Abstract
Human activities accelerate global nitrogen (N) deposition, and elevated N availability may alter the stoichiometric balance of nutrients and then affect nutrient absorption by plants. The boreal forest is considered one of the world’s most N-limited ecosystems, and its response to N deposition is already a hot issue. In order to explore how long-term nitrogen addition influences nutrient uptake and distribution in Larix gmelinii in a boreal forest, four N treatment levels (0, 25, 50 and 75 kg N ha−1 yr−1) have been applied in a boreal forest since May 2011. Nitrogen addition significantly reduced the soil pH, significantly changed the soil N availability, increased the total N and N/P in needles and fine roots, and decreased the total P in needles and the C/N in soil. Nitrogen addition significantly reduced nitrogen resorption efficiency, and its impacts on P resorption efficiency were not significant. Nitrogen addition significantly increased the root length, surface area and diameter of 4th- and 5th-order transport fine roots. The N and N/P of needles showed seasonal variation. The needle N concentration and N/P were positively correlated with N addition, while the needle P was negatively correlated with nitrogen addition. With increase in nitrogen addition, Larix gmelinii increased its investment in its belowground parts, which may explain why Larix gmelinii tended to put more C in long-lived roots to improve its C utilization efficiency. Given the P deficiency caused by N addition, Larix gmelinii may be more likely to absorb P from the soil and adjust its C distribution to meet its P demand rather than relying on internal nutrient resorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past several decades, human activities have doubled the global reactive nitrogen (N) supply as fertilizer use and fossil fuel combustion have increased (Galloway et al. 2008; Bobbink et al. 2010). Increased N deposition may lead to unbalanced inputs of N and phosphorus (P) in ecosystems (Clark and Tilman 2008), altering the environment for plants and affecting nutrient uptake and resorption by plants (Stevens et al. 2010; Zhan et al. 2017). Changes in soil and plant nutrient status can have an impact on plant nutrient conservation strategies (Vergutz et al. 2012; See et al. 2015). It is generally considered that soil nutrient availability has a negative impact on nutrient uptake (Tully et al. 2013; See et al. 2015; Yuan and Chen 2015). However, studies have shown that plants growing in low-fertility soils do not have higher nutrient uptake rates than plants in fertile soils (Aerts 1996), and fertilization studies have not shown a consistent response of nutrient uptake to an increase in soil nutrient availability (Midgley et al. 2016; Han et al. 2019).
Nitrogen and phosphorus are generally considered to be the two most limiting elements for net primary productivity (NPP) in terrestrial ecosystems (Koerselman and Meuleman 1996; Güsewell 2004; Kang et al. 2011; Zhu et al. 2016). The nitrogen to phosphorus ratio in plants can be used as an indicator of nutrient supply, and it can also be characterized as the plant nutrient assimilation status of nitrogen and phosphorus (Zhang et al. 2018). Touratier et al. (2001) found that leaf N/P ratio was affected by sampling time (growing month). Agren (2008) showed that N/P ratio of leaves decreased with plant growth in terrestrial ecosystems. However, some studies had shown a seasonal variation pattern of increasing in summer then decreasing in autumn (Orgeas et al. 2003), which indicated that the changes of N and P patterns in plant leaves were regulated by many factors. Plants absorb nitrogen and phosphorus in a certain proportion for their own growth. When one element is scarce and another element is relatively abundant, the scarce element becomes the main limiting factor for plant growth. Carbon (C), N and P in plants and soils are interrelated due to their atmospheric and terrestrial cycling (Elser et al. 2010). The continuous increase in anthropogenic N deposition leads to an imbalance in N and P inputs in ecosystems, changes plant resource acquisition strategies and affects the nutrient cycle of the ecosystem (Mahowald et al. 2008; Peñuelas et al. 2012).
In theory, plants exhibit a certain degree of morphological plasticity and nutritional balance in individual organisms (Sterner and Elser 2002; Augusto et al. 2008). Through long-term natural selection, the whole plants have developed a set of absorption strategies to adapt to environmental changes (Reich et al. 2003). Plants may maintain their stoichiometric homeostasis via regulating root nutrient capture (acquisition) and/or leaf nutrient resorption (Kobe et al. 2005; Vergutz et al. 2012; Zhang et al. 2017). Plant nutrient conservation efficiency in nutrient-poor environments is higher, which may be due to the increased nutrient absorption by leaves (Vergutz et al. 2012). The distribution of the nutrients captured through roots and leaf resorption depends on the availability of environmental nutrients and the costs involved in these processes (Wright and Westoby 2003; Wang et al. 2014). In nutrient-deficient environments, energy costs are usually proportional to the nutrient uptake, and exploring for nutrients requires a substantial investment to construct longer roots (Hodge 2004). Therefore, nutrient resorption may play a key role in balancing the stoichiometry of plants under these conditions. As the efficiency of nutrient use increases, the cost for plants to obtain nutrients from the soil decreases. Nutrients acquired from root uptake are less costly than those acquired from leaf nutrient resorption, and plants are more inclined to choose root capture strategies (Huang et al. 2015; Kou et al. 2017).
Fine roots, as the most active part of roots, account for more than 30% of the NPP in terrestrial ecosystems (Jackson et al. 1997). Their morphological and chemical characteristics are very sensitive to environmental changes, which can effectively indicate changes in the nutrient cycle (Bardgett et al. 2014; Wurzburger and Wright 2015). Fine root diameter, specific root length (SRL), specific root area, root surface area, branching rate and root tissue density are the key characteristics of root resource capture (Eissenstat et al. 2015; Wang et al. 2017). Plant roots play an important role in the belowground processes of ecosystems. Many studies have shown that different levels of fine roots have different physiological functions (Pregitzer et al. 2002; McCormack et al. 2015). In addition, the morphological characteristics of fine roots are also affected by atmospheric nitrogen deposition (Kou et al. 2015). Changes in soil nutrient cycles can be effectively reflected in root morphology and chemical changes (Zhu et al. 2013; Xia et al. 2017; Yan et al. 2017).
