Abstract
In general, trees growing at or near their limit of distribution are more sensitive to climate than those growing at their ecological core. Here we examined the growth–climate relationship of European beech (Fagus sylvatica L.) close to its northern distribution limit in southern Sweden. Tree-ring width chronologies were developed from four well-separated sites where the species was dominant (Djupeåsen, DJ; Baldringe, BLD; Komperskulla, KSK and Ryssberget Nature Reserve, RYSS). The chronologies extended from 52 years (BLD) to 150 years (RYSS). Significant negative relationships were found between tree growth and previous summer (July and August) temperatures at three sites. July temperature of the year of growth had a negative relationship with beech growth at BLD and DJ. In contrast, current summer (July and August) precipitation was positively correlated with beech growth at DJ and KSK. This sensitivity of European beech to drought at its northern limit is in line with the previous research. However, following the exceptionally dry summer in southern Sweden in 1970, a marked growth decrease was noted as well as a shift in the relationship between beech growth and current growing-season temperature. Our results show that that the radial growth of European beech has become more sensitive to drought and precipitation than temperature at its northern distribution limit in the last several decades.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
European beech (Fagus sylvatica L.) is a distinct shade-tolerant tree species which is able to grow in a wide range of environments and reduce the below-canopy irradiance, often to below 5% of available light (Emborg 1998; Collet et al. 2001; Bolte et al. 2007). This makes European beech a successful competitor with other species since it is able to regenerate under limited light conditions (Ellenberg 1988). Janík et al. (2016) found that the saplings of beech in Central Europe could grow up to 4 cm diameter at breast height under a closed canopy, but required canopy disturbances to grow larger. Moreover, European beech showed high competitive ability where moderate soil moisture and acidity are available. It is found on lower parts of mountain ranges, hills and plains (Bohn et al. 2004). Its distribution ranges from Central Europe to the maritime climates of Western Europe, although it has a special affinity to the former (Meusel 1965). Its northern limit of distribution has been defined in northern Polans and the southern regions of the Baltic countries (Bolte et al. 2007). Thus, its presence in southern Sweden means that the species is growing at its northern distribution limit.
Although several studies have been made on European beech across Europe, the results do not provide a clear-cut picture of potential limiting climate factors for distribution, especially at its northern margin (e.g., Dittmar et al. 2003; Czajkowski and Bolte 2006b; Beck and Müller 2007; Pretzsch et al. 2012; Zimmermann et al. 2015; Hacket-Pain et al. 2016). Spatial differences in responses to climatic stress may reflect the growth plasticity and competition ability of European beech in different regions (Tognetti et al. 1995; Pluess and Weber 2012). In southern Sweden, there was a decrease in growth superiority of spruce during the last 50 years, suggesting that European beech has been able to migrate northwardly compared to other species (Bolte et al. 2010).
European beech is sensitive to drought and extreme soil water depletion, reflected by its distribution area of mainly suboceanic climates (Bohn et al. 2004; Jump et al. 2006; Beck and Müller 2007). Furthermore, studies have shown greater susceptibility of European beech to embolism in its conducting system than other associated species (Cochard et al. 2005; Bréda et al. 2006), radial growth reduction and early leaf shedding during drought events (Granier et al. 2007). In European beech, stomatal control alone is often insufficient to maintain the shoot water potential above the critical limit and thereby prevent the loss of hydraulic conductivity (Schraml and Rennenberg 2002; Czajkowski and Bolte 2006a). Water shortage has an adverse effect on the species water budget and restricts its nitrogen supply (Geßler et al. 2004) significantly limited its growth as well as sap flow rates (Sitková et al. 2014). Soil water holding capacity was one of the limiting factors influencing the vitality of beech trees in drought-stressed forests in Europe (Chakraborty et al. 2017). However, high phenotypic plasticity of the species may contribute to its adaptability to drought and its competitiveness (Bolte et al. 2007; Stojnić et al. 2017).
