Abstract
Numerous oak species have wide geographical distribution and canopy dominance and they may influence the structure and functioning of the community. Therefore, they can be considered as foundation species. In the present study, we tested the potential association between individual genetic diversity of the host plant (microsatellites), host tree species (Quercus castanea and Q. crassipes), and seasonality (dry vs. rainy) with their arthropod community structure [diversity (H′), density, biomass and composition]. Forty individual oak canopies were fogged (10 individuals per species per season) in the Parque Ecológico Ciudad de México. We identified 338 arthropod morphospecies belonging to 21 orders. NMDS showed a separation of host tree species during both seasons, suggesting a different community structure between host tree species. Q. crassipes showed the highest values for H′, richness, and density in comparison with Q. castanea. In contrast, Q. castanea showed the highest values for arthropod biomass. Homozygosity by locus index (HL), a measure of individual diversity, showed that the more genetically diverse host individuals registered more H′ and density of arthropods, independently of the season. On the contrary, arthropod biomass registered an inverse pattern. This study suggests that genetic diversity of foundation species is an important element in the structuring of the canopy arthropod community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatially, genetic diversity can be distributed at different scales, such as populations, subpopulations, or among neighboring individuals (Escudero et al. 2003). This distribution may be the result of environmental influences, including human activities (Knowles 1991), geological history (Vakkari et al. 2006), phylogeographic patterns (Kelleher et al. 2004), past demographic history of plant species (Templeton et al. 1995), among others. Also, it has been documented that the life history characteristics influence the distribution of genetic diversity (Hamrick et al. 1992). For example, tree species sharing certain characteristics of life history (e.g. wide geographical distribution, long life time, spread by wind, and outcrossing) have more genetic diversity than tree species with different combination of features (Hamrick et al. 1992). Also, in these species, the highest genetic diversity is contained within rather than between populations (Siegismund and Jensen 2001).
In the last decade, various studies have documented that the genetic diversity in plants can affect the community of dependent species (e.g. microbes, plants, arthropods, birds, and mammals), especially when these plant species are considered foundation species (e.g. cottonwoods, eucalyptus, oaks, and willows; Whitham et al. 2006). Foundation species have been defined as species that structure a community by creating locally stable conditions for other species and by modulating and stabilizing fundamental ecosystem processes (e.g. Dayton 1972; Whitham et al. 2003).
Oak canopies represent an ideal system to study their associated communities, since they can be physically delimited (Moran and Southwood 1982). In general, it has been documented that canopies support a great diversity of organisms, and arthropods are considered the main component of their associated communities, in terms of abundance and diversity species (Stork and Hammond 1997). Recent estimates suggest that global average richness of this group is between 2.5 and 3.7 millions of species (Hamilton et al. 2010). Therefore, a first way to better understanding the mechanisms that generate and maintain such patterns may be a comprehensive overview of a local canopy arthropods fauna. Also, the quality of different host tree individuals may vary from each other, with considerable consequences for their associated arthropods (Riipi et al. 2004). In response, arthropods may form local demes on individual trees and gradually adapt to the host’s characteristics over time (Mopper 2005).
To evaluate the relationship between host plant genetic diversity and arthropod community structure, studies have been done at the intraspecific [e.g. among populations or patch of mixed genotypes vs. monocultives] (Johnson et al. 2006) and interspecific level (Shuster et al. 2006), as well as in hybrid complexes (Wimp et al. 2004; Tovar-Sánchez and Oyama 2006a, b). In general, two patterns have been reported in these studies: (1) more genetically, diverse host plants will support more species richness and diversity (Wimp et al. 2004; Tovar-Sánchez and Oyama 2006b) and (2) genetically, similar host plant species will hold more similar communities (Bangert et al. 2005; Johnson and Agrawal 2007). These last observations suggest that an increase in the host plant genetic diversity can generate changes in their morphological (Lambert et al. 1995; González-Rodríguez et al. 2004; Tovar-Sánchez and Oyama 2004), phenological (Hunter et al. 1997), and plant architecture (Martinsen and Whitham 1994; Whitham et al. 1999; Bangert et al. 2005), as well as in their secondary chemistry (Fritz 1999; Wimp et al. 2004). These characters constitute a wide array of resources and conditions that can be exploited by their associated herbivore.
Knowledge of the fine-scale associations between arthropods and plants plays a key role in our understanding of the impact of plant genetic diversity on dependent arthropod communities. However, knowledge of the mechanisms that may be driving these relationships remains poorly understood (Wimp et al. 2007). Nevertheless, it has been documented that the plant phenology affects both the quantity and the quality of resources available to arthropods (Murakami et al. 2005, 2007). Also, plants may protect themselves from herbivores with physical defenses (Lambert et al. 1995) and with a wide array of secondary chemicals (Forkner et al. 2004). All of these traits have a genetic basis (Wimp et al. 2007) that changes between host plant species and seasons, but have been rarely linked with arthropod community structure (Johnson and Agrawal 2005; Bangert et al. 2006). Understanding the strength of these associations is important as they provide a mechanistic approach to comprehend the relationship between plant genetic diversity and arthropod community structure.
It has been documented that canopy arthropod community structure varies spatially (Tovar-Sánchez et al. 2003). Hence, in order to evaluate the potential association between individual genetic diversity of host plant species and their arthropod community structure, it is important to conduct studies at a local scale. This approach minimizes the effect of confounding factors such as host plant origin (Tovar-Sánchez and Oyama 2006a), spatial variation in environmental gradients (altitude, latitude, topography) (Gering et al. 2003; Price et al. 2004), vegetation type (Jukes et al. 2001), forest age (Schowalter 1995; Jeffries et al. 2006), and disturbance (Tovar-Sánchez et al. 2003; Forkner et al. 2008).
Quercus (Fagaceae) genus has high species diversity (531) worldwide (Govaerts and Frodin 1998), and Mexico is considered its main center of diversification, containing 161 species, of which 109 are endemic (Valencia 2004). Because many oak species are considered as foundation species due to their wide geographical distribution and canopy dominance, these trees are an excellent system to study the relationship between the host plant genetic diversity and their canopy communities. Also, because relatively few studies have analyzed the influence of individual genetic diversity of foundation species in natural communities, today, the potential ecological consequences of this diversity are not yet clear (Hughes et al. 2008).
In this study, we evaluated whether the individual genetic diversity of Quercus castanea and Q. crassipes in the same locality is associated with canopy arthropod community structure in terms of diversity, density, and biomass. Also, we determined whether individuals of the same host tree species support a similar arthropod fauna and whether the arboreal community structure changes seasonally between oak species.
