in case of oxidative stress. Genotypes MP 26 reflecting higher levels of Proline with considerable levels of control sample i.e., 7.7 μmol/g for drought and 4.2 μmol/g with control levels as shown in Fig. 1b. The proline is a vital osmolyte frequently used by cells to accommodate osmotic pressure within the effected cells to avoid cellular injury due oxidation phenomenon.
The values of all synthetic population genotypes showed higher levels of proline in drought situation as compared to their respective controls indicating the high need of this osmolyte rise in case of oxidative stress. Genotypes Synthetic 7-47D reflecting higher levels of proline with lower levels of control sample i.e., 7.8 μmol/g for drought and 3.8 μmol/g with control levels as shown in Fig. 1c. The proline is a vital osmolyte frequently used by cells to accommodate osmotic pressure within the effected cells to avoid cellular injury due to oxidation phenomenon.
The values of four NIBGE genotypes showed higher levels of proline in drought situation as compared to their respective controls indicating the high need of this osmolyte rise in case of oxidative stress. Genotypes NIBGE 2 reflecting higher levels of proline with lower levels of control sample i.e., 7.8 μmol/g for drought and 5.2 μmol/g in control samples as shown in Fig. 1d. Genotype NIBGE 4 and NIBGE 6 indicating lower levels of drought samples as compared to control which showed elevated levels for these genotypes. The lower levels of proline in these genotypes indicate less production of the osmolyte required for drought tolerance reflecting somewhat drought resistant varieties. The proline is a vital osmolyte frequently used by cells to accommodate osmotic pressure within the effected cells to avoid cellular injury due to oxidation phenomenon.
The mean standard deviation of the presented genotypes in case of proline has shown that there was a marked difference between drought and control samples except NIBGE genotype have not shown significant difference (Table 2). The proline levels in all the genotypes had higher values as compared to their respective control samples. Higher levels of drought samples in all genotypes have shown indication of stress induced during the experiment in plants. On the other hand, control samples showed normal range of proline released under Hoagland solution medium.
By using Minitab tool for one-way ANOVA for Proline have shown non- significant effect on each other as shown in Table 3.
DNA Fingerprinting
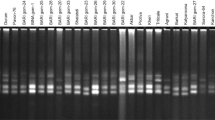
The SSR marker system was used to explore the extent of genetic diversity among presented wheat genotypes. PCR Primers of KSUM 66 series were designed and used to study polymorphism among different wheat genotypes. Concentration of the isolated DNA of all the genotypes was found ranging from 1668 to 328 ng/uL at 260 nm absorbance A few representative figures are reported as Fig. 2a–d.
a Polymorphism in Local genotypes with KSUM 66. Legends: M = ladder, 1 = LG 37, 2 = LG 51, 3 = LG 03, 4 = LG 34, 5 = LG 24, 6 = LG 22, 7 = LG 25, 8 = LG 64, 9 = LG 58, 10 = LG 01, 11 = LG 15, 12 = LG 60. b Polymorphism in Mapping population genotypes with KSUM 66. M = ladder, 1 = MP 46, 2 = MP 78, 3 = MP 41, 4 = MP 91, 5 = MP 63, 6 = MP 73, 7 = MP 37, 8 = MP 75, 9 = MP 64, 10 = MP 26, 11 = MP 120, 12 = MP 60, 13 = MP 82. c Polymorphism in Synthetic genotypes with KSUM 66. M = Ladder, 1 = Syn 7–5, 2 = Syn 7–7, 3 = Syn 7–19, 4 = Syn 7–75, 5 = Syn 7–54, 6 = Syn 7–65, 7 = Syn 7–32, 8 = Syn 7–22, 9 = Syn 7–47, 10 = Syn 7–45, 11 = Syn 6–12, 12 = Syn 6–10, 13 = Syn 6–6. d Polymorphism in NIBGE genotypes with KSUM 66. Legend: M = Marker, NIBGE 1 → 6
Statistical Analysis
All polymorphic loci were scored with 1 for presence and 0 for absence among all genotypes of wheat germplasm lines. The bivariate 0–1 data was used to construct the similarity matrix based on the Nei and Li [20] coefficient to estimate the genetic diversity among four wheat genotypes, i.e., LG, MP, Syn, and NIBGE. The genetic association among wheat genotypes has been graphically presented in the form of an individual dendrogram using an unweighted pair group of arithmetic means (UPGMA).