Recent studies on the climate–growth relationship of beech in Western Europe have revealed prominent positive responses to current summer precipitation and negative responses to temperature in the current and previous growing season (Scharnweber et al. 2011; Zimmermann et al. 2015; Hacket-Pain et al. 2016). On the contrary, Cavin and Jump (2017) found that beech has higher drought sensitivity at more northerly sites than close to its southern range edge in Western Europe. Dittmar et al. (2003) could not identify any single climatic parameter as the primary growth-limiting factor, but found that extreme low growth years were often associated with anomalously low precipitation in southern Sweden. In contrast, Grundmann et al. (2008) recognized that in southern Sweden, the most prominent climate parameters were summer (July–August) temperature and (June–August) precipitation of the previous year. In mixed beech and spruce forest stands in southern Sweden, the radial growth of beech was mainly controlled by late summer conditions of the year preceding growth, and an increased importance of July temperature from the 1950s, especially after the 1990s (Grundmann et al. 2011). They inferred that European beech growth potential would benefit from warm summers and prolonged vegetation periods and increase its mean diameter in relation to spruce. Besides, the higher competitive vigor and changed vitality of beech and the lower vitality of spruce may alter the distribution boundaries of both species in Sweden.
The main goal of the present study was to shed light on the influence of climate on beech (F. sylvatica) growth at four sites located in its northern margin of distribution in southern Sweden, using dendrochronological methods. Our specific objectives were to (1) develop tree-ring width chronologies for F. sylvatica at the studied sites; (2) analyze the relationships between climate variables and radial tree growth; (3) compare the sensitivity of stands at the northern range limit to those throughout its distribution range.
Materials and methods
Study area and site characteristics
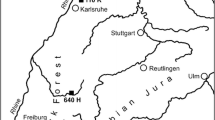
We sampled European beech (F. sylvatica L.) individuals from four sites in southern Sweden in late August and early September 2014. The sites were (from west to east): Djupeåsen (in the county of Halland), Baldringe (county of Skåne), Komperskulla and Ryssberget (county of Blekinge) (Fig. 1). The studied sites were characterized by being slightly elevated in the landscape, with altitudes ranging from 50 m a.s.l. at Baldringe to 160 m a.s.l. at Ryssberget. At all sites, F. sylvatica was the dominant species, but at Ryssberget, only a few scattered Norway spruce individuals were observed. At these sites, plots (40 × 70 m) have been assigned for long-term monitoring of air pollutions, managed by SWETHRO (the Swedish Throughfall Monitoring Network). Inside each plot, a subplot (30 × 30 m) has been designed in accordance with the guidelines for Swedish Forest Agency observation plots. The corners of the 30 × 30 m subplots were in most cases marked with poles.
The study area displays a gradient of decreasing precipitation and soil moisture from the west coast site in Halland (Djupeåsen) to the site in southernmost Sweden (Baldringe in Skåne), with the sites in Blekinge (Komperskulla and Ryssberget) being somewhere in between (Table 1). To describe the relationships between climate and radial beech growth, we used meteorological data from the nearest meteorological station operated by the Swedish Meteorological and Hydrological Institute (SMHI) for each site (Table 1). Mean annual temperature ranges from 6.5 °C at Komperskulla (KSK) and Ryssberget (RYSS) to 8.1 °C at Baldringe (BLD), while the mean temperature range during the growing season (May to September) is 13.6 °C at KSK and RYSS to 14.3 °C at BLD. Annual precipitation ranged from 851 mm year−1 at BLD to 1348 mm year−1 at Djupeåsen (DJ) (Fig. 2, Table 1).