The boreal forest area accounts for about 14.5% of the total land area and 30% of the forest area, which is the second largest forest biome on land, and it is recognized as one of the most N-limited ecosystems (Vitousek et al. 2002; T Randerson et al. 2006), mainly because of its remoteness from N emission sources. In this context, increased N deposition on boreal forests is thought to act as a fertilizer (Flechard et al. 2010) that can stimulate tree growth (Pregitzer et al. 2010; Stuart et al. 2015). However, the evidence is lacking about the impact of increasing N deposition as well as of N accumulation in forest ecosystems over longtime scales (Bontemps et al. 2011).Therefore, it is of great significance to study the effects of nitrogen deposition on the main ecosystem processes of boreal forests, especially on the nutrient cycling processes below- and aboveground.
The Greater Khingan Mountains area is the only open coniferous forest area in the cold temperate zone in China, and Larix gmelinii is the main dominant tree species in the Greater Khingan Mountains forest community. To reveal the response of leaf nutrient uptake, root morphology and chemical characteristics to nitrogen deposition and their relationship with soil in a Larix gmelinii forest, a long-term nitrogen addition experiment was conducted in the Greater Khingan Mountains, Northeast China. The purpose of this study was to answer the following questions: (1) Does N addition alter the nutrient concentrations and stoichiometry in soil, fine roots, litter and needles? (2) Does N addition alter nutrient resorption by Larix gmelinii? (3) Does N addition alter various aspects of fine root morphology? We hypothesized that (1) nitrogen addition might reduce the nitrogen resorption efficiency (NRE) and phosphorus resorption efficiency (PRE); (2) TN in needles would show seasonal variation (3) The SRL of fine roots would be reduced by nitrogen addition, and the morphology of fine roots of different root orders would respond differently to different levels of nitrogen addition.
Materials and methods
Site description and experimental design
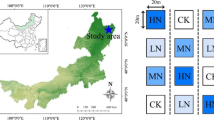
This study was carried out in a Larix gmelinii forest of the Nanwenghe National Natural Reserve in the Greater Khingan Mountains, Northeast China (51°05′-51°39′ N, 125°07′-125°50′ E). The climate in this area is a typical continental climate in the cold temperate zone. Affected by the Siberian cold current, the mean annual temperature is approximately −2.7 °C. The peak high temperature is 36 °C, and the minimum low temperature is −48 °C. The accumulated temperature above 10 °C is 1400–1600 °C, the annual sunshine time is approximately 2500 h, and the plant growth period is approximately 110 days. The mean annual precipitation is approximately 500 mm, mostly concentrated in July and August. The Larix gmelinii forest is the dominant plant community in the experimental area. The growth density of trees in the plot is 2852 (± 99) trees/ha, with an average DBH of 8.98 (± 0.32) cm. The main soil types in the experimental plot are sandy loam (0–20 cm) and gravel sand (20–40 cm).
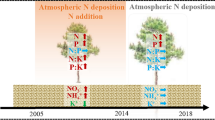
To study the effect of nitrogen deposition, a nitrogen addition experiment was established. We randomly established 12 plots of 20 m × 20 m (Fig. 1). Based on the current nitrogen deposition rate (25 kg N ha−1 yr−1) in northern China (Liu et al. 2013), a total of 4 treatments were set: control (0 kg N ha−1 yr−1), low nitrogen (25 kg N ha−1 yr−1), medium nitrogen (50 kg N ha−1 yr−1) and high nitrogen (75 kg N ha−1 yr−1) to simulate the response of plants to climate change against the backdrop of future atmospheric nitrogen deposition levels that are 1, 2 and 3 times the current rate, respectively, and three repetitions were set for each treatment. A 10-m-wide buffer belt was set up between the plots to prevent interference between the plots. The artificial application of nitrogen began in May 2011. Nitrogen was evenly sprayed once per month every year in the growing season of the local forest (May–September). Before each application, NH4NO3 was weighed according to the rate of nitrogen addition, mixed with 32 L water and then evenly sprayed with a sprayer on the forestland in each plot. To compensate water supply, the control plot was sprayed with the same amount of pure water.
Soil properties and plant nutrients
From May to September 2019, we used stainless steel samplers (5 cm in diameter and 20 cm in length) to take soil samples from five random locations in each plot once each month. Subsequently, each soil core was divided into 0–10 cm (surface soil layer) and 10–20 cm (deep soil layer) depth intervals. Representative soil samples were obtained from the different soil cores by combining and homogenizing the soil samples from the same soil layer. The soil samples were air-dried, and roots and other debris were removed. The pH value, total N, total C and total P of the soil were measured after grinding.
Five litter collectors (baskets made of PVC pipe and gauze, 1 m × 1 m × 1 m) were placed in each plot according to the five-point method, for a total of 60 litter collectors. The litter was collected once a month, and the litter from the five collectors in the same plot was combined evenly to obtain mixed samples. After being taken back to the laboratory, the litter samples were dried and crushed for analysis. The needles of Larix gmelinii were sampled monthly. Three healthy mature trees (with DBH and height similar to the average level) were randomly selected, and typical individual branches were collected from the upper two-thirds of the crown with a high pole pruner. The needles of three trees in each sample plot were mixed evenly, and a composite sample (approximately 300 g) was obtained. Once in the laboratory, the needles were dried and pulverized before analysis. This study excluded needles with any visible damage. We used the green leaves collected in summer and litter collected in autumn to calculate the nutrient reabsorption of leaves.
A stainless steel sampler was used to collect fine root samples within a 50-cm radius of Larix gmelinii (near the same trees that were selected for foliar sampling). The soil blocks were divided into the surface soil layer (0–10 cm) and the deep soil layer (10–20 cm). Living fine roots were extracted from soil blocks, washed with tap water on a 2-mm sieve cloth, washed with deionized water and stored at 4 °C. Fine root samples were scanned with a scanner (Expression 11000XL, Epson, NSW, Australia) at a resolution of 600 dpi. Based on a stream-order approach for the study of root branch orders (Pregitzer et al. 2002), the morphological changes in all fine root orders were studied. Distal roots were classified as first-order roots, while the joining of two first-order roots was classified as a second-order root. Third-, fourth- and fifth-order roots were defined in a similar manner. After scanning, the roots were dried and ground for further analysis.
The total N concentration in the soil, needles, litter and whole fine root samples was detected by a continuous flow analyzer (Skalar san+, Netherlands). Total C (TC) was determined by an automatic TC/TN analyzer (Analytik Jena AG, multi N/C 3100, Germany). Total P was determined by an ultraviolet–visible spectrophotometer (SHIMADZU UV-1780, Japan), and soil pH was measured by a pH meter (SX7150, China) in a 1:2.5 (soil/water) suspension. WinRHIZO TronMF 2012 software (Regent Instrument Inc., Quebec, Canada) was used to analyze the roots in the captured images and to perform fine root classification using a stream-order approach.
