Abstract
Thirty families of Eucalyptus globulus L., established in a first-generation open-pollinated progeny test, were evaluated for the production of heartwood. Five trees of each family were harvested at 9 years of age, total tree height was measured and a cross-sectional disc was removed at 25 % stem height to estimate the amount of heartwood. The heartwood proportion of the stemwood cross-sectional area averaged 41 % with significant between-family variation (P = 0.016) ranging from 27 to 53 %. There were also important within-family differences with coefficients of variation of the mean between 4 and 48 %. Moderate heritability values were obtained for heartwood diameter and proportion (h 2 = 0.31 and 0.23, respectively) but low estimates were found for sapwood width (h 2 = 0.17). Strong positive genetic and phenotypic correlations of heartwood diameter were found with stem DBH and with heartwood proportion. Both correlation estimates indicated that larger trees tended to have more heartwood. The results indicate that there is an opportunity to reduce heartwood content in E. globulus through selection and breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalyptus globulus contains a substantial proportion of heartwood at the harvest age for pulping (Gominho and Pereira 2000; Gominho et al. 2001). The proportion of heartwood within the tree has a significant impact on wood pulping due to different heartwood and sapwood properties (Pereira et al. 2003). Heartwood contains a larger amount of extractives, and its presence therefore reduces the wood assortment quality at the pulp mill because of lower pulp yields and brightness (Gominho et al. 2001; Miranda et al. 2007; Lourenço et al. 2010, 2011, 2008; Pereira et al. 2003).
Heartwood development occurs with tree ageing and varies between and within species (reviews can be found at Bamber and Fukazawa 1985; Hillis 1987; Taylor et al. 2002). It seems to be age and growth related, probably linked to, or resulting from, the regulation of sapwood amount (Pinto et al. 2004; Sellin 1994; Wilkes 1991; Climent et al. 1993).
A positive influence of radial growth on heartwood diameter was reported for E. globulus (Gominho and Pereira 2000, 2005; Miranda et al. 2006; Morais and Pereira 2007) and E. grandis (Wilkins 1991). Early radial growth was shown to be a relevant trait for predicting heartwood dimension in Pinus radiata (Hillis and Ditchburne 1974; Wilkes 1991) and P. canariensis (Climent et al. 1993, 2002). Tree age was important to define heartwood diameter in Eucalyptus spp. and P. radiata (Bamber 1976), P. banksiana (Yang and Hazenberg 1991) and Picea abies (Sellin 1994).
For E. globulus, several studies have documented the effect of site, tree growth, and silvicultural factors on heartwood content (Gominho and Pereira 2005; Miranda et al. 2006, 2009). The genetic influence on heartwood content has been less studied. However, the between-tree natural variability found within one site suggests that a genetic factor is involved in eucalypt heartwood development. Differences between E. globulus clones were noticed by Miranda et al. (2007), namely regarding the vertical development of heartwood.
The aim of the present study was to investigate whether there are genetic differences between families on heartwood and sapwood traits, using a first-generation progeny trial of E. globulus with 30 open-pollinated families from different origins in Australia and Portugal. Heartwood and sapwood relationship with growth traits was also analysed.
Materials and methods
The E. globulus L. trees that were used for this study were raised from seeds representing 30 open-pollinated families selected in stands across the natural range of the species in Australia and in the area of distribution in Portugal. The samples were obtained from a first-generation open-pollinated progeny test of E. globulus established by Celbi (now Altri) at Quinta do Furadouro, in Portugal. The site is located in the central coastal region, approximately 10 km from the Atlantic coast (39°20′N; 9°15′W, 50 m altitude). The climate is of the Mediterranean type tempered by oceanic influence, with an annual rainfall of 607 mm and mean temperature of 15.2 °C. The soils are eutric cambisols developed on sandstones. A detailed description of the site was presented elsewhere (Pereira et al. 1989).
The trial was installed in March 1989 according to the usual practices in eucalypt forestry and harvested at 8.9 years of age. Five trees of each family were sampled, totalling 150 trees. The over-bark diameter at breast height (DBH) and tree height of all trees were measured.
A stem cross-sectional disc was taken at 25 % of total tree height. The heartwood delimitation was made by visual observation since the heartwood in E. globulus differentiates by colour from the sapwood. The total disc wood cross-sectional area and the heartwood area were measured using an image analysis system (Gominho and Pereira 2000). The sapwood area was obtained by difference, and the mean heartwood diameter and sapwood radial width were calculated subsequently.