Methods
Study site
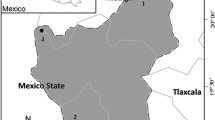
The study site is located at the Parque Ecológico de la Ciudad de Mexico (PECM) (19°14′ and 19°16′N, 99°15′ and 99°10′W) which has been decreed as an area of ecological conservation since 1989 (Diario Oficial 1989). It is situated in the piedmont of Ajusco Hills with an altitudinal range between 2,500 and 2,800 m (Tovar-Sánchez 2009) with an approximate area of 728 ha (Diario Oficial 1989) in central Mexico. Annual average temperature oscillates between 12 and 14 °C and registers an annual precipitation of 1,000 mm, falling mainly in rainy season from June to October; meanwhile, dry season is from November to May (White et al. 1990). Some area of this site was covered by lava from the Xitle cone approximately 2000 years ago (Cordova et al. 1994).
At the PECM, there are three well-defined types of vegetation (Soberón et al. 1991; Rzedowski 1994), a pine oak (Pinus—Quercus) forest is located at the higher parts, and a dense oak forest and a xerophytic shrub are located at the middle part (Quercus spp.) (Soberón et al. 1991; Rzedowski 1994). Within Quercus species in the PECM, the dominating species are Q. castanea Née, Q. crassipes Humb. & Bonpl., Q. frutex Trel., Q. glabrescens Benth., Q. laeta Liebm., Q. laurina Humb. & Bonpl., Q. obtusata Humb. & Bonpl. and Q. rugosa Née. In order to minimize the effects of environmental factors, the study sites were chosen at a fine geographical scale and according to their common characteristics such as similar edaphic conditions, altitude, climate, and vegetation type (mature oak).
The oak host species
Q. castanea and Q. crassipes (section Lobatae) are two abundant species with a wide distribution in Mexico (Valencia 2004). Both species can be distinguished by the shape, size, coloration, and pubescence of their leaves.
Q. castanea includes trees from 5 to 15 m in height with a trunk diameter of 30–60 cm. These trees present gray-greenish leaves with aristate and underside with the veins conspicuously elevated and reticulate. It is located between 1,900 and 3,500 m a.s.l., and it distributes in more than 15 Mexican states trough the major mountain ranges (Sierra Madre Oriental, Sierra Madre Occidental, Sierra Madre del Sur and Transmexican Volcanic Belt; Valencia 2004). It is found frequently in perturbed areas with a xerophytic scrub type of vegetation, and it is also localized in mountain cloud forests (Rzedowski and Rzedowski 2001). On the other hand, Q. crassipes include trees from 3 to 20 m in height and 0.40–1 m in trunk diameter. Leaves are deciduous, coriaceous, narrowly elliptic, and lanceolate, their surface is barely lustrous, and the lower surface is tomentose, whitegrayish (Rzedowski and Rzedowski 2001). It is found in altitudes from 1,900 to 3,500 m a.s.l. in Pinus-Quercus, Quercus-Cupressus, Quercus, Pinus, xerophytic scrubs, in transition between grassland vegetation and mixed forest, mountain cloud forests, and perturbed vegetation (Romero et al. 2002). It is distributed in 12 Mexican states, mainly in the Transmexican Volcanic Belt, although it can be found at the Sierra Madre del Sur (Rzedowski and Rzedowski 2001).
Molecular data
Leaves with no apparent damage from twenty individuals per species were collected. Leaf tissue was frozen in liquid nitrogen and transported to the laboratory for DNA extraction. Total DNA was extracted and purified by using the DNAeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). DNA quantification was done by fluorometric analysis, and DNA quality was visualized by comparing the intensity of bands with known standards of lambda DNA on agarose gels at 0.8 %.
Genetic analyses were performed using microsatellite loci. Primers 1/5, Zag 46, and Zag 110 were obtained according to Steinkellner et al. (1997). PCRs were done as follows: 15 ng of DNA template, 50 mM KCl, 20 mM Tris–HCl (pH 8.4), 2 mM MgCl2, 0.13 mM of each dNTP, 25 mM of each primer, and 0.8 U of Taq polymerase, in a final volume of 25 μl. Reaction conditions were an initial denaturation step at 95 °C for 6 min, followed by 30 cycles at 94 °C for 1 min, 1 min at the appropriate annealing temperature, followed by 30 s at 72 °C, and a final extension at 72 °C for 8 min. Annealing temperature differed for each primer pair. 55 °C for Zag1/5, 48 °C for Zag46 and Zag110. PCR products were resolved on polyacrylamide gels at 6 % (7 M urea) at 60 W for 3 h. Polymorphic fragments were sequenced on an automatic sequencer AFL (Pharmacia) at 35 W for 80–90 min. External molecular weight (50, 100, 150 and 250 pb) was used in conjunction with the Fragment Manager (v1.1) conversion software (Pharmacia) to size the amplified fragments. Initial alignment was performed in Sequencer (GeneCodes, Ann Arbor, MI, USA), and all base pair changes were verified by chromatograms observation.
Canopy arthropod community
Canopy arthropod community structure of Q. castanea and Q. crassipes at the PECM was determined using 10 trees of each species sampled in overlapping zones during dry season (February 2005) and 10 trees during rainy season (August 2005). In total, 20 trees per species were sampled inside the dense oak forest where plant species composition of the surrounding trees was similar. We selected individuals of 8–10 m (mean ± SE, 9.12 ± 0.17 m) in height and between 18.3 and 20.1 m2 (mean 19.20 ± 2.08 m2) of crown cover. Crowns that do not overlap with any other tree within the oak forest were sampled. Arthropods were collected by fogging with 750 ml of non-persistent insecticide (AquaPy, AgrEvo, Mexico) the entire canopy of a single tree. AquaPy is composed by 30 g/l of natural pyrethrins, synergized with 150 g/l of piperonyl-but-oxide. One of the advantages of this insecticide is that it does not present residual activity for long periods of time due to the nature of pyrethrins that are photo-labile and biodegradable (with the dosage used in this study, the biological residual effects may last less than 24 h). Fogging activity was done between 4:00 and 4:30 a.m. (lasting approximately 1:30 min.) in order to avoid denaturalization of the insecticide by solar radiation, avoiding windy or rainy days. Arthropods fallen from each fogged tree were collected in 10 plastic trays (1.0 m2 area per tray) that were randomly distributed under tree crowns. The arthropods were kept in 70 % ethanol except adult organisms of Lepidoptera, which were kept in glassine paper bags. The content of each sample was counted and separated into morphospecies and was sent to specialists for taxonomic identification.
Statistical analysis
Genetic diversity of host plant
Individual genetic diversity of Q. castanea and Q. crassipes was estimated using homozygosity by loci index (HL), a microsatellite-derived measure that improves heterozygosity estimates in natural populations by weighting the contribution of each locus to the homozygosity value depending on their allelic variability (Aparicio et al. 2006). HL is calculated as follows: HL = (ΣE h )/(ΣE h + ΣE j ), where E h and E j are the expected heterozygosity of the loci that an individual bears in homozygosis (h) and in heterozygosis (j), respectively. This index varies between 0 when all loci are heterozygous and 1 when all loci are homozygous. HL was estimated using CERNICALIN, an Excel spreadsheet available on request.