Data analysis
The nutrient resorption efficiency (RE) was calculated as [(NM—NS)/NM] × 100, where NM is the nutrient concentration of fresh leaves and NS is the nutrient concentration of senescent leaves. Specific root length (SRL) is the scanning root length divided by the mass after drying. The normal distribution of all the data was tested by the Kolmogorov–Smirnov test, and the homogeneity of variances was tested by Levene's test. We used one-way analysis of variance (ANOVA) to analyze the effects of nitrogen addition on the total C, total N and total P in the needles. We used two-way analysis of variance (ANOVA) to analyze the effects of N addition on soil and fine root TC, TN and TP at different soil depths, as well as the effect of fine roots length, diameter and surface area. General linear model-multivariate analysis of variance (ANOVA) was used to study the effects of months, treatments and their interactions on the physicochemical properties of Larix gmelinii needles. Pearson correlation was used to analyze the correlation between needles and soil and fine roots at different soil depths, and we performed regression analysis. All analyses were performed with SPSS 22.0 software package (SPSS, Inc., Chicago, Illinois, USA). Diagrams were drawn using Sigmaplot 13.0 software (Systat Software Inc., Chicago, IL, USA). Tukey’s post hoc test was used to test differences between treatments. Statistically significant differences were accepted at P < 0.05.
Results
Response of soil stoichiometric characteristics to N addition
Compared with the control, nitrogen addition had no significant effect on the soil TC or TP concentrations in all treatments but significantly increased the soil TN concentration and soil N/P ratio and decreased the soil C/N ratio (Table 1). Nitrogen addition significantly reduced soil pH values (Table 1). High-N treatment significantly increased the C/P in 10–20 cm soil. Total N, TC, TP, C/N, N/P, pH in different soil layers had the same response to nitrogen addition (Table 1).
Effects of N addition on plant nutrient stoichiometry
Nitrogen addition significantly affected the N and P concentrations of needles compared to the control, but had a small effect on the C concentration, only the high-N treatment in July and August significantly increased the needle TC (Fig. 2a–c). The N and P concentrations in needles showed seasonal changes, and the values in July were higher compared to the rest of the period studied. The needle N concentration in September was the lowest compared with those in other months (Fig. 2b, c). Nitrogen addition (high- and medium-N) significantly increased TN, N/P and C/P and decreased TP and C/N in needles compared to the control treatment (Fig. 2b–f), and the needle N/P ratio under nitrogen addition was in the range of 7.76–13.08 (Fig. 2f). Nitrogen addition significantly increased TN and decreased TP in litter (Table 2). For the fine roots, N addition had no significant effect on TC, TP or the C/P ratio (Table 3). High-N treatment significantly increased the N concentration and N/P ratio and decreased the C/N ratio at both soil depths (Table 3). Moreover, the interaction of N addition and month had a significant impact on needle TN, C/N and N/P (Fig. 2b, d, f). High-N treatment significantly reduced the N resorption efficiency (NRE) (Fig. 3a). PRE was significantly higher in the medium-N treatment than in the control, but the PRE in the control treatment was very low, less than 10% (Fig. 3b).
Effects of N addition on C, N and P concentrations (a–c) and the C/N, C/P and N/P ratios (d–f) in fresh needles of Larix gmelinii. Bars with different letters are significantly different from each other (P < 0.05). Data are expressed as the mean ± standard error. Lowercase letters indicate the significance among different treatments, and uppercase letters indicate the significance among different months
Effects of N addition on fine root morphological traits
In this study, fine roots of Larix gmelinii were categorized into five root orders. High-N treatment significantly reduced the specific root length (SRL) of fine roots in the 0–10 cm soil layer (Table 3). Medium-N treatment significantly increased the mean diameter of the fourth- and fifth-order fine roots at both soil depths, and medium-N treatment significantly increased the mean diameter of the first- and third-order fine roots in the 0–10 cm soil layer but had no significant effect on the mean diameter of the fine roots in the 10–20 cm soil layer (Fig. 4). Medium-N treatment significantly increased the root length and surface area of fourth- and fifth-order transport fine roots but had no significant effect on lower-order fine roots (Fig. 4). The average diameter, length and surface area of fine roots were positively correlated with root order.
Effect of N addition on fine root mean diameter (a: 0–10 cm soil; b: 10–20 cm soil), mean length (c: 0–10 cm soil) and mean surface area (d: 0–10 cm soil) of Larix gmelinii by root order (mean ± SE). Different letters indicate significant differences among N addition treatments (P < 0.05, Tukey’s honestly significant difference test)
The relationships between soil and plant nutrients
There was a significant positive correlation between needle TN and soil TP concentration (Fig. 5b), and a negative correlation between needle TP and fine root TN concentration (Fig. 5a). There was a significant negative correlation between fine root TC and soil TP (Fig. 5c). There was no correlation between soil TP and fine root TN (Fig. 5d).