Differences between families were tested with a one-way ANOVA, by applying pairwise analysis (Tukey’s test, P < 0.05). The following model was used: y = μ + a + ε, where y are the observed values, μ the overall mean, a the family effect and ε the residual. A simple linear regression equation was fitted for heartwood area and diameter, and for sapwood area and width against DBH.
Heritability, meaning the proportion of the total observed variation that is of genetic origin, and additive genetic correlations between each assessment were estimated. Individual narrow-sense heritability (h 2) was estimated for each measured trait as:
where σ 2a represents the additive genetic variation and σ 2p the total phenotypic variance. The additive genetic variation was calculated as σ 2a = 2.5σ 2f , where σ 2f is the family component of variation. Following Griffin and Cotterill (1988), a coefficient of relatedness of 2.5 was used to account for the fact that open-pollinated eucalypt progenies may comprise a mixture of selfs (an average rate of 30 %) and outcrosses. Phenotypic variance was estimated as σ 2p = σ 2f + σ 2 ε , where σ 2 ε is the error variance (representing both environmental and non-additive genetic variation).
Genetic correlations between measured traits (x and y) were evaluated as follows:
where cova(x, y) is the additive genetic covariance between x and y, estimated as \(\text{cov}_{\text{a}} (x,y) = {{\left( {\sigma^{2}_{{{\text{a}}\left( {x + y} \right)}} - \sigma_{{{\text{a}}x}}^{2} - \sigma_{{{\text{a}}y}}^{2} } \right)} \mathord{\left/ {\vphantom {{\left( {\sigma^{2}_{{{\text{a}}\left( {x + y} \right)}} - \sigma_{{{\text{a}}x}}^{2} - \sigma_{{{\text{a}}y}}^{2} } \right)} 2}} \right. \kern-0pt} 2}\), and σ 2ax and σ 2ay are the additive variance components for traits x and y, respectively, and \(\sigma_{{{\text{a}}\left( {x + y} \right)}}^{2}\) is the family variance component of the sum of traits x and y.
The phenotypic correlation between traits (x and y) was estimated as:
where covp(x, y) is the phenotypic covariance between x and y, and \(\sigma_{{{\text{p}}x}}^{2}\) and \(\sigma_{{{\text{p}}y}}^{2}\) are the phenotypic variances for traits x and y, respectively.
The standard errors for h 2 were calculated using:
where
SE (h 2) is the standard error of the heritability estimate, k is the number of offsprings per family, s is the number of families and t = h 2/2.5 (MacDonald et al. 1997).
Results
The mean biometric characteristics of the sampled trees from the 30 E. globulus families are listed in Table 1 as family mean values and coefficient of variation of the mean. There was considerable variation of tree diameter between families with significant differences (P = 0.004), with mean values ranging from 14.1 to 21.0 cm. There was also between-tree variation within each family, as indicated by an average coefficient of variation of the family means of 16.5 %, although the differences varied between families (coefficients of variation ranging from 3 to 33 %).
Tree height, with a mean value of 19.7 m, did not show statistical differences between families and varied little within the families (mean coefficient of variation of 10 %).
Heartwood and sapwood development
Table 2 summarises the mean family values of stem cross-sectional area of total wood, heartwood diameter and sapwood radial width, as well as of heartwood proportion.
The between-family comparison of heartwood development showed a significant difference in heartwood diameter (P = 0.012) that ranged from 6.1 to 9.8 cm. Heartwood proportion, that was on average of 41 % of the stem wood cross-sectional area (ranging 28.7–50.5 %), also showed between-family differences (P = 0.016). There was also an important between-tree variation within each family that corresponded to an average 23.4 % coefficient of variation of the mean (ranging 6.8–45.1 %) for heartwood diameter and between 3.6 and 47.6 % for heartwood proportion.
The sapwood radial width was in the range of 1.6–2.9 cm. The differences between families were not statistically significant (P = 0.06).