Community structure of canopy arthropods
Analysis of variance (ANOVA) was used to test the effect of host species (S), season (s), individual tree, and the interaction host species × season (S × s) on canopy arthropod density (Zar 2010). Density values of canopy arthropods were estimated in square meters (individuals/m2), and the data were log-transformed (X′ = log X + 1) (Zar 2010).
The diversity of canopy arthropod community of Q. castanea and Q. crassipes at the PECM was estimated using the Shannon–Wiener index (H′), and after the index was compared between pairs of trees with a randomization test as described by Solow (1993), this test re-samples 10,000 times from a distribution of species abundances produced by a summation of the two samples.
Differences in arthropod species composition between different host species and seasons were tested using non-metric multidimensional scaling (NMDS) based on the presence of 338 arthropods. NMDS is a robust ordination technique for community analysis (Clarke 1993), which has been used to analyze differences in arthropod community composition (e.g. Wimp et al. 2004; Tovar-Sánchez and Oyama 2006a). NMDS was used to generate a dissimilarity matrix among the host categories (Q. castanea and Q. crassipes) using the Bray–Curtis dissimilarity coefficient (Faith et al. 1987). Analysis of similarity (ANOSIM) was used to evaluate the differences between arthropods community composition and host species. ANOSIM was employed to test for differences between groups using 1,000 random reassignments and to determine whether the generated dissimilarity matrix is significantly different than chance (Warwick et al. 1990).
Similarity in canopy arthropod community composition (at the morphospecies level) between individual trees and species was estimated using the Jaccard index (I.S.J.) (Southwood 1978). The biomass of canopy arthropods associated with Q. castanea and Q. crassipes was calculated as Tovar-Sánchez (2009). Diversity (H′), density, and biomass variables were not correlated with each other, excepting species richness (S). Therefore, regression analyses were done independently for each variable, in order to assess the relationship between individual genetic diversity of host plant [to Q. crassipes, Q. castanea, and total (both species)] and diversity, density, and biomass (Zar 2010). The softwares used for statistical analysis were STATISTICA 6.0 (StatSoft 1998), Species diversity and richness version 3.03 (Henderson and Seaby 2002), and PC-ORD version 4.28 (McCune and Mefford 1999).
Results
Arthropod community
In total, 13,782 arthropods were collected, 10,622 during rainy season and 3,160 during dry season. Canopy arthropods composition of Q. crassipes and Q. castanea is composed by 338 morphospecies belonging to 21 orders (Araneae, Arthropleona, Astigmata, Coleoptera, Cryptostigmata, Dermaptera, Diptera, Hemiptera, Hymenoptera, Isoptera, Lepidoptera, Mecoptera, Mesostigmata, Neuroptera, Opiliones, Orthoptera, Pseudoscorpiones, Prostigmata, Psocoptera, Symphypleona, and Thysanoptera, nomenclature based on Evans 1992; Hopkin 1997; Borror et al. 2005).
In general, the number of morphospecies was lower during dry season in comparison with rainy season. Q. crassipes trees supported the highest number of morphospecies during both seasons (dry = 36, rainy = 187, total = 201), while Q. castanea showed the lowest species richness for both seasons (dry = 25, rainy = 123, total = 137, Table 1). Also, significant differences in epiphyte canopy arthropod composition were registered for both seasons (dry F 1,5,300 = 45.29, P < 0.01; rainy F 1,20,700 = 67.13, P < 0.001).
Relative abundance of morphospecies grouped into arthropod orders changed between seasons and within oak species for Q. castanea but not for Q. crassipes (Fig. 1). In Q. crassipes, the most abundant groups were Arthropleona, Prostigmata, and Cryptostigmata during both seasons, in comparison with Q. castanea which supported the highest relative abundance of Cryptostigmata, Hemiptera, and Diptera during dry season and of Arthropleona, Prostigmata, and Cryptostigmata during rainy season. In general, Arthropleona, Prostigmata, and Cryptostigmata were the most common groups in both oaks species.
Canopy arthropod composition in Quercus crassipes and Q. castanea at the Parque Ecológico de la Ciudad de México in dry (February, 2005) and rainy (August, 2005) seasons. ART Arthropleona, PRO Prostigmata, CRY Cryptostigmata, HYM Hymenoptera, PSO Psocoptera COL Coleoptera, DIP Diptera, HEM Hemiptera, SYM Symphypleona, MES Mesostigmata, ARA Araneae and OTHERS: (Astigmata, Dermaptera, Isoptera, Lepidoptera, Mecoptera, Neuroptera, Opiliones, Orthoptera, Pseudoscorpiones, and Thysanoptera)
In dry season, NMDS ordination explained 73 % of the variance between sampling points (axis 1 = 31 % and axis 2 = 42 %), and in rainy season explained 67 % (axis 1 = 29 % and axis 2 = 38 %) (Fig. 2). In general, we found significant differences in oak canopy arthropod community composition for both species (ANOSIM r = 0.3151, n = 40, P < 0.001, Fig. 2), showing that these communities on each host species are significantly different from one another. Also, the differences between categories were significant after correcting the critical value of alpha for inflated type II errors (P = 0.02 for all comparisons): Q. castanea versus Q. crassipes in dry season, r = 0.6049, P < 0.001; and in rainy season, r = 0.5318, P < 0.001.
Arthropod community composition among oak hosts Quercus crassipes and Q. castanea. Each point is a two-dimensional (axis 1 and axis 2) representation of arthropod species composition on an individual tree based on global, non-metric multidimensional scaling (NMDS). Distances between points reflect a dissimilarity matrix created using the Bray–Curtis dissimilarity coefficient (Faith et al. 1987). Points that are close together have arthropod communities that are more similar in composition compared to points that are far apart
Arthropod density
The density of canopy arthropod species differed significantly between tree species (F = 99.98, df = 1, P < 0.001), between seasons (F = 179.76, df = 1, P < 0.001), and among individual trees (F = 2.05, df = 36, P < 0.001). The interaction species × season was statistically significant (F = 10.47, df = 1, P < 0.001). In general, we found that canopy arthropod densities (mean ± SE individuals m2) were higher during rainy season. Q. crassipes supported the highest arthropod densities for both seasons (dry = 52.0 ± 5.3, rainy = 372.5 ± 36.7) in comparison with Q. castanea that registered the lowest density (dry = 30.8 ± 4.6; rainy = 346.5 ± 30.1, Table 2).