a Monthly mean annual air temperature (T) and total precipitation (P) in the studied sites during the reference period 1890–2001 (source: http://opendata-catalog.smhi.se/explore/). b Long-term climate fluctuations in the sampled plots. Data were obtained from the nearest meteorological stations for the studied plots of beech trees in southern Sweden (see Table 1)
Tree-ring sampling, processing and standardization
At each of the four study sites, 20 dominant beech trees growing outside the perimeters of the permanent Swedish Forest Agency observation subplots were selected for sampling. Two increment cores were extracted from each tree in opposite directions at a height of ca. 1.3 m. Subsequently, the cores were mounted and examined with the aid of a microscope to identify the different patterns of rings and other diagnostic features that could be used to cross-date all series according to Hart et al. (2010). All individual ring widths were measured at a precision of 0.001 mm using a LINTAB table and the TSAP software (Rinntech, Heidelberg), and average series established for each individual tree were statistically checked using the COFECHA software (Holmes 1983). The ARSTAN software was used to remove the age-related growth trend from each raw tree-ring width (TRW) series in a process called standardization (Cook 1985). The TRW series were standardized by fitting a 30-year smoothing spline to the growth curve of each sample using the least squares technique. We chose this spline because it removes the majority of non-climatic noise and approximates the default length of 32 years for detrending in COFECHA. The standardized series were then averaged into site chronologies, and their quality evaluated by the following statistics: average mean sensitivity, the average interseries correlation (Rbar) and the expressed population signal (EPS). Mean sensitivity is an indicator of climate sensitivity in the tree-ring record (Fritts 1976). The average interseries correlation is the average of all Pearson correlation coefficients calculated for each TRW series against the composite chronology once the series being tested is removed (Grissino-Mayer 2001). EPS describes how a finite tree average estimates the hypothetical infinite population, where a value of 0.85 is a good EPS value (Wigley et al. 1984). Rbar and EPS values were calculated for 30-year moving windows with 15-year overlaps along the standardized chronologies. The standardized beech TRW chronologies for each site were then used for analysis of the climate–growth relationship.
Growth–climate relationship
We analyzed the relationships between the standardized beech TRW chronologies and climate using instrumental monthly mean temperature and total monthly precipitation from the nearest meteorological stations at the sites (Table 1). The period of analyses depended on the lengths of the chronologies and was set to 1952–2001 for BLD, 1907–2001 for DJ, 1909–2001 for KSK, and 1890–2001 for RYSS. The correlations between the TRW indices and temperature and precipitation were calculated using the software DendroClim 2002 (Biondi and Waikul 2004). A 16-month analysis window was used, from July of the year preceding growth to October of the growth year, to incorporate the effect of previous summers and winter’s climate on beech growth as well as climate during the growth season. The temporal variability in the relationship between the monthly mean temperature, total monthly precipitation and TRW indices was calculated by moving interval correlation analysis for the same months (i.e., previous July–current October), with a base length of 40 years.
Results
Chronology characteristics
The total number of cores was 155, consisting of 39 cores from BLD, 36 from DJ and 40 each for KSK and RYSS (Table 2, Fig. 3). Mean segment length (MSL) of the four TRW records varied from 52 years at BLD to 150 years at RYSS. Average growth rates (AGR) ranged from 1.4 mm year−1 at DJ to 3.2 mm year−1 at BLD. Beech growth at BLD showed the highest individual AGR variability, ranging from 0.94 to 4.52 mm year−1 (Fig. 3). The average mean sensitivity was higher at DJ and RYSS sites compared to BLD and KSK, ranging from 0.15 to 0.22. The EPS values were above the threshold of 0.85 for all sites for the entire chronology lengths. The standardized chronologies showed distinct growth declines, which were largely site specific. In BLD, obvious growth declines lasted from 1980 to 1992 and from 2000 to 2008. At DJ and KSK, low growth rates were found from the 1940s to 1980, followed by slight growth increases in 1990 to 2000, and then, a short period of declined growth was observed after 2000, particularly in 2006 (Fig. 3). At RYSS, the trees suffered from many pronounced periods of growth declines, particularly after 1950 (e.g., 1954, 1976, 1992–1993, 1995 and 2006).