Heritability estimates
Individual heritabilities and genetic and phenotypic correlations are presented in Table 3. Moderate heritability values were obtained for heartwood diameter (h 2 = 0.31, SE 0.19) and heartwood proportion (h 2 = 0.23, SE 0.19) and low for sapwood width (h 2 = 0.17, SE 0.17). DBH also had a moderate heritability of h 2 = 0.26 (SE 0.19).
Strong positive genetic (r a = 0.99) and phenotypic (r p = 0.77) correlations were found between heartwood diameter and stem diameter at breast height. Heartwood diameter showed also high genetic and phenotypic correlations with heartwood proportion, respectively, r a = 0.76 and r p = 0.67. Both phenotypic and genetic correlations indicated that a large heartwood in trees tended to correspond to a higher heartwood proportion. For instance, family F8 had a large heartwood diameter of 9.8 cm and a heartwood proportion of 45.5 %, while family F20 had a heartwood diameter of 5.5 cm and a proportion of 31.5 %.
The genetic correlations of sapwood width with DBH were moderate (r a = 0.24) and very low with heartwood diameter (r a = 0.05). Negative genetic and phenotypic correlations were found between sapwood width and heartwood proportion.
Influence of tree growth on heartwood and sapwood
The influence of tree growth on heartwood and sapwood development was assessed considering tree breast height diameter as the growth indicator, and the respective models are summarised in Table 4. Heartwood diameter, area and proportion, and sapwood width and area were found to have highly significant positive relationship with tree breast height diameter.
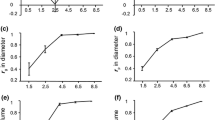
Heartwood diameter and area correlated positively with tree diameter at breast height, as graphically represented on Fig. 1 for heartwood diameter, for which the model explained 59 % of the total variation. Sapwood area also showed a good linear regression with tree diameter (Fig. 2) with a positive correlation that explained 53 % of the total variation. On the contrary, the correlation of sapwood radial width with tree diameter was weak and accounted only for 14 % of the variation, as shown in Fig. 3.
As regards the proportion of heartwood in the cross-section, only a very weak correlation was found with tree DBH (Fig. 4).
Discussion
The 9-year-old E. globulus trees in this trial possessed a large proportion of heartwood at 25 % of stem height (Table 2), in general agreement with previous reports for the species at the same age (Gominho and Pereira 2000). The large sampling that was made here allows the conclusion that this substantial and early stage heartwood development is a general across-family feature of E. globulus and therefore strengthens previous recommendations of including heartwood content as a stem quality parameter for pulping (Miranda et al. 2007; Pereira et al. 2003).
There were statistical significant differences between families regarding heartwood development both in absolute dimensions (diameter) as well as in proportion of the cross-sectional area. Overall, the proportion of heartwood ranged from a maximal value of 50.5 % (family F17) to a minimal value of 28.7 % (family F9). The families could be divided into three groups of heartwood proportion, for instance <35, 35–45 and >45 %, which included, respectively 4, 19 and 7 families.
This family difference in heartwood content may have a practical impact regarding the pulping quality of the material. In the assumption that heartwood will have a pulp yield that is 4 % points lower than sapwood (47.9 vs. 52.1 %, respectively for heartwood and sapwood pulp yield, as reported by Miranda et al. 2007), the stems with less than 35 % heartwood would yield at least 6 kg pulp more per ton of raw material than the stems with over 50 % heartwood. Similarly, a correlation between increased accumulation of extractives and lower pulp yields was found for E. globulus trees at 18 years of age (Miranda et al. 2003). Benefits from lower content of heartwood will also include lower chemical consumption and higher pulp brightness (Miranda et al. 2006; Lourenço 2008; Lourenço et al. 2010, 2011; Gominho et al. 2005).
The results suggest that heartwood development in E. globulus is genetically influenced only to a moderate extent, e.g., with heritability estimates of h 2 = 0.23 for heartwood proportion. This contradicts the overall statement that heartwood formation is under a strong genetic control though influenced by environment and forest practices (Hillis 1987).
The heritability estimates reported here should be viewed with caution. Since these estimates were based on families grown on only one location, the family × environment interaction variance could not be assessed and is added to the estimate of family variance on that particular site. Thus, the single-site heritability is biased because it estimates the sum of additive plus additive × environment variance relative to the total phenotypic variance. The standard errors of heritability estimates were therefore high (approximately 0.19).