At the individual tree level, it is observed that during dry season, the epiphyte arthropod densities (mean ± SE individuals m2) associated with Q. crassipes varied from 20.6 ± 5.5 to 83.8 ± 13.1. On the other hand, densities for Q. castanea ranged between 18.9 ± 5.3 and 48.1 ± 6.6 (Table 2). In rainy season, densities for Q. crassipes oscillated between 158.9 ± 30.6 and 560.2 ± 48.5, and for Q. castanea, densities varied from 204.7 ± 19.4 to 473.6 ± 32.7 (Table 2). However, only significant differences in total epiphyte arthropod densities between oak species during dry season were obtained (Table 2).
Diversity of arthropods
The total number of morphospecies found in this study was 338. In general, both the diversity index and the species richness of the associated fauna with Q. crassipes and Q. castanea were higher during rainy season (Table 1). However, Q. castanea supported the lowest species richness and diversity (dry: S = 25, H′ = 2.5; rainy: S = 123, H′ = 3.9) in comparison with Q. crassipes (dry: S = 36, H′ = 3.2; rainy: S = 187, H′ = 4.4) for both seasons. Total diversity (dry + rainy) showed significant differences between oak host species (Q. castanea and Q. crassipes, Table 1).
Similarity among taxa
The Jaccard similarity index showed that during dry season, Q. castanea and Q. crassipes shared 10 % of the arthropod community, while in rainy season, this percentage increased to 15 % of shared species.
Biomass of arthropods
Biomass of canopy arthropods (mg DW/m2) differed significantly between oak species (F = 87.68, df = 1, P < 0.001), between seasons (F = 154.90, df = 1, P < 0.001), among individual trees (F = 2.11, df = 36, P < 0.001), and the interaction species x season was statistically significant (F = 16.16, df = 1, P < 0.001). In general, arthropod biomass was higher during rainy season in comparison with dry season. Q. castanea supported the highest arthropod biomass (mean mg DW/m2 ± SE) in both seasons (dry = 30.9 ± 0.003, rainy = 1108.9 ± 0.044), while Q. crassipes supported the lowest biomass (dry = 14.4 ± 0.002, rainy = 318.2 ± 0.002) (Table 3).
Arthropod composition (biomass)
In general, biomass of morphospecies grouped into arthropod orders changed between host oak species (Fig. 3). The order Lepidoptera was dominant in Q. castanea and Q. crassipes during both seasons. Particularly, during dry season, Thysanoptera, Hymenoptera, Coleoptera, and Hemiptera were the most important arthropod groups in Q. crassipes; meanwhile, in Q. castanea were Hemiptera, Diptera, Hymenoptera and Coleoptera. During rainy season, Coleoptera, Thysanoptera, Orthoptera, and Psocoptera were the orders that registered the greatest biomass in Q. crassipes; meanwhile, Psocoptera, Coleoptera, Hymenoptera, and Diptera were the most common in Q. castanea (Fig. 3).
Canopy arthropod biomass associated with Q. crassipes and Q. castanea at the Parque Ecológico de la Ciudad de México during dry (February 2005) and rainy season (August, 2005). ARA Araneae, COL Coleoptera, DIP Diptera, HEM Hemiptera, HYM Hymenoptera, LEP Lepidoptera, ORT Orthoptera, PSO Psocoptera, THY Thysanoptera and OTHERS = (Arthropleona, Astigmata, Cryptostigmata, Dermaptera, Isoptera, Mecoptera, Mesostigmata, Neuroptera, Opiliones, Pseudoscorpiones, Prostigmata, Symphypleona)
Association between oak host plant genetic diversity and canopy arthropod community
This study revealed that individual genetic diversity of host plant affects significantly the community structure of canopy arthropods. In general, our results showed a negative and significant relationship between genetic diversity of oak host plant (Q. crassipes, Q. castanea, and total) and the Shannon–Wiener diversity index (H′) and density, independently of the season (Fig. 4). This result showed that the more genetically diverse host plant supports more H′ and density of their arthropod communities. The exception to this pattern was arthropod density in Q. castanea during rainy season. On the contrary, a positive and significant relationship was observed between biomass and genetic diversity for both species and for the total during rainy season (Table 4).
Correlation between individual genetic diversity of host plant (HL) (Quercus crassipes and Q. castanea) in dry season, rainy season, and total (both seasons), and canopy arthropod community structure [Shannon–Wiener diversity index (H′), density and biomass] obtained with microsatellites markers (SSRs). *P < 0.05, **P < 0.01, ***P < 0.001, ns no statistically significant differences
Discussion
The results obtained in the present study showed that oak host species (Q. castanea and Q. crassipes), seasonality (rainy vs. dry), and the levels of genetic diversity of the oak host plant were significantly associated with canopy arthropod community structure. We found a different community structure among oak host species in terms of composition, diversity, density, and biomass. Also, the values of Shannon–Wiener diversity index (H′), species richness (S), density, and biomass of arthropodofauna were higher during rainy season in comparison with dry season for both oak host species. Finally, we found that with increasing genetic diversity of the oak host plant, values of H′ and density also increase. In contrast, arthropod biomass registered an inverse pattern.
Association between oak host species and canopy arthropod community
Our study revealed that oak host species affect significantly the community structure of canopy arthropods. NMDS showed that canopy arthropod composition was different among oak host species during both seasons. This suggests that canopy arthropods of Q. crassipes and Q. castanea behave as natural communities; therefore, differences between oaks species result in phenotypes that structure the composition of the arthropod community. This separation between taxa among different oak host species was also found in Q. alba and Q. velutina in Missouri (Le Corff and Marquis 1999), in Q. crassifolia, Q. crassipes, and their hybrids in the center of Mexico (Tovar-Sánchez and Oyama 2006a), in Eucaliptus moluccana, E. cebra, E. marginata, and E. calophylla in Australia (Recher et al. 1996), and in four dominant conifers (Jeffrey pine, sugar pine, white fir, incense cedar) and in three angiosperms species (California black oak, manzanita and White-thorn ceanothus) in Sierra Nevada in California (Schowalter and Zhang 2005). Probably, phylogenetic origin of the host species may influence their arthropod community structure, due to differences in their canopy architecture, genetic pool, chemical substances, phenological and historical events (Tovar-Sánchez and Oyama 2006a; Gilbert and Webb 2007; Gossner et al. 2009). The aforementioned suggests that most arthropod species are associated with a set of more or less phylogenetically closely related hosts, rather than a random set of hosts present in their geographic distribution range (Gilbert and Webb 2007; Gossner et al. 2009).
This implies that Q. castanea and Q. crassipes are important reservoirs of canopy arthropods; then, each oak host species supports a different canopy arthropod community and that seasonality is not a factor that modifies the assemblage of species in each community.