a: The correlation analysis of needle P concentration and fine-root N concentration in 0–10 cm soil; b: the correlation analysis of needle N concentration and P concentration in 0–10 cm soil; c: the correlation analysis of fine-root C concentration in 0–10 cm soil and P concentration in 0–10 cm soil; d: the correlation analysis of P concentration in 0–10 cm soil and fine-root N concentration in 0–10 cm soil
Discussion
Effect of N addition on elemental and nutrient stoichiometry in plant-soil systems
Forests in temperate regions are generally considered to be N-limited (Vitousek and Howarth 1991; Vitousek et al. 2010). Our results showed that the needle N/P ratio was in the range of 7.76–13.08 (Fig. 2f), which was slightly higher than the European coniferous forest of 9.76 (Kang et al. 2011) and slightly lower than the Chinese temperate Larix gmelinii of 8.08–13.81 (Li et al. 2017). This result seems to be similar to the global research of Peter et al. (2004) that as the environmental temperature increases and the latitude decreases, the N/P of plant leaves increases. Previous studies in our laboratory (Yan et al. 2018) found that N addition significantly increased the total biomass of Larix gmelinii trees, increasing also the rate of annual growth. The addition of nitrogen increased the total solid stock of carbon, but as the amount of N deposited increased, the net effect of N addition diminished, suggesting that N might be a limiting factor in boreal forests of Larix gmelinii. Our results showed that nitrogen deposition caused a nutrient imbalance in the Larix gmelinii forest ecosystem in the Greater Khingan Mountains, especially in the soil. Nitrogen deposition significantly increased the ratio of N/P in the soil and decreased the soil pH and soil C/N (Table 1). Soil acidification may lead to the degradation of root function, increasing the toxicity of Al3+ and affecting the resorption of nutrients by roots (Chen et al. 2017). A decrease in the soil C/N ratio may also be beneficial for plant growth and increase soil microbial biomass and activity (Li et al. 2016). Our results also showed that the response of C, N and P in different soil depths to N addition was consistent, which was different from the results of Park and Ro (2018). This may be related to the local soil that is very thin, only 20–30 cm thick, and the underlying layer is full of stones. In terrestrial ecosystems, N and P in plants were derived from soil pools, and the C, N and P concentration in the soil mainly depends on the release of C, N and P through litter decomposition by microbes (Luciola et al. 2012). However, litter is difficult to decompose by microbial action in soil with a C/N ratio > 25 (Tian et al. 2002). During the growing season of the study, the C/N ratio of Larix gmelinii leaves was greater than 25, which could affect the return of nutrients from litter degradation. This phenomenon may explain the N-limited growth pattern of Larix gmelinii. After 8 years of artificial nitrogen application, the needle N concentration increased significantly, while the needle P concentration decreased significantly in the growing season, which was consistent with the research results of Kou et al. (2017). Green leaf nutrient concentration can reflect soil fertility in some cases (Lambers et al. 2008), which indicates that N addition may change the availability of soil nutrients.
The TN and N/P of needles showed a seasonal pattern that increased in summer and then decreased in autumn, reaching its highest value in July, which was similar to the results of Li et al. (2017). The nitrogen concentration in the needles increased significantly in May and June. TN and N/P increased significantly under the high nitrogen treatment in July, August and September. Seasonal patterns in the nitrogen concentration and nitrogen/phosphorus ratio showed a strong effect of the needle development stage on the needle nutrient concentration (Fig. 2b, f). Under high-N treatment, needle TN increased significantly. With the rapid growth of needles, the accumulated nitrogen becomes diluted, resulting in a downward trend of total nitrogen. In July and August, the needles matured gradually, and the nutrient dilution process stopped. At the same time, the strong photosynthetic activity still requires a large amount of N to meet its own consumption needs. The growth slowed down or even stopped gradually after autumn, but the effect of nitrogen application on the nutrient resorption rate was still observable, and growth still showed an increasing trend under high nitrogen addition. The seasonal variation in needle N/P and the seasonal response of needle N to nitrogen addition indicate that the threshold for nutrient limitation in needles of Larix gmelinii varies with the season. This may be due to the differences in the nutrient requirements of plants in different seasons. At the same time, the cost of nutrient acquisition varies among the different growth periods.
We also found that there was a linear relationship between the needle N and the total P concentration in soil as well as a linear relationship between the fine root N and the needle P concentration (Fig. 5b, a). This indicates that the essential elements are not cycling independently. Nitrogen and P are closely linked through soil nutrient dynamics and cycling within plants (Sardans et al. 2012).
Effects of N addition on fine root morphology and stoichiometry
Plant roots have evolved to maximize the resource acquisition while minimizing the energy required for root tissue growth and maintenance. In this study, nearly all the morphological and chemical properties of Larix gmelinii roots exhibited trends similar to those reported in many other forest plant species (Pregitzer et al. 2002; Xiong et al. 2012). The experimental results showed that high-N addition significantly reduced the SRL (Table 3) of fine roots in the 0–10 cm soil layer. SRL is considered an indicator of the cost–benefit relationship in the root system (Fitter et al. 1991). Many studies have found that SRL is positively correlated with root respiration and negatively correlated with root longevity (Eissenstat et al. 2000; McCormack et al. 2012). Fine root turnover is an important way for plant C sinks to export C to the soil. The lower SRL value might indicate that high-N addition reduces the growth and metabolism of roots, thus prolonging the life of fine roots and slowing down the carbon input from fine roots to soil. Medium-N treatment significantly increased the root length and surface area of fourth- and fifth-order transport fine roots but had no significant effect on lower-order fine roots, which suggested that the N increase might have altered the exchange rates of resources across the plant-soil interface, resulting in an increase in the transport capacity of roots. Changes in root length and surface area can reflect the nutrient uptake efficiency of individual roots. This result indicates that plants prefer to invest more C in long-lived roots (i.e., higher-order roots) with the increase in nitrogen deposition to improve the C utilization efficiency.
Medium-N treatment significantly increased the fine roots diameter of 0–10 cm soil layer, significantly increased the fine root diameter of fourth- and fifth-order roots in 10–20 cm soil layer, but had no significant effect on first- and third-order roots (Fig. 4), which can be explained in two ways. First, plants can adjust and optimize their resource transportation strategies by increasing their fine root diameters. Nitrogen addition may lead to higher nitrogen uptake by roots and enhanced transport, especially in nitrogen-limited ecosystems. Second, previous studies have shown that soil acidification caused by N addition can aggravate soil aluminum release, and then may lead to an increase in root diameter (Zobel et al. 2007). The low-order roots diameter in deep soil did not respond to N addition, which may be due to the energy cost involved. Because the N concentration in deep soils is lower (Table 1), plants get nutrients at a lower cost from shallow soils, thus increasing investment in shallow fine roots.
Li et al. (2015) found that simulated nitrogen addition increased the availability of inorganic nitrogen absorbed by roots, which was consistent with our results. High-N treatment significantly increased the N concentration and N/P ratio of fine roots (Table 3). Significant positive correlations were observed between aboveground plant and root stoichiometry (Fig. 5), indicating that the nutrient concentrations in plants are closely related to the transport to aboveground plant parts and to fine roots. Freschet et al. (2010) reported that the nutritional concentrations of root tissue tended to be similar to those in leaves and stems. The above study further showed that the aboveground and fine root nitrogen and phosphorus concentrations and the ratio of nitrogen to phosphorus had a consistent correlation. Our results provide strong evidence that the internal specific changes in the main plant nutrient characteristics (N, P and N: P) are consistent not only at the level of a single plant organ but also the whole plant.