Very few studies have been made on the genetic variation of heartwood and sapwood traits. Santos et al. (2004) estimated a heritability coefficient of h 2 = 0.39 for sapwood/heartwood ratio in E. grandis. In Pinus sylvestris, Ericsson and Fries (1999) found narrow-sense heritabilities for heartwood diameter ranging from 0.30 to 0.54. In Juglans nigra, Woeste (2002) indicated narrow-sense heritability of 0.40 for heartwood area. Pâques (2001) found in Larix a narrow-sense heritability of 0.75–0.83 for heartwood length and 0.63–0.78 for heartwood radial proportion.
Variability associated with the individual trees within each family was of significant magnitude (Table 2), and the within-site environmental variation may therefore be one non-negligible cause of variation. It is known that E. globulus responds strongly to micro-climatic and soil differences (Soares et al. 2007; Pereira et al. 1996) but the experimental design of the trial did not allow discriminating this effect.
As indicated in Table 3, heartwood development is directly linked to radial growth: correlations of heartwood diameter are positive and very high, both at the phenotypic and at genetic levels (0.77 and 0.99, respectively), and correlations of heartwood proportion are moderate (0.24 and 0.45). Sapwood width is also positively linked to radial growth.
The influence of tree growth in heartwood formation has been reported with positive correlations for different species, i.e., P. radiata (Wilkes 1991), P. pinaster (Pinto et al. 2004; Knapic and Pereira 2005), P. contorta (Yang and Murchison 1992), P. canariensis (Climent et al. 2002), J. nigra (Woeste 2002), Tectona grandis (Bhat 1995), Larix decidua (Leibundgut 1983), Acacia melanoxylon (Knapic et al. 2006) and Eucalyptus grandis (Wilkins 1991). This was reported also for E. globulus (Gominho and Pereira 2000, 2005) and again confirmed in the present study.
The heartwood diameter increased with tree diameter (Fig. 1), and 59 % of the individual variation of heartwood area was explained by the tree growth in diameter. However, family effects affect this relation of heartwood dimension with tree growth, as shown by the differences between genetic and phenotypic correlations (Table 3).
The analysis of the effect of tree radial growth on the cross-sectional heartwood proportion is more difficult, since it combines the superposed effects of tree growth on the additive accumulation of heartwood area and the sapwood circumferential enlargement. Therefore, although heartwood diameter is positively correlated with tree diameter (Fig. 1), the same does not occur for heartwood proportion, and tree diameter does not explain heartwood proportion (Fig. 4). This means that it is possible to have large trees with a low heartwood proportion and vice versa.
Heartwood formation and development have been rationalised as a regulating process associated with tree growth, to maintain an optimum sapwood volume that conserves the nutritional balance in the living part of the tree, since essential elements are translocated to the sapwood. The amount of sapwood should be related to the tree’s conductive needs associated with its crown development, and therefore, the formation and development of heartwood progresses within the tree to regulate the amount of sapwood (Bamber 1976). Larger trees, eventually with bigger crowns, will have more sapwood area, as it has been reported for Cryptomeria japonica (Wang and Chen 1992), E. grandis (Wilkins 1991), Picea mariana and P. glauca (Yang and Hazenberg 1992), P. abies (Sellin 1996), P. sylvestris (Mörling and Valinger 1999) and Larix species (Pâques 2001).
This study also found a positive and significant correlation between sapwood area and tree growth as depicted in Fig. 2. However, the radial dimension of the conductive area, i.e., the sapwood radial width, is particularly constant within the species and not explained by the tree diameter as shown in Fig. 3. The sapwood radial width found in this study was independent on families and ranged between 1.6 and 2.9 cm (Table 2). This is in accordance with previous reports that E. globulus sapwood width is rather homogeneous within and between trees in the range of 1.5–3.7 cm (Gominho and Pereira 2005; Miranda et al. 2006).
Conclusions
At harvest for the pulp industry, E. globulus trees have a significant proportion of heartwood. The heartwood development of E. globulus showed genetic variation, and moderate heritability levels were found for heartwood diameter and proportion, indicating a potential in this species for improving wood quality in terms of less heartwood development. Breeding programs could therefore include heartwood proportion by using, e.g., core sampling.
The correlation of heartwood traits with tree growth is positive, and the factors that will result into a higher tree growth will increase heartwood. The silvicultural management of E. globulus plantations used for pulping should take into account the presence of heartwood in the trees and the factors of its variation at genetic and phenotypic levels.