Growth–climate relationships
The correlation analyses revealed differences in beech growth responses to climate among the sites. At BLD, TRW showed significant (p < 0.05 throughout) positive correlations with June temperature (T) and precipitation (P) of the year prior to growth, and March P of the year of growth, while it was negatively correlated with current July T (Fig. 4). At DJ, TRW showed significant correlations with June T (negative), July–August P (positive) of the current year, and with previous July and August T (negative), and previous June, August and October P (positive). Significant correlations at KSK in the growth year were only found for July P (positive) and in previous year for July–August T (negative), October T (positive) and August P (positive). No significant correlations were found between TRW and the chosen climate variables of the current season at RYSS. However, significant negative correlations were found with previous July and August T, and positive correlations with previous July P and October T (Fig. 4).
Current and previous correlation between the standardized chronologies of beech trees in the sampled sites and monthly mean temperature and total monthly precipitation. Climatic data for each site were obtained from the nearest meteorological station (see Table 1). Sites: BLD Baldringe, DJ Djupeåsen, KSK Komperskulla, RYSS Ryssberget. Asterisks show significant coefficients at p < 0.05
Moving correlation analysis revealed a strengthening of the relationship between previous August T and June P and TRW at BLD since the 1990s (Fig. 5). On the contrary, there was a temporal decline in the relationship between tree growth and current July, August and September T, with no significant relationship with precipitation of the current season during the period 1990–2013 (Fig. 6). At the other three sites, significant negative relationships with previous summer T and increased positive relationships with previous summer P were observed (Fig. 5). A weakening of the relationship between current season T and beech growth was observed at DJ, KSK and RYSS after the mid-1970s. The temporal correlation between beech growth and current season P varied from site to site. No relationship between TRW and precipitation of the current season was found at BLD. In contrast, there was a weak association between TRW and precipitation of the current season at the DJ and RYSS sites after 1950s (Fig. 6). At DJ, April, June and September P of the current season showed a weak association with TRW, while at RYSS, April and May P of the current season showed similar evolution as the TRW data.
Moving correlation analysis between the standardized chronologies of beech trees in the studied sites and the mean air temperature (°C) and annual precipitation (mm year−1), calculated for the period of June to December for the growing season prior to ring formation. Plots are: BLD Baldringe, DJ Djupeåsen, KSK Komperskulla, RYSS Ryssberget
Discussion
Spatial variability in beech growth
Given the wide distribution across Europe, beech grows in a variety of climate zones despite its sensitivity to water deficit (Rozas et al. 2015). It seems that other factors (e.g., genetic adaptations, competitive interactions, dispersion capacities and habitat disturbance) facilitate the expansion of beech (Saltre et al. 2013; Sánchez de Dios et al. 2016). However, the growth of trees near their species margin of distribution is more sensitive to environmental forcing such as climate (Fritts and Swetnam 1989). This dendrochronological principle is based on existence of an environmental gradient throughout the range of a species distribution, where growing conditions are assumed to become less favorable with increased distance from the optimal position until reaching the species external range boundary where physiological and ecological requirements are favorable (Murphy et al. 2006; Hart et al. 2010; Farahat et al. 2015). Consequently, the ecological performance and sensitivity to climatic conditions of European beech are expected to be highly variable throughout its distribution range.
In this study, we showed that the growth variability of European beech in southern Sweden is site specific and likely depends more on local environmental conditions than regional climate. This is somewhat contradictory to recent studies that have showed common geographical patterns in beech growth responses to climate, depending on local environmental gradients such as altitude, latitude and/or soil moisture (Di Filippo et al. 2007; Rozas et al. 2015; Chakraborty et al. 2017). Still, common growth decline events were obvious at most sites, especially after 2005. These are likely related to major events that affected southern Sweden, such as the “January storm” (named Gudrun in Sweden) in 2005, which was followed by drought and led to growth declines at southern Sweden in 2006. A sharp growth decline was also noted at DJ, KSK and RYSS in the 1970 s, likely corresponding to the exceptionally dry summer in southern Sweden in 1976. Growth decline in European beech was also observed during 1976 in Germany (Beck 2009). In contrast to our results, Bolte et al. (2010) found that the beech growth seems unaffected or even slightly increased since the drought year 1976.