In general, Q. crassipes showed the highest richness and diversity of canopy arthropods (dry: S = 36, H′ = 3.2; rainy: S = 187, H′ = 4.4) during both seasons in comparison with Q. castanea (dry: S = 25, H′ = 2.5; rainy: S = 123, H′ = 3.9). The precise causes of these differences between host tree species are not fully understood; however, it is possible that differences between crown structure of host species may generate differences in resource and niche diversity that may be used by arthropods (Tovar-Sánchez and Oyama 2006b; Tovar-Sánchez 2009). For example, Guerrero et al. (2003) recorded differences in canopy arthropod abundance in nine tree species of Sapotaceae and Lecythidaceae in a tropical forest, as a result of differences in canopy density of host species. Probably, these differences may generate changes in microclimate conditions that arthropod communities that inhabit them are exposed to (Tassone and Majer 1997). Another factor that has been considered is the variability between chemical substances among host taxa (Orians 2000; Osier and Lindroth 2001). In terms of species richness of canopy arthropods (dry + rainy season), the results obtained for Q. crassipes (201) are similar to those reported in Great Britain for Q. petraea (204) (Southwood et al. 2004). While those registered in Q. castanea (137) from Mexico are similar to Q. cerris (144) from Great Britain (Southwood et al. 2004). However, these values are lower than those reported for Q. robur (285) in Great Britain (Southwood et al. 2004), Q. laurina (342) and Q. rugosa (272) in the Center of Mexico (Tovar-Sánchez 2009). In contrast, our results are higher than those reported for Q. ilex (84) in Great Britain (Southwood et al. 2004). Moreover, total species diversity values (dry + rainy) for Q. crassipes (4.6) and Q. castanea (4.0) are similar to those reported for Q. laeta (4.3), Q. rugosa (4.4), Q. crassifolia (4.4), and Q. greggi (4.9) in the Center of Mexico (Tovar-Sánchez et al. 2003). According to the literature, there are many factors that may influence the richness and diversity of oak canopy arthropod communities, for example, host plant origin (native, introduced or hybrid; Tovar-Sánchez and Oyama 2006a, b), geographical range (Lawton 1982), habitat heterogeneity (Summerville and Crist 2002, 2003), disturbance (Jeffries et al. 2006), host plant secondary chemistry (Murakami et al. 2007), spatial variation (Tovar-Sánchez et al. 2003), temporal variation (Southwood et al. 2004, 2005), and the level of genetic variation of the host plant (Tovar-Sánchez and Oyama 2006b).
Association between seasonality and canopy arthropod community
The results obtained in the present study showed that seasonality (rainy vs. dry) was significantly associated with canopy arthropod community structure at the PECM. The general pattern registered was that H′, S, density, and biomass of arthropodofauna was higher during rainy season in comparison with dry season for both oak host species. These results are similar to those reported in other studies of tropical (Guerrero et al. 2003; Stuntz et al. 2003; Wagner 2003) and temperate forests in Mexico (Palacios-Vargas et al. 2003; Tovar-Sánchez et al. 2003; Tovar-Sánchez and Oyama 2006a, b; Tovar-Sánchez 2009), South France, and Great Britain (Southwood et al. 2004, 2005), and in Japan (Murakami et al. 2005). It has been proposed that this pattern may be the result of an increase in resource availability during rainy season, which is in accordance with seasonal growth of forest ecosystems (Strong et al. 1984; Barbosa et al. 2000). In this sense, Rzedowski (1978) mentions that Q. castanea and Q. crassipes are deciduous species, which in turn increase branch, leaves, and fruit formation and the development of epiphyte plants during rainy season, a fact that can favor a great possibility of resources that may be used by canopy arthropods. The formation of these resources can promote changes at the microclimate level (Basset and Burckhardt 1992; Basset and Novotny 1999; Peeters et al. 2001), which in turn increases the conditions that may be exploited by arthropods in this particular habitat (Yarnes and Boecklen 2005). Moreover, during rainy season, younger leaves are more abundant, nutritive, softer and have less chemical substances for defense (Kursar and Coley 2003; Boege 2004; Coley et al. 2006). An increase in host plant nutritional quality as well as the availability of canopy resources may explain the increase in density, species richness, and biomass of the oak canopy arthropods at the PECM during rainy season. This last scenario was also observed experimentally by Agosta (2008) who reported a larger growth and survival in more than 200 caterpillars in 238 host trees during rainy season in comparison with dry season. This increase in primary consumers (herbivores) adds the possibility that predators may feed from them, so when the number of prays arise, we would expect that predator populations will be favored (Siemann et al. 1998). Hence, we would think that parasitoid populations (wasps and flays) would also increase their relative abundance when the number of herbivore and carnivore hosts also increases (Walker et al. 2008). Finally, the increase in forest vegetation during rainy season may serve as a bridge in order to increase the possibility of colonizing new trees by new arthropod species (Basset et al. 1992; Campos et al. 2006). This situation presumes that the quality of host plant and resource availability during rainy season promotes a cascade effect on canopy arthropod community.
On the other side, seasonality influenced the H′ and S of Q. castanea and Q. crassipes canopy arthropod community, because the highest values were obtained during rainy season. Similar results have been reported for eucalyptus in west Australia (Recher et al. 1996) and for other Mexican oaks in the Mexican valley (Tovar-Sánchez et al. 2003; Tovar-Sánchez and Oyama 2006a; Tovar-Sánchez 2009). These observations are probably the result of an increase in host plant quality and availability of resources during rainy season, as already mentioned.
Arthropod density (number of individuals/m2) registered in rainy season is similar to the observations made by Southwood et al. (1982) for Q. robur in South Africa (591.3), and by Tovar-Sánchez (2009) for Q. laurina (406.7) and Q. rugosa (312. 12), but is lower than the reported for Criptomeria japonica (3500) in Japan (Hijii 1989) or intermediate in comparison with other studies where canopy fogging technique was used in temperate and tropical forests (Southwood et al. 1982; Adis et al. 1997; Guerrero et al. 2003). It is probable that these differences are the result of sampling effects in different studies (canopy fogging efficiency) or differences in volume and density of foliage sampled (Guerrero et al. 2003). In particular, arthropod dominant orders showed changes in terms of density in response to seasonal variation. During dry season, Arthropleona, Prostigmata and Cryptostigamata were the groups that presented the highest densities in Q. crassipes, while Cryptostigamata, Hemiptera, and Diptera dominated in Q. castanea. During rainy season, the most important groups were Arthropleona, Prostigmata, and Cryptostigamata in both host species. Our results are consistent with other studies where mites and springtails associated with canopies of diverse plant species in different environments, presented high dominance (Guerrero et al. 2003; Palacios-Vargas and Castaño-Meneses 2003; Thunes et al. 2004; Tovar-Sánchez 2009). The relative dominance of mites and springtails may be explained because Q. crassipes and Q. castanea have the necessary conditions for the establishment of epiphytes vascular plants as well as the presence of a rugose cortex that facilitates organic matter accumulation, circumstances that provide food, and a great diversity of microhabitats for these organisms that are degraders (Kitching et al. 1993; Palacios-Vargas and Castaño-Meneses 2003). Microarthropods particularly mites and springtails play a key role in ecosystem management because they participate in organic matter decomposition and nutrient recycling (Blair et al. 1992; Vu and Nguyen 2000). These facts reflect the importance that this group has in terms of ecosystem restoration and conservation. Specifically, Cryptostigmata was one of the most abundant groups in both oak host species during dry and rainy seasons. These observations are in agreement with other studies of tropical (Palacios-Vargas et al. 1999; Palacios-Vargas and Castaño-Meneses 2003) and temperate forests (Palacios-Vargas et al. 2003; Tovar-Sánchez 2009) where canopy fogging techniques were used, a fact that may be explained in terms of the heterogeneity that each canopy microhabitat presents (epiphytes, trunks, branches) (Karasawa and Hijii 2004). Also, Arthropleona was another important group in both seasons. Cutz-Pool et al. (2005) have suggested that the presence of this group in canopies depends on the diversity of epiphyte plants, algae, lichens, and moss that are present in this habitat. Moreover, it has been proposed that humus accumulation on oak canopies is another factor that favors the abundance of Arthropleona (Nadkarni and Longino 1990; Paoletti et al. 1991; Tovar-Sánchez 2009).