References
Bamber RK (1976) Heartwood, its function and formation. Wood Sci Technol 10:1–8
Bamber RK, Fukazawa K (1985) Sapwood and heartwood. A review. For Abstr 46:567–580
Bhat KM (1995) A note on heartwood proportion and wood density of 8-year-old teak. Indian For 121:515–517
Climent J, Gil L, Pardos J (1993) Heartwood and sapwood development and its relationship to growth and environment in Pinus canariensis Chr.Sm ex DC. For Ecol Manag 59:165–174
Climent J, Chambel MR, Pêrez E, Gil L, Pardos J (2002) Relationship between heartwood radius and early radial growth, tree age, and climate in Pinus canariensis. Can J For Res 32:10–111
Ericsson T, Fries A (1999) High heritability for heartwood in north Swedish Scots pine. Theor Appl Genet 98:732–735
Gominho J, Pereira H (2000) Variability of heartwood content in plantation grown Eucalyptus globulus Labill. Wood Fiber Sci 32:189–195
Gominho J, Figueira J, Rodrigues J, Pereira H (2001) Within-tree variation of heartwood, extractives and wood density in the eucalypt hybrid urograndis (Eucalyptus grandis x E. urophyla). Wood Fiber Sci 33:3–8
Gominho J, Pereira H (2005) The influence of tree spacing in heartwood content in Eucalyptus globulus Labill. Wood Fiber Sci 37:582–590
Gominho J, Rodrigues J, Pereira H (2005) Clonal variation and influence of extractives in Eucalyptus globulus Labill. kraft pulping. In: Proceedings of the 9th international chemical engineering conference, ChemPor 2005, 21–23 September, Coimbra, Portugal, p 54
Griffin AR, Cotterill PP (1988) Genetic variation in growth of outcrossed, selfed and open-pollinated progenies of Eucalyptus regnans and some implications for breeding strategy. Silvae Genet 37:124–131
Hillis WE (1987) Heartwood and tree exudantes. Springer, Berlin
Hillis WE, Ditchburne N (1974) The prediction of heartwood diameter in radiata pine trees. Can J For Res 4:524–529
Knapic S, Pereira H (2005) Within-tree variation of heartwood and ring width in maritime pine (Pinus pinaster Ait.). For Ecol Manag 210:81–89
Knapic S, Tavares F, Pereira H (2006) Heartwood and sapwood variation in Acacia melanoxylon R. Br. trees in Portugal. Forestry 79:371–380
Leibundgut H (1983) Untersuchungen verschiedener Provenanzen von Larix decidua. Schweiz. Z Forstwes 134:61–62
Lourenço A (2008) The influence of Eucalyptus globulus heartwood in pulp production. Master degree Dissertation in Forestry Engineering, Instituto Superior de Agronomia, Universidade Técnica de Lisboa, Lisboa, Portugal
Lourenço A, Gominho J, Pereira H (2010) Pulping and delignification of sapwood and heartwood from Eucalyptus globulus. J Pulp Pap Sci 36:63–69
Lourenço A, Gominho J, Pereira H (2011) Modeling of sapwood and heartwood delignification kinetics of Eucalyptus globulus using consecutive and simultaneous approaches. J Wood Sci 57:20–26
MacDonald AC, Borralho NMG, Potts BM (1997) Genetic variation for growth and wood density in Eucalyptus globulus ssp. globulus in Tasmania (Australia). Silvae Genet 46:236–241
Miranda I, Tomé M, Pereira H (2003) The influence of spacing on wood properties for Eucalyptus globulus Labill pulpwood. Appita J 56:140–144
Miranda I, Gominho J, Lourenço A, Pereira H (2006) The influence of irrigation and fertilization on heartwood and sapwood contents in 18-year-old Eucalyptus globulus trees. Can J For Res 36:2675–2683
Miranda I, Gominho J, Lourenço A, Pereira H (2007) Heartwood, extractives and pulp yield of three Eucalyptus globulus clones grown in two sites. Appita J 60:485–488
Miranda I, Gominho J, Pereira H (2009) Variation of heartwood and sapwood in 18-year-old Eucalyptus globulus trees grown with different spacings. Trees 23:367–372