Effects of N addition on plant nutrient capture and resorption
Nutrient resorption mainly occurs during leaf senescence. Through this process, plants can reabsorb nutrients from senescing leaves and reduce their dependence on soil nutrient availability (Gonzales et al. 2019; Seidel et al. 2019). A previous meta-analysis (You et al. 2018) showed that the global average values of NRE and PRE under natural conditions were 47.4% and 53.6%, respectively. Nitrogen addition significantly reduced NRE by 13.3%, but had no significant effect on PRE globally. The NRE and PRE of temperate coniferous forests in China were 57% and 60% (Zheng et al. 2020); the NRE and PRE of European coniferous forests were 43% and 48%, respectively (Primicia et al. 2014). Our results showed that high nitrogen application significantly reduced the NRE of plant leaves by 35.7%, but the PRE was very low. Even at the medium nitrogen application level, PRE was only 5.7% (Fig. 3), which indicated that phosphorus resorption had little change under the condition of unbalanced nitrogen and phosphorus input. Nitrogen addition significantly reduced the concentration of needle P (Fig. 2c). For the phosphorus deficiency caused by nitrogen deposition, species with high stability can maintain their phosphorus supply by increasing plant phosphorus resorption and/or phosphorus uptake from soil. However, the PRE in our study is much lower than the global average PRE, 52.0%–46.9% (Aerts 1996; Vergutz et al. 2012; Yuan and Chen 2015), suggesting that Larix gmelinii might prefer to absorb phosphorus from the soils under nitrogen addition. When nitrogen deposition eases nitrogen limitations, plant growth depends more on whether the plant can maintain a balanced supply of phosphorus. Phosphatase activity can affect the release rate of phosphorus into soil. However, due to the high concentration of nitrogen in phosphatase, phosphatase activity is often limited by the nitrogen supply. Marklein and Houlton (2012) found that nitrogen addition increased phosphatase activity in soil. Fujita et al. (2010) showed that increased N stimulated phosphatase activity through the N/P stoichiometric effect, thereby increasing plant P uptake. Although nitrogen application did not significantly increase the P concentration in the soil in our experiment (Table 1), our study showed that the P concentration in the soil was significantly positively correlated with the N concentration in the needles (Fig. 5b). We believe that nitrogen addition may reduce the dependence of plants on internal P cycle to a certain extent. There is a balance between nutrients acquired by plants through nutrient resorption and nutrients acquired from soil, and this balance depends on the relative energy consumption of these two processes; plants tend to use the process that consumes less energy (Wright and Cannon 2001; Mao et al. 2013; Wang et al. 2014). Because Larix gmelinii can obtain more phosphorus from the outside environment and obtain more nutrients from its roots than from its needles, Larix gmelinii may be more likely to absorb nutrients from the soil.
We also found that nitrogen addition significantly increased the N/P ratio in needles and fine roots (Fig. 2, Table 3). To maintain their P supply, plants depend to a large extent on P uptake from soil and organic P mineralization. Nasto et al. (2014) found that plants can distribute more C to root production and/or root exudates, which can alleviate P limitation by increasing the mineralization and absorption of P in the rhizosphere. In our study, nitrogen addition significantly increased the investment of Larix gmelinii in their belowground parts, significantly reduced the SRL of fine roots, and increased the fine root diameter of fourth- and fifth-order fine roots (Table 3; Fig. 4), which was more conducive to the acquisition of nutrients in the soil of larch forests. Deng et al. (2016) also found that nitrogen addition significantly increased the ratio of arbuscular mycorrhizal (AM) to ectomycorrhizal in a young stand of Larix principis-rupprechtii, and soil acid phosphatase activity was positively correlated with the biomass of AM. Mycorrhizal fungi can mediate resorption and reduce dependence on absorption in aging plants, and arbuscular mycorrhizae are more important and effective than ectomycorrhizae in promoting plant P acquisition (Lambers et al. 2008). All this evidence suggests that Larix gmelinii may be more likely to consume nitrogen and adjust its C distribution to meet its phosphorus needs than to rely on internal nutrient cycling.
Under different nutrient constraints, the effect of N addition on NRE is greater than that of PRE (Fig. 3), which can be attributed to the enrichment of N in soil. Increasing the nitrogen input can reduce plant dependence on internal cycling because plants can obtain more nitrogen and phosphorus from the external environment than from resorption. In addition, the reduction in NRE and the increase in the nitrogen concentration in needles will increase the concentration of nitrogen returned to soil by plants, which will significantly affect the litter decomposition rate (Zhang et al. 2016). Numerous studies have shown that nitrogen addition can improve N use efficiency in the soil and litter layer, and high-quality litter has a higher decomposition rate than low-quality litter (Janssens et al. 2010; Lv et al. 2014; Gong et al. 2015); therefore, nitrogen deposition may accelerate the carbon and nitrogen cycles in Larix gmelinii forests.
Under N limitation, high-N addition significantly changed soil N availability, thereby reducing NRE (Mayor et al. 2014; Lü et al. 2016). To maintain a balance with NRE, PRE should decrease correspondingly when soil P availability is relatively high (Reed et al. 2012; Han et al. 2013). Although excessive nitrogen addition may stimulate phosphatase activity to increase the availability of soil phosphorus (Fujita et al. 2010), plants can also increase their PRE to rapidly compensate for phosphorus deficiency. Therefore, changes in soil nutrient status may affect plant nutrient use strategies. These adjustments substantially improve the plant nutrient balance and adaptability. These results may be further attributed to the changes in soil nutrient patterns, plant growth strategies and nutrient cycling in ecosystems caused by nitrogen addition.
Conclusions
In this study, we attempted to clarify the response of nutrient uptake strategies to N deposition in boreal forest ecosystems through long-term nitrogen addition field experiments. With the increase of N addition, plants tend to invest more C in long-lived roots (i.e. higher order roots) to improve C utilization efficiency. At the same time, increasing N input can reduce the dependence of Larix gmelinii on internal circulation, because plants get more nitrogen and phosphorus from external environment than from absorption. Nitrogen addition may change the availability of soil nitrogen and reduce NRE. Under different nutrient constraints, the effect of N application on NRE is greater than that of PRE. These results indicate that soil nutrient status affects the nutrient utilization strategy and uptake efficiency of Larix gmelinii, and improved our ability to predict Larix gmelinii growth with increase in N deposition in the future.