The dendrogram resulting from cluster analysis of NIBGE genotypes revealed 2 clusters holding all 6 NIBGE genotypes in such a way that NIBGE 4 and 3 have same genetic makeup (Fig. 3a). In the second cluster, NIBGE 5 and 2 while NIBGE 6 and 1 were under this dandrogram showing variable similarity. This small group included 6 genotypes of NIBGE origin presenting linkage or association among these genotypes. A maximum genetic similarity value of 0.84 was observed between NIBGE 6 and 1, which revealed a high degree of similarity to the extent of 84 % existing between them. A minimum genetic similarity value of 0.76 was observed between NIBGE 5 and 2, which showed that they are 24 % dissimilar. Similar studies were conducted by different investigators using SSR markers [21].
The dendrogram resulting from cluster analysis of local genotypes revealed 3 main clusters (Fig. 3b). Cluster I holding 5 genotypes as LG 35, LG 47, LG 39, LG 30, and LG 44. Cluster II was the largest and included 10 genotypes namely LG 10, LG 01, LG 58, LG 41, LG 64, LG 22, LG 34, LG 25, LG 57, and LG 15. Cluster III comprised the remaining 5 genotypes as LG 24, LG 60, LG 03, LG 51 and LG 37. A maximum genetic similarity value of 0.98 was observed between LG 53 and 47, which revealed a high degree of similarity to the extent of 98 % existing between them. A minimum genetic similarity value of 0.54 was observed between LG 01 and LG 58, which showed that they are 42 % dissimilar. Similar studies were conducted by different investigators using SSR markers [22, 23].
The dendrogram resulting from cluster analysis revealed 3 main clusters for mapping population genotype (Fig. 3c). Cluster I holding 8 genotypes as MP 40, MP 134, MP 61, MP 82, MP 51, MP 64, and MP 71. Cluster II was the largest and included 7 genotypes namely MP 75, MP 73, MP 27, MP 63, MP 26, MP 59, and MP 91. Cluster III comprised the remaining 6 genotypes as MP 37, MP 120, MP 41, MP 60, MP 78, and MP 46. A maximum genetic similarity value of 0.82 was observed between MP 61 and MP 82, which revealed a high degree of similarity to the extent of 82 % existing between them. A minimum genetic similarity value of 0.46 was observed between MP 78 and MP 46, which showed that they are 54 % dissimilar. Similar studies were conducted by different investigators using SSR markers [21, 22].
The dendrogram resulting from cluster analysis revealed 3 main clusters for synthetic genotype (Fig. 3d). Cluster I holding 9 genotypes as Syn 6–7, Syn 7–47, Syn 3–21, Syn 6–14, Syn 7–65, Syn 7–54, Syn 6–6, Syn 7–45, and Syn 7–22. Cluster II included 7 genotypes namely Syn 3–19, Syn 6–11, Syn 7–19, Syn 6–9, Syn 7–32, Syn 6–12, and Syn 7–75. Cluster III comprised the remaining 4 genotypes as Syn 6–16, Syn 6–10, Syn 7–7, and Syn 7–5. A maximum genetic similarity value of 0.80 was observed between Syn 6–16 and Syn 6–10, which revealed a high degree of similarity to the extent of 80 % existing between them. A minimum genetic similarity value of 0.63 was observed between Syn 6–9 and Syn 7–32, which showed that they are 37 % dissimilar. Similar studies were conducted by different investigators using SSR markers [23].
Palombi and Damiano [24] have also studied comparison between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit where they have found that SSR are better markers as compared to RAPD. The correlation was also established within all groups and genotypes along with other variables responsible for change in drought stress. Data were analyzed by using Minitab 15 for one way ANOVA as there was no blocking available in this experiment. Correlation was used to study root/shoot ratio variation in comparison to osmolyte proline [25].