In the present study, we found that concerning biomass, Lepidoptera was the dominant order in Q. castanea and Q. crassipes canopies for both seasons. Comparable results were reported for Q. robur and Q. petreae in Great Britain (Southwood et al. 2004) and for Larix kaempferi, a coniferous plant in Japan (Hijii et al. 2001). However, a decrease in biomass values was detected during dry season. These results are consistent with those reported for Q. laurina and Q. rugosa in the same study site (PECM) (Tovar-Sánchez 2009). It has been suggested that this decrease in Lepidoterans fauna (mainly during larvae stage) is an animal strategy to avoid high temperatures in order to prevent dryness during this season (Southwood 1978; Wolda 1979). Probably, the increase in Lepidoptera fauna during rainy season may be due to the tight relationship that these kind of organisms present between their developmental stages and changes in resources and conditions during this season (Betzholtz 2003; Hättenschwiler and Schafellner 2004; Yarnes and Boecklen 2005). Furthermore, this type of organisms consumes great amounts of food, because their high metabolic rate (Arnett and Louda 2002). In addition, the high Lepidoptera dominance may be the result of their high degree of competitivity, the great amount of resources in host species, and the low number of predators (Begon et al. 2006), permitting lepidopterans to increase their dominance, and some might even be considered as plague (Guedes et al. 2000; Arnett and Louda 2002). In general, mean biomass (mg DW/m2) values observed in this study during rainy season (723.5) are similar to those reported for Q. robur in South Africa (498) and in Great Britain (600) by Moran and Southwood (1982), and for Q. laurina (494.6) and Q. rugosa (647.4) in Mexico (Tovar-Sánchez 2009) during the same season.
Association between oak host plant genetic diversity and canopy arthropod community
Our results showed that with increasing genetic diversity (HL) of the oak host plant, the values of H′, S, and density of canopy arthropod communities increases. In particular, results of HL versus H′ explained from 63 to 79 % of the variation for Q. crassipes and from 56 to 69 % for Q. castanea. These results are consistent with those reported by Wimp et al. (2004), who found that the cottonwoods genetic diversity (Populus fremontii × P. angustifolia) has a positive and significant relationship with the diversity (H′) of their endophagous insect community, explaining about 60 % of the variability in the community. Similarly, Tovar-Sánchez and Oyama (2006b) reported that the oak genetic diversity (Quercus crassipes × Q. crassifolia) explained about 78 % of the diversity (H′) of their endophagous insect community. However, in the aforementioned studies, genetic diversity has been measured at the population level. On the other hand, some studies have interpreted the genetic diversity of host individuals according to the genotype which they belong (e.g. parental species, F1 hybrids, and backcross hybrid) (Dungey et al. 2000; Hochwender and Fritz 2004; Wimp et al. 2004) or to the genotypic diversity of the stand or patch (mixed genotypes vs. monoculture) (Johnson et al. 2006; Crutsinger et al. 2007) which were correlated with the diversity of arthropods associated. For example, Dungey et al. (2000) found that arthropod herbivores species richness was greater on eucalyptus hybrids than parental species. Similarly, Wimp et al. (2005) found that species richness was significantly greater in F1 hybrids compared to pure cottonwoods. On the other hand, Crutsinger et al. (2007) found that the number of species in the associated arthropod community increased as the number of host plant genotypes of Solidago altissima in experimental plots increased. In addition, most of these studies have been under experimental conditions (Hochwender and Fritz 2004; Johnson et al. 2006) and not in natural systems.
In this work, we evaluated the individual genetic diversity of host plant in one locality, and this experimental design allowed us to control for variables that have been documented as factors that modify the arthropod community structure, for example, forest age (Marquis et al. 2000), altitude (Summerville et al. 2003), soil type (Gering et al. 2003), climate (Price et al. 2004), vegetation type (Jukes et al. 2001), disturbances (Tovar-Sánchez et al. 2003; Forkner et al. 2008), among others.
In general, it has been suggested that arthropod communities are favored in the more genetically diverse host plants, creating a plethora of niches (Wimp et al. 2004), habitats with a more complex architecture (Martinsen and Whitham 1994; Whitham et al. 1999; Bangert et al. 2005), an increase in food resources, and a low resistance to herbivorous attack (Fritz 1999). Therefore, the variation in community composition was linked to genetically based variation in these oak traits.
Host plant genetic diversity not only has direct impact on the community of associated herbivores, but also can be extended to the following trophic levels indirectly, by promoting a cascade effect throughout the community (Whitham et al. 2006). For example, an increase in host plant genetic diversity can promote an augment in their architectural complexity and nutritional quality (Bailey et al. 2004; Glynn et al. 2004). This may favor herbivores density (Bailey et al. 2006), predation intensity, and parasitism degree (Sarfraz et al. 2008).
Interestingly, arthropod biomass registered an inverse pattern. This means that as the genetic diversity of the oak host plant increases, the biomass of the associated arthropods decreases. Similar results were obtained by Tovar-Sánchez et al. (unpublished data) who found that the biomass of arthropod communities associated with canopies of Q. crassipes and Q. rugosa decreases as the genetic diversity of the oak host species increases. This result suggests that the genetically less diverse host plant may have a lower amount of defense mechanisms against herbivores. This last scenario may promote the increase in the dominance of certain groups (e.g. Lepidoptera larvae), favoring an increase in abundance and in body size due to competitive superiority (Tovar-Sánchez 2009) which may become a plague (Arnett and Louda 2002).