Growth–climate relationship
Our results indicate that beech growth in southern Sweden is in general slightly more sensitive to temperature and precipitation during the year prior to growth than the actual year of growth. Precipitation of the previous year was positively associated with beech growth at the studied sites, but with discrepancies in the months with significant correlations among sites (Fig. 5). The importance of precipitation in July of the current year was noted at DJ and KSK. Also, two sites displayed a significant negative correlation with July temperatures during the year of growth: DJ (the wettest), BLD (the driest). These positive precipitation and negative temperature association are typical drought responses. Rainfall during the growing season is essential for photosynthesis and wood increment by providing water needed for these processes. The importance of water for plants depends on the soil moisture content in the studied sites, in addition to other soil variables (e.g., soil organic carbon) that help in retaining enough water for beech growth during the growing season. In contrast to Grundmann et al. (2011), our results confirm once again the sensitivity of beech trees at its northern limits to drought and it could not benefit from warm summers without the availability of enough soil water for growth. The negative correlations with previous summer temperature at DJ and KSK suggest carryover effects at those sites, where warm temperatures cause carbohydrate depletion and consequently growth decline (Hacket-Pain et al. 2015). This explanation is more likely than the impact of masting, which seems limited in southern Sweden and mainly concentrated to the year of actual masting (Drobyshev et al. 2010). Our results show that previous August T and June P have become more increasingly important for trees at BLD since 1990 s. The decreasing importance for current summer T and P during the same period suggests a positive impact of previous summer conditions on the growth of beech trees compared to current season conditions at this site. Conversely, the significant negative relationship with previous summer temperatures and increased positive relationship with previous summer precipitation at the other three sites (DJ, KSK and RYSS) suggests that local environmental conditions caused beech trees at these sites to become more sensitive to increasing summer temperatures or drought. As the sampled trees were distributed on slopes at these three sites, the ability of the soils to retain water and reduce the evaporation was lower compared to flat growth environment at BLD. Regardless of tree ages at the different sites, we attributed the high average growth rates at BLD to enhanced soil moisture retaining capacity at this site. It is worth noting the “inverse relationship” between beech growth and current summer temperature after the famous drought in 1976 in southern Sweden, which leads to marked decreases in growth for discontinuous periods after 1976 at DJ, KSK and RYSS. A similar shift (decrease) in the growth trend was recorded for spruce in a mixed spruce–beech forest in southern Sweden (Bolte et al. 2010).
Adaptability and plasticity of beech trees
In line with our findings, other studies have revealed the sensitivity of mature beech to drought and decreases in soil water potential (e.g., Dittmar et al. 2003; Jump et al. 2006; Beck and Müller 2007; Sitková et al. 2014; Kühn et al. 2015). Moreover, beech is characterized by high susceptibility to xylem cavitation compared to other species (Maherali et al. 2004; Bréda et al. 2006). The change in the ecological performance of European beech in different studied sites in Europe, including southern Sweden, reflects a high plasticity and adaptability for the species in most environmental conditions (e.g., Dittmar et al. 2003; Weber et al. 2013; Latte et al. 2015; Sánchez de Dios et al. 2016). The ability of European beech to counterweight between its transpiring leaf area and the water-absorbing root surface area protects it against drought. European beech has many functional traits enabling it to adapt to local microclimatic and edaphic conditions (e.g., Bolte et al. 2004; Bréda et al. 2006; Bolte and Villanueva 2006; Sitková et al. 2014). Likely, the varying growth responses to climate of different European beech populations reflect regional variations in the adaptation and competition status of populations within its distribution range (Tognetti et al. 1995; Dittmar et al. 2003; Bolte et al. 2007). The variation in the site macro- and microclimatic conditions may be the reason for the wide variations in radial growth–climate relationship of European beech between studies. Moreover, the genetic variation plays a major role for the adaptive potential of beech across Europe (Bolte et al. 2016), and marginal populations of beech in Europe are characterized by significant embolism resistance with increasing temperature and aridity (Stojnić et al. 2017). Beech in mixed stands has been found to exhibit superior growth performances compared to those in monospecific stands, e.g., by improved water use efficiency (González de Andrés et al. 2018), even during dry years (Metz et al. 2016). Thus, silvicultural practices may contribute to mitigate the effects of substantial rainfall deficits on growth of the species.