References
Asif, M., Rahman, M., & Zafar, Y. (2005). DNA fingerprinting studies of some wheat (Triticum aestivum L.) genotypes using RAPD analysis. Pakistan Journal of Botany, 37(2), 271–277.
Food and Agriculture Organization 2012. Annual Wheat Report. Internet source: www.fao.org/worldfoodsituation
Zhu, Y., Chen, H., Fan, J., Wang, Y., Li, Y., Chen, J., Fan, J., Yang, S., Hu, L., Leung, H., Mew, T. W., Teng, P. S., Wang, Z., & Mundt, C. C. (2000). Nature, 406, 718–722.
Nayyar, H. (2004). Variation in osmoregulation in differentially drought sensitive wheat genotype involves calcium. Journal of Biologia Plantarum, 47, 541–547.
Stewart, G. R., & Lee, J. A. (1974). Proline accumulation: biochemical aspects. In “Physiology and Biochemistry of Drought Resistance in Plants”. Planta, 120, 279–289.
Briens, M., & Larher, F. (1982). Osmoregulation in halophytic higher plants: a comparative study of soluble carbohydrates, polyols, betaines and free proline. Plant, Cell and Environment, 5, 287–292.
Smirnoff, N., & Cumbes, Q. J. (1989). Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry, 28, 1057–1060.
Bartels, D. (2002). Drought and desiccation induced modulation of gene expression in plants. Journal of Plant Cell and Environment, 25, 141–151.
Cushman, J. C. (2001). Osmoregulation in plants: implications for agriculture. American Zoologist, 41, 758–769.
Steudle, E. (2000). Advances in the studies on water uptake by plant roots. Journal of Experimental Botany, 51(530), 1531–1542.
Iqbal, M. J., Rahman, M., Ashraf, M., Sheikh, M. A., & Jamil, A. (2012). Trehalose expression in hexaploid wheat (Triticum aestivum L.) germplasm under drought stress. Pakistan Journal of life Social Sciences, 10(2), 106–110.
Aziz, A., & Khan, M. A. (2003). Proline and water status of some desert shrubs before and after rains. Pakistan Journal of Botany, 35, 902–906.
Hare, P. D., Cress, W. A., & Staden, J. V. (1996). Dissecting the roles of osmolyte accumulation during stress. Plant, Cell and Environment, 21, 535–553.
Boyer, J. S. (1982). Plant productivity and environment. Science, 218(4571), 443–448.
Abraham, E., Cabassa, C. H., Erdei, L., & Szabados, L. (2010). Methods for determination of proline in plants. Methods in Molecular Biology, 639, 317–331.
Hoagland, D.R., & Arnon. D.I. (1950). The water culture method for growing plant without soil. CA. USA, pp. 347.
Doyle, J. J., & Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin, 19, 11–15.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning, a laboratory manual (3rd ed.). New York: Cold Spring Harbor.
Stewart, C.R. (1981). Proline accumulation: biochemical aspects. In “Physiology and Biochemistry of Drought Resistance in Plants,” Academic Press, Sydney, pp 243–259.
Nei, M., & Li, W. H. (1979). Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences of the United States of America, 76, 5269–5273.
Chakravarthy, B. K., & Naravaneni, R. (2006). SSR marker based DNA fingerprinting and diversity study in rice (Oryza sativa. L). African Journal of Biotechnology, 8, 684–688.
Panaud, O., Chen, X., & McCouch, S. R. (1996). Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oriza stiva L.). Molecular and General Genetics, 252, 597–607.
Ram, S. G., Thiruvengadam, V., & Vinod, K. K. (2007). Genetic diversity among cultivars, landraces and wild relatives of rice as revealed by microsatellite markers. Journal of Applied Genetics, 48, 337–345.
Palombi, M. A., & Damiano, C. (2002). Comparison between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Reports, 20, 1061–1066.
Steel, R. G. D., & Torrie, J. H. (1990). Principles and procedures of statistics. New York. USA: McGraw.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iqbal, M.J., Maqsood, Y., Abdin, Z.U. et al. SSR Markers Associated with Proline in Drought Tolerant Wheat Germplasm. Appl Biochem Biotechnol 178, 1042–1052 (2016). https://doi.org/10.1007/s12010-015-1927-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1927-1