Availability of data and material
Data are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol. https://doi.org/10.2307/2261481
Agren GI (2008) Stoichiometry and nutrition of plant growth in natural communities. Annu Rev Ecol Evol Syst. https://doi.org/10.1146/annurev.ecolsys.39.110707.173515
Augusto L, Meredieu C, Bert D et al (2008) Improving models of forest nutrient export with equations that predict the nutrient concentration of tree compartments. Ann For Sci 65(8):808–808. https://doi.org/10.1051/forest:2008059
Bardgett RD, Mommer L, De Vries FT (2014) Going belowground: root traits as drivers of ecosystem processes. Trends Ecol Evol 29(12):692–699. https://doi.org/10.1016/j.tree.2014.10.006
Bobbink R, Hicks K, Galloway J et al (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20(1):30–59. https://doi.org/10.1890/08-1140.1
Bontemps JD, Hervé JC, Leban JM, Dhôte JF (2011) Nitrogen footprint in a long-term observation of forest growth over the twentieth century. Trees 25:237–251. https://doi.org/10.1007/s00468-010-0501-2
Chen G, Tu L, Peng Y et al (2017) Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 412(1–2):441–451. https://doi.org/10.1007/s11104-016-3074-z
Clark CM, Tilman D (2008) Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451(7179):712–715. https://doi.org/10.1038/nature06503
Deng M, Liu L, Sun Z et al (2016) Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol 212(4):1019–1029. https://doi.org/10.1111/nph.14083
Eissenstat DM, Wells CE, Yanai RD et al (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147(1):33–42. https://doi.org/10.1046/j.1469-8137.2000.00686.x
Eissenstat DM, Kucharski JM, Zadworny M et al (2015) Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol 208(1):114–124. https://doi.org/10.1111/nph.13451
Elser JJ, Fagan WF, Kerkhoff AJ et al (2010) Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol 186(3):593–608. https://doi.org/10.1111/j.1469-8137.2010.03214.x
Fitter AH, Stickland TR, Harvey ML et al (1991) Architectural analysis of plant root systems 1. architectural correlates of exploitation efficiency. New Phytol 118(3):375–382. https://doi.org/10.1111/j.1469-8137.1991.tb00018.x
Flechard CR, Nemitz E, Smith RI, Fowler D, Sutton MA (2010) Dry deposition of reactive nitrogen to european ecosystems: a comparison of inferential models across the nitroeurope network. Atmos Chem Phys. https://doi.org/10.5194/acpd-10-29291-2010
Freschet GT, Cornelissen JHC, Van Logtestijn RSP et al (2010) Evidence of the ‘plant economics spectrum’ in a subarctic flora. J Ecol 98(2):362–373. https://doi.org/10.1111/j.1365-2745.2009.01615.x
Fujita Y, Robroek BJM, De Ruiter PC et al (2010) Increased N affects P uptake of eight grassland species: the role of root surface phosphatase activity. Oikos 119(10):1665–1673. https://doi.org/10.1111/j.1600-0706.2010.18427.x
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320(5878):889–892. https://doi.org/10.1126/science.1136674
Gong S, Guo R, Zhang T et al (2015) Warming and nitrogen addition increase litter decomposition in a temperate meadow ecosystem. PLoS ONE. https://doi.org/10.1371/journal.pone.0116013
Gonzales K, Yanai R (2019) Nitrogen–phosphorous interactions in young northern hardwoods indicate P limitation: foliar concentrations and resorption in a factorial N by P addition experiment. Oecologia 189(3):829–840. https://doi.org/10.1007/s00442-019-04350-y
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164(2):243–266. https://doi.org/10.1111/j.1469-8137.2004.01192.x
Han W, Tang L, Chen Y et al (2013) Relationship between the relative limitation and resorption efficiency of nitrogen vs phosphorus in woody plants. PLoS ONE. https://doi.org/10.1371/journal.pone.0083366
Han Y, Dong S, Zhao Z et al (2019) Response of soil nutrients and stoichiometry to elevated nitrogen deposition in alpine grassland on the Qinghai-Tibetan Plateau. Geoderma 343:263–268. https://doi.org/10.1016/j.geoderma.2018.12.050
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162(1):9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Huang W, Zhou G, Liu J et al (2015) Mineral elements of subtropical tree seedlings in response to elevated carbon dioxide and nitrogen addition. PLoS ONE. https://doi.org/10.1371/journal.pone.0120190
Jackson R, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient concentrations. Proc Natl Acad Sci 94(14):7362–7366. https://doi.org/10.1073/pnas.94.14.7362
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3(5):315–322. https://doi.org/10.1038/ngeo844
Kang H, Zhuang H, Wu L et al (2011) Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: an analysis based on local observations. For Ecol Manage 261(2):195–202. https://doi.org/10.1016/j.foreco.2010.10.004
Kobe RK, Lepczyk CA, Iyer M (2005) Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 86(10):2780–2792. https://doi.org/10.1890/04-1830
Koerselman W, Meuleman AF (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol. https://doi.org/10.2307/2404783
Kou L, Guo D, Yang H et al (2015) Growth, morphological traits and mycorrhizal colonization of fine roots respond differently to nitrogen addition in a slash pine plantation in subtropical China. Plant Soil 391(1–2):207–218. https://doi.org/10.1007/s11104-015-2420-x
Kou L, Wang H, Gao W et al (2017) Nitrogen addition regulates tradeoff between root capture and foliar resorption of nitrogen and phosphorus in a subtropical pine plantation. Trees 31(1):77–91. https://doi.org/10.1007/s00468-016-1457-7
Lambers H, Raven JA, Shaver GR et al (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23(2):95–103. https://doi.org/10.1016/j.tree.2007.10.008
Lannes LS, Bustamante MMC, Edwards PJ, Venterink HO (2012) Alien and endangered plants in the Brazilian Cerrado exhibit contrasting relationships with vegetation biomass and N: P stoichiometry. New Phytol. https://doi.org/10.1111/j.1469-8137.2012.04363.x