Conclusions
We conclude from the present study that the radial growth of beech trees at its northern limits in southern Sweden is mainly determined by climatic conditions (temperature and precipitation) from the previous year’s summer and that current July temperature was negatively associated with growth. Current summer precipitation had positive effects on beech growth and may counteract on the negative impact of summer temperatures. Our results confirm the sensitivity of growth in beech trees to drought at its northern limits, as they cannot benefit from warm summers if sufficient soil moisture is unavailable. A recent shift in the relationship between radial growth of beech and current season temperature occurred in the end of 1970s, followed by marked periodical decreases in beech growth. The observed climate–growth associations indicate that drought and water availability are more limiting for European beech’s growth than temperature in southern Sweden.
References
Beck W (2009) Growth patterns of forest stands—the response towards pollutants and climatic impact. iForest 2: 4–6. http://www.sisef.it/iforest/show.php?id=472. 21 Jan 2009
Beck W, Müller J (2007) Impact of heat and drought on tree and stand vitality—dendroecological methods and first results from Level II-plots in southern Germany. Schriftenr Forstl Fak Univ Göttingen ud Nordwestdtsch ForstlVersuchsanst 142:120–128
Biondi F, Waikul K (2004) DENDROCLIM2002: a C++ program for statistical calibration of climate signals in tree-ring chronologies. Comp Geosci 30:303–311
Bohn U, Gollub G, Hettwer Ch, Neuhäuslová Z, Raus Th, Schlüter H (2004) Karte der natürlichen Vegetation Europas/Map of the natural vegetation of Europe. BfN, Bonn-Bad Godesberg, Germany. CD-ROM
Bolte A, Villanueva I (2006) Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur J For Res 125(1):15–26
Bolte A, Rahmann T, Kuhr M, Pogoda P, Murach D, Von Gadow K (2004) Relationships between tree dimension and coarse root biomass in mixed stands of European beech (Fagus sylvatica L.) and Norway spruce (Picea abies [L.] Karst.). Plant Soil 264(1–2):1–11
Bolte A, Czajkowski T, Kompa T (2007) The northeastern distribution range of European beech: a review. Forestry 80(4):413–429. https://doi.org/10.1093/forestry/cpm028
Bolte A, Hilbrig L, Grundmann B, Kampf F, Brunet J, Roloff A (2010) Climate change impacts on stand structure and competitive interactions in a southern Swedish spruce–beech forest. Eur J Forest Res 129:261–276
Bolte A, Czajkowski T, Cocozza C et al (2016) Desiccation and mortality dynamics in seedlings of different European Beech (Fagus sylvatica L.) populations under extreme drought conditions. Front Plant Sci 7:751
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625–644
Cavin L, Jump AS (2017) Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial edge. Glob Change Biol 23:362–379
Chakraborty T, Saha S, Matzarakis A, Reif A (2017) Influence of multiple biotic and abiotic factors on the crown die-back of European beech trees at their drought limit. Flora 229:58–70
Cochard H, Coste S, Chanson B, Guehl JM, Nicolini E (2005) Hydraulic architecture correlates with bud organogenesis and primary shoot growth in beech (Fagus sylvatica). Tree Physiol 25:1545–1552
Collet C, Lanter O, Pardos M (2001) Effects of canopy opening on height and diameter growth in naturally regenerated beech seedlings. Ann For Sci 58:127–134
Cook ER (1985) A time-series analysis approach to tree-ring standardization. Ph.D. Dissertation, University of Arizona, Tucson
Czajkowski T, Bolte A (2006a) Frosttoleranz deutscher und polnischer Herkünfte der Buche (Fagus sylvatica L.) und ihre Beeinfl ussung durch Trockenheit. Arch Forstwes Landschökol 40:119–126 (in German with English summary)