Conclusions and perspectives
The data presented here showed that oak host species (Q. castanea and Q. crassipes), seasonality (rainy vs. dry), and genetic diversity of oak host plants were significantly associated with canopy arthropod community structure. Interestingly, the genetic diversity pattern found in this study was independent of the oak host species and of the seasonality. It is now important to demonstrate these associations in other foundation species and in other spatial scales to understand their generality.
In terms of conservation, if arthropod species respond to genetic differences among host plants, as our study showed, to conserve genetic diversity in the host plant population is fundamental to preserve the diversity of the associated arthropod communities. This community genetic perspective serves as a guide for future conservation efforts. It is necessary to propose strategies to reduce the impact of factors that can negatively alter the genetic diversity and genetic structure, especially in foundation species that are community and ecosystem drivers. For example, it is important to regulate the species use in restoration programs, because, although they are native species, they can be invasive. Under this scenario, vegetal community structure can be modified and therefore the distribution and abundance of foundation species. Since oaks represent dominant trees in Mexican temperate forest, these findings may be important locally and at a landscape level. The consideration of the genetic diversity of the foundation species can be a general and efficient approach to conserving processes and diverse assemblages in nature.
References
Adis J, Paarmann W, da Fonseca CRV, Rafael JA (1997) Knock-down efficiency of natural pyrethrum and survival rate of living arthropods obtained by fogging in Central Amazonia. In: Stork NE, Adis J, Didham RK (eds) Canopy arthropods. Chapman and Hall, London, pp 67–81
Agosta SJ (2008) Fitness consequences of host use in the field: temporal variation in performance and a life history tradeoff in the moth Rothschildia lebeau (Saturniidae). Oecologia 157:69–82
Aparicio JM, Ortego J, Cordero PJ (2006) What should we weigh to estimate heterozygosity, alleles or loci? Mol Ecol 15:4659–4665
Arnett AE, Louda SM (2002) Re-test of Rhinocyllus conicus host specificity, and the prediction of ecological risk in biological control. Biol Conserv 106:251–257
Bailey JK, Bangert RK, Schweitzer JA, Trotter RT III, Shuster SM, Whitham TG (2004) Fractal geometry is heritable in trees. Evolution 59:2100–2102
Bailey JK, Wooley SC, Lindroth RL, Whitham TG (2006) Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol Lett 9:78–85
Bangert RK, Turek RJ, Martinsen GD, Wimp GM, Bailey JK, Whitham TG (2005) Benefits of conservation of plant genetic diversity on arthropod diversity. Conserv Biol 19:379–390
Bangert JK, Allan GJ, Turek RJ, Wimp GM, Meneses N, Martinsen GD, Keim P, Whitham T (2006) From genes to geography: a genetic similarity rule for arthropod community structure at multiple geographic scales. Mol Ecol 15:4215–4228
Barbosa P, Segarra A, Gross P (2000) Structure of two macrolepidopteran assemblages on Salix nigra (Marsh) and Acer negundo L.: abundance, diversity, richness, and persistence of scarce species. Ecol Entomol 25:374–379
Basset Y, Burckhardt D (1992) Abundance, species richness, host utilization and host specificity of insect folivores from a woodland site, with particular reference to host architecture. Rev Suisse Zool 99:771–791
Basset Y, Novotny V (1999) Species richness of insect herbivore communities on Ficus in Papua New Guinea. Biol J Linn Soc 67:477–499
Basset Y, Aberlenc HP, Delvare G (1992) Abundance and stratification of foliage arthropods in a lowland rainforest of Cameroon. Ecol Entomol 17:310–318
Begon M, Townsend CR, Harper JL (2006) Ecology from individuals to ecosystems. Blackwell Publishing, Australia
Betzholtz PE (2003) Habitat utilization by ovipositing females and larvae in an endangered population of the moth Dysauxes ancilla (Lepidoptera: Ctenuchidae). J Res Lep 37:47–54
Blair JM, Crossley DA, Callaham LC (1992) Effects of litter quality and microarthropods on N dynamics and retention of exogenous 15N in decomposing litter. Biol Fertil Soils 12:241–252
Boege K (2004) Herbivore attack in Casearia nitida influenced by plant ontogenetic variation in foliage quality and plant architecture. Oecologia 143:117–125
Borror DJ, Triplehorn AC, Johnson FN (2005) Study of insects. Thomson Brooks/Cole, USA
Campos RI, Vasconcelos HL, Ribeiro SP, Neves FS, Soares JP (2006) Relationship between tree size and insect assemblages associated with Anadenanthera macrocarpa. Ecography 29:442–450
Clarke KR (1993) Non-parametric multivariate analysis of changes in community structure. Aus J Ecol 18:117–143
Coley PD, Bateman LM, Kursar A (2006) The effects of plant quality on caterpillar growth and defense against natural enemies. Oikos 115:218–228
Cordova C, Martín del Pozo AL, López-Camacho J (1994) Palaeolandsforms and volcanic impact in the environment of Prehistoric Cuicuilco, southern Mexico City. J Archaeol Sci 21:585–596
Crutsinger GM, Collins MD, Fordyce JA, Sanders NJ (2007) Temporal dynamics in non-additive responses of arthropods to host-plant genotypic diversity. Oikos 117:255–264
Cutz-Pool LQ, Palacios-Vargas JG, Castaño-Meneses G (2005) Diversidad y abundancia de colémbolos de musgos corticícolas en el Volcán Iztaccíhuatl. Folia Entomol Mex 4:171–175
Dayton PK (1972) Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In: Parker BC (ed) Proceedings of the colloquium on conservation problems in Antarctica. Allen Press, Lawrence, pp 81–89
Dungey HS, Potts BM, Whitham TG, Li HF (2000) Plant genetics affects arthropod community richness and composition: evidence from a synthetic eucalypt hybrid population. Evolution 54:1938–1946
Escudero A, Iriondo JM, Torres ME (2003) Spatial analysis of genetic diversity as tool for plant conservation. Biol Conserv 113:351–365
Evans GO (1992) Principles of Acarology. University Press, CAB International, Cambridge
Faith DP, Minchin PR, Belbin L (1987) Composition dissimilarity as a robust measure of ecological distance. Vegetation 69:57–68
Forkner RE, Marquis RJ, Lill JT (2004) Feeny revisited: condensed tannins as anti-herbivore defenses in leaf-chewing herbivore communities of Quercus. Ecol Entomol 29:174–187