Li W, Jin C, Guan D et al (2015) The effects of simulated nitrogen deposition on plant root traits: a meta-analysis. Soil Biol Biochem 82:112–118. https://doi.org/10.1016/j.soilbio.2015.01.001
Li Y, Niu S, Yu G (2016) Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: a meta-analysis. Glob Change Biol 22(2):934–943. https://doi.org/10.1111/gcb.13125
Li H, Crabbe MJC, Xu F, Wang W, Chen H (2017) Seasonal variations in carbon, nitrogen and phosphorus concentrations and c:n:p stoichiometry in the leaves of differently aged larix principis-rupprechtii mayr. plantations. Forests. https://doi.org/10.3390/f8100373
Liu X, Zhang Y, Han W et al (2013) Enhanced nitrogen deposition over China. Nature 494(7438):459–462. https://doi.org/10.1038/nature11917
Lü XT, Reed SC, Yu Q et al (2016) Nutrient resorption helps drive intra-specific coupling of foliar nitrogen and phosphorus under nutrient-enriched conditions. Plant Soil 398(1–2):111–120. https://doi.org/10.1007/s11104-015-2642-y
Lv Y, Wang C, Jia Y et al (2014) Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China. Appl Soil Ecol 79:1–9. https://doi.org/10.1016/j.apsoil.2013.12.002
Mahowald N, Jickells TD, Baker AR et al (2008) Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Glob Biogeochem Cycles. https://doi.org/10.1029/2008GB003240
Mao R, Song CC, Zhang XH et al (2013) Response of leaf, sheath and stem nutrient resorption to 7 years of N addition in freshwater wetland of Northeast China. Plant Soil 364(1–2):385–394. https://doi.org/10.1007/s11104-012-1370-9
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193(3):696–704. https://doi.org/10.1111/j.1469-8137.2011.03967.x
Mayor JR, Wright SJ, Turner BL (2014) Species-specific responses of foliar nutrients to long-term nitrogen and phosphorus additions in a lowland tropical forest. J Ecol 102(1):36–44. https://doi.org/10.1111/1365-2745.12190
McCormack ML, Adams TS, Smithwick EAH et al (2012) Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195(4):823–831. https://doi.org/10.1111/j.1469-8137.2012.04198.x
McCormack ML, Dickie IA, Eissenstat DM et al (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207(3):505–518. https://doi.org/10.1111/nph.13363
Midgley MG, Phillips RP (2016) Resource stoichiometry and the biogeochemical consequences of nitrogen deposition in a mixed deciduous forest. Ecology 97(12):3369–3378. https://doi.org/10.1002/ecy.1595
Nasto MK, Alvarez-Clare S, Lekberg Y et al (2014) Interactions among nitrogen fixation and soil phosphorus acquisition strategies in lowland tropical rain forests. Ecol Lett 17(10):1282–1289. https://doi.org/10.1111/ele.12335
Orgeas J, Ourcival J-M, Bonin G (2003) Seasonal and spatial patterns of foliar nutrients in cork oak (Quercus suber L.) growing on siliceous soils in Provence (France). Plant Ecol. https://doi.org/10.1023/A:1021278421821
Peñuelas J, Sardans J, Rivas-ubach A et al (2012) The human-induced imbalance between C, N and P in Earth’s life system. Glob Change Biol 18(1):3–6. https://doi.org/10.1111/j.1365-2486.2011.02568.x
Peter B, Reich JO, David G, Tilman (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.0403588101
Pregitzer KS, DeForest JL, Burton AJ et al (2002) Fine root architecture of nine North American trees. Ecol Monogr 72(2):293–309. https://doi.org/10.1890/0012-9615(2002)072[0293:FRAONN]2.0.CO;2
Pregitzer KS, Burton AJ, Zak DR, Talhelm AF (2010) Simulated chronic nitrogen deposition increases carbon storage in northern temperate forests. Glob Change Biol 14(1):142–153. https://doi.org/10.1111/j.1365-2486.2007.01465.x
Primicia I, Imbert JB, Traver MC, Castillo FJ (2014) Inter-specific competition and management modify the morphology, nutrient content and resorption in scots pine needles. Eur J For Res 133(1):141–151. https://doi.org/10.1007/s10342-013-0753-7
Randerson T, Liu H, Flanner MG, Chambers SD, Jin Y, Hess PG, Zender CS (2006) The impact of boreal forest fire on climate warming. Science. https://doi.org/10.1126/science.1132075
Reed SC, Townsend AR, Davidson EA et al (2012) Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytol 196(1):173–180. https://doi.org/10.1111/j.1469-8137.2012.04249.x
Reich PB, Wright IJ, Cavender-Bares J et al (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164(S3):S143–S164. https://doi.org/10.1086/374368
Sardans J, Rivas-Ubach A, Peñuelas J (2012) The C: N: P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect Plant Ecol, Evol Syst 14(1):33–47. https://doi.org/10.1016/j.ppees.2011.08.002
See CR, Yanai RD, Fisk MC et al (2015) Soil nitrogen affects phosphorus recycling: foliar resorption and plant–soil feedbacks in a northern hardwood forest. Ecology 96(9):2488–2498. https://doi.org/10.1890/15-0188.1
Seidel F, Lopez C, Larry M et al (2019) N isotope fractionation in tree tissues during N reabsorption and remobilization in Fagus crenata blume. Forests 10(4):330. https://doi.org/10.3390/f10040330
Smith SW, Johnson D, Quin SLO, Woodin SJ (2015) Combination of herbivore removal and nitrogen deposition increases upland carbon storage. Glob Change Biol. https://doi.org/10.1111/gcb.12902
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Stevens CJ, Thompson K, Grime JP et al (2010) Contribution of acidification and eutrophication to declines in species richness of calcifuge grasslands along a gradient of atmospheric nitrogen deposition. Funct Ecol 24(2):478–484. https://doi.org/10.1111/j.1365-2435.2009.01663.x
Tian X, Takahiro T (2002) Relative roles of microorganisms and soil animals on needle litter decomposition in a subalpine coniferous forest. Acta Phytoecol Sin 26:257–263. https://doi.org/10.3321/j.issn:1005-264X.2002.03.001
Touratier F, Field JG, Moloney CL (2001) A stoichiometric model relating growth substrate quality (C:N: P ratios) to N: P ratios in the products of heterotrophic release and excretion. Ecol Model 139(2–3):265–291. https://doi.org/10.1016/S0304-3800(01)00237-X
Tully KL, Wood TE, Schwantes AM et al (2013) Soil nutrient availability and reproductive effort drive patterns in nutrient resorption in Pentaclethra macroloba. Ecology 94(4):930–940. https://doi.org/10.1890/12-0781.1