Czajkowski T, Bolte A (2006b) Unterschiedliche Reaktion deutscher und polnischer Herkünfte der Buche (Fagus sylvatica L.) auf Trockenheit. Allg Forst Jagdztg 177:30–40 (in German with English summary)
Di Filippo A, Biondi F, Čufar K, De Luis M, Grabner M, Maugeri M, Presutti Saba E, Schirone B, Piovesan G (2007) Bioclimatology of beech (Fagus sylvatica L.) in the Eastern Alps: spatial and altitudinal climatic signals identified through a tree-ring network. J Biogeogr 34:1873–1892
Dittmar C, Zech W, Elling W (2003) Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe -a dendroecological study. For Ecol Manag 173:63–78
Drobyshev I, Övergaard R, Saygin I, Niklasson M, Hickler T, Karlsson M, Sykes MT (2010) Masting behaviour and dendrochronology of European beech (Fagus sylvatica L.) in southern Sweden. For Ecol Manag 259(11):2160–2171
Ellenberg H (1988) Vegetation ecology of central Europe, 4th edn. Cambridge University Press, Cambridge
Emborg J (1998) Understorey light conditions and regeneration with respect to the structural dynamics of a near-natural deciduous forest in Denmark. For Ecol Manag 106:83–95
Farahat EA, Linderholm HW, Lechowicz MJ (2015) Influence of dust deposition and climate on the radial growth of Tsuga canadensis near its northern range limit. Eur J For Res 135(1):69–76
Fritts HC (1976) Tree rings and climate. Academic Press, London
Fritts HC, Swetnam TW (1989) Dendroecology: a tool for evaluating variations in past and present forest environments. Adv Ecol Res 19:111–188
Geßler A, Keitel C, Nahm M, Rennenberg H (2004) Water shortage affects the water and nitrogen balance in central European beech forests. Plant Biol 6:289–298
González de Andrés E, Camarero JJ, Blanco JA, Imbert JB, Lo YH, Sangüesa-Barreda G, Castillo FJ (2018) Tree-to-tree competition in mixed European beech-Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J Ecol 109:59–75
Granier A, Reichstein M, Breda N, Janssens IA, Falge E, Ciais P et al (2007) Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agric For Meteorol 143:123–145
Grissino-Mayer HD (2001) Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Res 57:205–221
Grundmann BM, Bonn S, Roloff A (2008) Cross-dating of highly sensitive Common beech (Fagus sylvatica L.) tree-ring series with numerous missing rings. Dendrochronologia 26:109–113
Grundmann BM, Bolte A, Bonn S, Roloff A (2011) Impact of climatic variation on growth of Fagus sylvatica and Picea abies in Southern Sweden. Scand J For Res 26(S11):64–71
Hacket-Pain AJ, Friend AD, Lageard JGA, Thomas PA (2015) The influence of masting phenomenon on growth–climate relationships in trees: explaining the influence of previous summers’ climate on ring width. Tree Physiol 35:319–330
Hacket-Pain AJ, Cavin L, Friend AD, Jump AS (2016) Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur J For Res 135(5):897–909
Hart JL, van de Gevel SL, Sakulich J, Grissino-Mayer HD (2010) Influence of climate and disturbance on the growth of Tsuga canadensis at its southern limit in eastern North America. Trees 24:621–633
Holmes R (1983) Computer-assisted quality control in tree-ring dating and measuring. Tree-Ring Bull 43:69–78
Janík D, Král K, Adam D, Hort L, Samonil P, Unar P, Vrska T, McMahon S (2016) Tree spatial patterns of Fagus sylvatica expansion over 37 years. For Ecol Manag 375:134–145
Jump AS, Hunt JM, Martinez-Izquierdo JA, Penuelas J (2006) Natural selection and climate change: temperature-linked spatial and temporal trends in gene frequency in Fagus sylvatica. Mol Ecol 15:3469–3480
Kühn AR, Grill S, Baumgarten M, Ankerst DP, Matyssek R (2015) Daily growth of European beech (Fagus sylvatica L.) on moist sites is affected by short-term drought rather than ozone uptake. Trees 29:1501–1519
Latte N, Lebourgeois F, Claessens H (2015) Increased tree-growth synchronization of beech (Fagus sylvatica L.) in response to climate change in northwestern Europe. Dendrochronologia 33:69–77