Morais CM, Pereira H (2007) Heartwood and sapwood variation in Eucalyptus globulus Labill. trees at the end of rotation for pulpwood production. Ann For Sci 64:665–671
Mörling T, Valinger E (1999) Effects of fertilization and thinning on heartwood area, sapwood area and growth in Scots pine. Scand J For Res 14:462–469
Pâques LE (2001) Genetic control of heartwood content in larch. Silvae Genet 50:69–75
Pereira H, Pardos J, Boudet AM, Mitchell P, Mughini G, Kyritsis S, Dalianis C (1996) Eucalypt plantations for production of raw-material for industry and energy in Europe. In: Cartier P, Ferrero GL, Henius UM, Hultberg S, Sachau J, Wiinblad M (eds) Biomass for energy and the environment. Pergamon/Elsevier, Oxford, pp 84–89
Pereira H, Graça J, Rodrigues JC (2003) Wood chemistry in relation to quality. In: Barnett JR, Jeronimidis G (eds) Wood quality and its biological basis, chap 3. CRC Press/Blackwell, Oxford, pp 5–83
Pereira JS, Linder S, Araújo MC, Pereira H, Ericsson T, Burralho N, Leal L (1989) Optimisation of biomass production in Eucalyptus globulus plantation. A case study. In: Pereira JS, Landsberg JJ (eds) Biomass production by fast-growing trees. Kluwer, Dordrecht, pp 101–121
Pinto I, Pereira H, Usenius A (2004) Heartwood and sapwood development within maritime pine (Pinus pinaster Ait.) stems. Trees 18:284–294
Santos PET, Geraldi IO, Garcia JN (2004) Estimated of genetic parameters of wood traits for sawn timber production in Eucalyptus grandis. Genet Mol Biol 27:567–573
Sellin A (1994) Sapwood-heartwood proportion related to tree diameter, age, and growth rate in Picea abies. Can J For Res 24:1022–1028
Sellin A (1996) Sapwood amount in Picea abies (L.) Karst. determined by tree age and radial growth rate. Holzforschung 50:291–296
Soares P, Tomé M, Pereira JS (2007) A produtividade do eucaliptal. In: Alves AM, Pereira JS, Silva JMN (eds) O eucaliptal em Portugal—impactes ambientais e investigação científica, chap 2. ISA Press, Lisboa, pp 27–55
Taylor AM, Gartner BL, Morrell JJ (2002) Heartwood formation and natural durability—a review. Wood Fiber Sci 34:587–611
Wang SY, Chen KN (1992) Effects of plantation spacings on tracheid lengths, annual ring widths, and percentages of latewood and heartwood of Taiwan-grown Japanese cedar. Mokuzai Gakkaishi 38:645–656
Wilkes J (1991) Heartwood development and its relationship to growth in Pinus radiata. Wood Sci Technol 25:85–90
Wilkins AP (1991) Sapwood, heartwood and bark thickness of silviculturally treated Eucalyptus grandis. Wood Sci Technol 25:415–423
Woeste KE (2002) Heartwood production in a 35-year-old black walnut progeny test. Can J For Res 32:177–181
Yang KC, Hazenberg G (1991) Sapwood and heartwood width relationship to tree age in Pinusbanksiana Michx. Wood Fiber Sci 23:247–252
Yang KC, Murchison HG (1992) Sapwood thickness in Pinus contorta var. latifolia. Can J For Res 22:2004–2006
Yang KC, Hazenberg G (1992) Impact of spacing on sapwood and heartwood in Picea mariana (Mill.)B.S.P. and Picea glauca (Moench.) Voss. Wood Fiber Sci 24:330–336
Acknowledgments
The authors acknowledge the support of Celbi (now Altri) in sample supply and stand and tree data and the assistance of our colleague Lídia Silva in image analysis. The work was carried out with the base funding to Centro de Estudos Florestais given by Fundação para a Ciência e Tecnologia, Portugal, under the FEDER/POCI 2010 programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Meincken and T. Seifert.
Rights and permissions
About this article
Cite this article
Miranda, I., Gominho, J., Araújo, C. et al. Family effects in heartwood content of Eucalyptus globulus L.. Eur J Forest Res 133, 81–87 (2014). https://doi.org/10.1007/s10342-013-0741-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-013-0741-y