Vergutz L, Manzoni S, Porporato A et al (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82(2):205–220. https://doi.org/10.1890/11-0416.1
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13(2):87–115. https://doi.org/10.1007/BF00002772
Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB et al (2002) Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57(1):1–45. https://doi.org/10.1023/A:1015798428743
Vitousek PM, Porder S, Houlton BZ et al (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20(1):5–15. https://doi.org/10.1890/08-0127.1
Wang M, Murphy MT, Moore TR (2014) Nutrient resorption of two evergreen shrubs in response to long-term fertilization in a bog. Oecologia 174(2):365–377. https://doi.org/10.1007/s00442-013-2784-7
Wang G, Liu F, Xue S (2017) Nitrogen addition enhanced water uptake by affecting fine root morphology and coarse root anatomy of Chinese pine seedlings. Plant Soil 418(1–2):177–189. https://doi.org/10.1007/s11104-017-3283-0
Wright IJ, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Funct Ecol 15(3):351–359. https://doi.org/10.1046/j.1365-2435.2001.00522.x
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17(1):10–19. https://doi.org/10.1046/j.1365-2435.2003.00694.x
Wurzburger N, Wright SJ (2015) Fine-root responses to fertilization reveal multiple nutrient limitation in a lowland tropical forest. Ecology 96(8):2137–2146. https://doi.org/10.1890/14-1362.1
Xia M, Talhelm AF, Pregitzer KS (2017) Chronic nitrogen deposition influences the chemical dynamics of leaf litter and fine roots during decomposition. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2017.04.011
Xiong D, Huang J, Yang Z et al (2012) Fine root architecture and morphology among different branch orders of six subtropical tree species. Shengtai Xuebao/Acta Ecologica Sinica 32(6):1888–1897. https://doi.org/10.5846/stxb201103040263
Yan G, Chen F, Zhang X, Wang Q (2017) Spatial and temporal effects of nitrogen addition on root morphology and growth in a boreal forest. Geoderma. https://doi.org/10.1016/j.geoderma.2017.05.030
Yan G, Xing Y, Wang J et al (2018) Sequestration of atmospheric CO2 in boreal forest carbon pools in northeastern China: effects of nitrogen deposition. Agric For Meteorol 248:70–81. https://doi.org/10.1016/j.agrformet.2017.09.015
You C, Wu F, Yang W et al (2018) Does foliar nutrient resorption regulate the coupled relationship between nitrogen and phosphorus in plant leaves in response to nitrogen deposition? Sci Total Environ 645:733–742. https://doi.org/10.1016/j.scitotenv.2018.07.186
Yuan ZY, Chen HY (2015) Negative effects of fertilization on plant nutrient resorption. Ecology 96(2):373–380. https://doi.org/10.1890/14-0140.1
Zhan S, Wang Y, Zhu Z et al (2017) Nitrogen enrichment alters plant N: P stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environ Exp Bot 134:21–32. https://doi.org/10.1016/j.envexpbot.2016.10.014
Zhang W, Chao L, Yang Q et al (2016) Litter quality mediated nitrogen effect on plant litter decomposition regardless of soil fauna presence. Ecology 97(10):2834–2843. https://doi.org/10.1002/ecy.1515
Zhang J, Lv J, Li Q et al (2017) Effects of nitrogen deposition and management practices on leaf litterfall and N and P return in a Moso bamboo forest. Biogeochemistry 134(1–2):115–124. https://doi.org/10.1007/s10533-017-0349-2
Zhang R, Pan H, He B et al (2018) Nitrogen and phosphorus stoichiometry of Schima superba under nitrogen deposition. Sci Rep 8(1):1–8. https://doi.org/10.1038/s41598-018-32031-y
Zheng L, Zhao Q, Sun Q, Zeng D (2020) Nitrogen addition elevated autumn phosphorus retranslocation of living needles but not resorption in a nutrient-poor Pinus sylvestris var. mongolica plantation. For Ecol Manag. https://doi.org/10.1016/j.foreco.2020.118174
Zhu F, Yoh M, Gilliam FS, Mo J (2013) Nutrient limitation in three lowland tropical forests in Southern China receiving high nitrogen deposition: insights from fine root responses to nutrient additions. PLoS ONE. https://doi.org/10.1371/journal.pone.0082661
Zhu J, Wang Q, He N et al (2016) Imbalanced atmospheric nitrogen and phosphorus depositions in China: implications for nutrient limitation. J Geophys Res: Biogeosciences 121(6):1605–1616. https://doi.org/10.1002/2016JG003393
Zobel RW, Kinraide TB, Baligar VC (2007) Fine root diameters can change in response to changes in nutrient concentrations. Plant Soil 297(1–2):243–254. https://doi.org/10.1007/s11104-007-9341-2
Acknowledgements
We gratefully acknowledge Professor Ligong Wang from Daxinganling academy of agricultural and forestry sciences, China, for his advice about field experiment design and suggestions on an earlier draft of this manuscript.
Funding
This research was supported by grants from the National Key Research and Development Program of China "Global Change and Response" (2016YFA0600800), National Natural Science Foundation of China (41575137, 41773075, 31370494, 31170421) and Open Grant for Key Laboratory of Sustainable Forest Ecosystem Management (Northeast Forestry University), Ministry of Education (KFJJ2019ZD02).
Author information
Authors and Affiliations
Contributions
QW and TL designed the study, were awarded funding, supervised data collection and contributed to and edited manuscripts. QW, GL, YX, TL, LW and YF contributed the whole manuscript preparation and design and wrote the main manuscript text. QW, GL, YX, TL, ZY and XW prepared all Figures, GL, YX, TL, LW, YF, ZY, XW and QW prepared field experiments, prepared tables and collected literatures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Agustín Merino.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, G., Xing, Y., Wang, Q. et al. Long-term nitrogen addition regulates root nutrient capture and leaf nutrient resorption in Larix gmelinii in a boreal forest. Eur J Forest Res 140, 763–776 (2021). https://doi.org/10.1007/s10342-021-01364-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-021-01364-1