Maherali H, Pockman WT, Jackson RB (2004) Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85:2184–2199
Metz J, Annighöfer P, Schall P, Zimmermann J, Kahl T, Schulze E-D, Ammer C (2016) Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob Change Biol 22:903–920
Meusel H (1965) Vergleichende Chorologie der zentraleuropäischen Flora. Bd. 1. Fischer, Jena, Germany, 258 pp (maps). 583 p (text) (in German)
Murphy HT, VanDerWal J, Lovett-Doust J (2006) Distribution of abundance across the range of eastern North American trees. Glob Ecol Biogeogr 15:63–71
Pluess AR, Weber P (2012) Drought-adaptation potential in Fagus sylvatica: linking moisture availability with genetic diversity and dendrochronology. PLoS ONE 7(3):e33636. https://doi.org/10.1371/journal.pone.0033636
Pretzsch H, Dieler J, Seifert T, Rötzer T (2012) Climate effects on productivity and resource-use efficiency of Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica [L.]) in stands with different spatial mixing patterns. Trees 26:1343–1360
Rozas V, Camarero JJ, Sangüesa-Barreda G, Souto M, García-González I (2015) Summer drought and ENSO-related cloudiness distinctly drive Fagus sylvatica growth near the species rear-edge in northern Spain. Agric For Meteorol 201:153–164
Saltre F, St-Amant R, Gritti ES, Brewer S, Gaucherel C, Davis BAS, Chuine I (2013) Climate or migration, what limited European beech post-glacial colonization? Glob Ecol Biogeogr 22:1217–1227
Sánchez de Dios R, Hernández L, Montes F, Sainz-Ollero H, Cañellas I (2016) Tracking the leading edge of Fagus sylvatica in North-Western Iberia: Holocene migration inertia, forest succession and recent global change. PPEES 20:11–21
Scharnweber T, Manthey M, Criegee C, Bauwe A, Schröder A, Wilmking M (2011) Drought matters–declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For Ecol Manag 262:947–961
Schraml C, Rennenberg H (2002) Ökotypen der Buche (Fagus sylvatica L.) zeigen unterschiedliche Reaktionen auf Trockenstress. Forstwiss Centrabl 121:59–72 (in German with English summary)
Sitková Z, Nalevanková P, Střelcová K, Fleischer P Jr, Ježík M, Sitko R, Pavlenda P, Hlásny T (2014) How does soil water potential limit the seasonal dynamics of sap flow and circumference changes in European beech? Lesn Cas For J 60:19–31
Stojnić S, Suchocka M, Benito-Garzon M et al (2017) Variation in xylem vulnerability to embolism in European beech from geographically marginal populations. Tree Physiol. https://doi.org/10.1093/treephys/tpx128
Tognetti R, Michelozzi M, Borghetti M (1995) The response of European beech (Fagus sylvatica L.) seedlings from two Italian populations to drought and recovery. Trees 9:348–354
Weber P, Bugmann H, Pluess AR, Walthert L, Rigling A (2013) Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees 27:171–181
Wigley TML, Briffa KR, Jones PD (1984) On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J Clim Appl Meteorl 23:201–213
Zimmermann J, Hauck M, Dulamsuren Ch, Leuschner C (2015) Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in Central European mixed forests. Ecosystems 18:560–572
Acknowledgements
We thank the two anonymous reviewers whose critical comments helped to refine the manuscript. This study was supported by the Swedish Clean Air & Climate Research Program (SCAC) and contributes to the strategic research area Biodiversity and Ecosystem Services in a Changing Climate (BECC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Judy Simon.
Rights and permissions
About this article
Cite this article
Farahat, E., Linderholm, H.W. Growth–climate relationship of European beech at its northern distribution limit. Eur J Forest Res 137, 619–629 (2018). https://doi.org/10.1007/s10342-018-1129-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-018-1129-9