Forkner RE, Marquis RJ, Lill JT, Corff JL (2008) Timing is everything? Phenological synchrony and population variability in leaf-chewing herbivores of Quercus. Ecol Entomol 33:276–285
Fritz RS (1999) Resistance of hybrid plants to herbivores: genes, environment, both? Ecology 80:382–391
Gering JC, Veech JA, Crist TO (2003) Additive partitioning of species diversity across multiple spatial scales: implications for regional conservation of biodiversity. Conserv Biol 17:488–499
Gilbert GS, Webb CO (2007) Phylogenetic signal in plant pathogen-host range. Proc Natl Acad Sci 104:4979–4983
Glynn C, Rönneberg-Wästljun A, Julkunen-Tiitto R, Weih M (2004) Willow genotype, but not drought treatment, affects foliar phenolic concentrations and leaf- beetle resistance. Entomol Exp Appl 113:1–14
González-Rodríguez A, Arias DM, Valencia S, Oyama K (2004) Morphological and RAPD analysis of hybridization between Quercus affinis and Q. laurina (Fagaceae), two Mexican red oaks. Am J Bot 91:401–409
Gossner MM, Chao A, Bailey RI, Prinzing A (2009) Native fauna on exotic trees: phylogenetic conservatism and geographic contingency in two lineages of phytophages on two lineages of trees. Am Nat 173:599–614
Govaerts R, Frodin DG (1998) World checklist and bibliography of Fagales (Betulaceae, Corylaceae, Fagaceae and Ticodendraceae). Royal Botanic Gardens, Kew
Guedes RNC, Zanuncio TV, Zanuncio JC, Medeiros AGB (2000) Species richness and fluctuations of defoliator lepidoptera populations in Brazilian plantations of Eucalyptus grandis as affected by plant age and weather factors. For Ecol Manag 137:179–184
Guerrero JCH, da Fonseca CRV, Hammond PM, Stork NE (2003) Seasonal variation of canopy arthropods in Central Amazon. In: Basset Y, Novotny V, Miller S, Kitching RL (eds) Arthropods of tropical forest. Spatio-temporal dynamics and resource use in the canopy. Chapman and Hall, London, pp 170–175
Hamilton AJ, Basset Y, Benke KK, Grinbacher PS et al (2010) Quantifying uncertainty in estimation of tropical arthropod species richness. Am Nat 176:90–95
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plan species. New For 6:95–124
Hättenschwiler S, Schafellner C (2004) Gypsy moth feeding in the canopy of a CO2-enriched mature forest. Glob Change Biol 10:1899–1908
Henderson PA, Seaby MPH (2002) Species diversity and richness software 3.03. Pisces Conservation Ltd, Lymington
Hijii N (1989) Arthropod communities in a Japanese cedar (Cryptomeria japonica D. Don) plantation: abundance, biomass and some properties. Ecol Res 4:243–260
Hijii N, Umeda Y, Mizutani M (2001) Estimating density and biomass of canopy arthropods in coniferous plantations: an approach based on a tree-dimensional parameter. For Ecol Manag 144:147–157
Hochwender CG, Fritz RS (2004) Plant genetic differences influence herbivore community structure: evidence from a hybrid willow system. Oecologia 138:547–557
Hopkin PS (1997) Biology of the springtails (Insecta: Collembola). Oxford University Press, Oxford
Hughes AR, Inouye BD, Johnson TJ, Underwood N, Vellend M (2008) Ecological consequences of genetic diversity. Ecol Lett 11:1–15
Hunter MD, Varley GC, Gradwell GR (1997) Estimating the relative roles of top-down and bottom-up forces on insect herbivore populations: a classic study revisited. Proc Natl Acad Sci 94:9176–9181
Jeffries JM, Marquis RJ, Forkner RE (2006) Forest age influences oak insect herbivore community structure, richness and density. Ecol Appl 16:901–912
Johnson MTJ, Agrawal AA (2005) Plant genotype and environment interact to shape a diverse arthropod community on evening primrose (Oenothera biennis). Ecology 86:874–885
Johnson MT, Agrawal AA (2007) Covariation and composition of arthropod species across plant genotypes of evening primrose, Oenothera biennis. Oikos 116:941–956
Johnson MT, Lajeunesse MJ, Agrawal AA (2006) Additive and interactive effects of plant genotypic diversity on arthropod communities and plant fitness. Ecol Lett 9:24–34
Jukes MR, Peace AJ, Ferris R (2001) Carabid beetle communities associated with coniferous plantations in Britain: the influence of site, ground vegetation and stand structure. For Ecol Manag 148:271–286
Diario Oficial de la Federación: 28 de junio (1989) Decreto de Expropiación, pp 31–39
Karasawa S, Hijii N (2004) Morphological modifications among oribatid mites (Acari:Oribatida) in relation to habitat differentiation in mangrove forest. Pedobiologia 48:383–394
Kelleher CT, Hodkinson TR, Kelly DL, Douglas GC (2004) Characterization of chloroplast DNA haplotypes to reveal the provenance and genetic structure of oaks in Ireland. For Ecol Manag 189:123–131
Kitching RL, Bergelson JM, Lowman MD, McIntyre S, Carruthers S (1993) The biodiversity of arthropods from Australian rainforest canopies: general introduction, methods, sites and ordinal results. Aust J Ecol 18:181–191
Knowles P (1991) Spatial genetic structure within two natural stands of black spruce (Picea mariana (Mill.) B.S.P.). Silvae Genet 40:13–19
Kursar TA, Coley PD (2003) Convergence in defense syndromes of young leaves in tropical rainforest. Biochem Syst Ecol 21:929–949
Lambert L, McPherson RM, Espelie KE (1995) Soybean host plant resistance mechanisms that alter abundance of white-flies (Homoptera: Alyrodidae). Environ Ecol 24:1381–1386
Lawton JH (1982) Vacant niches and unsaturated communities: a comparison of bracken herbivores at sites on two continents. J Anim Ecol 51:573–595
Le Corff J, Marquis RJ (1999) Differences between understory and canopy in herbivore community composition and leaf quality for two oak species in Missouri. Ecol Entomol 24:46–58
Marquis RJ, Forkner RE, Lill J, Le Corff J (2000) Impact of timber harvest on species accumulation curves for oak herbivore communities of the Missouri Ozarks. Missouri Ozark Forest Ecosystem Project (MOFEP). In: Brookshire B, Shifley S (eds) Proceedings of the Second Missouri Ozark Forest Ecosystem Project Symposium, GTRNC-227 North Central Experiment. Department of Agriculture, Forest Service, Station, St. Paul, pp 183–195
Martinsen GD, Whitham TG (1994) More birds nest in hybrid cottonwood trees. Wilson Bull 106:474–481
McCune B, Mefford MJ (1999) PCORD. Multivariate analysis of ecological data, version 4.28. MjM Software. Gleneden Beach, Oregon
Mopper S (2005) Phenology-how the time creates spatial structure in endophagous insect population. Ann Zool Fenn 42:327–333
Moran VC, Southwood TRE (1982) The guild composition of arthropod communities in trees. J Anim Ecol 51:289–306
Murakami M, Yoshida K, Hara H, Toda MJ (2005) Spatio-temporal variation in lepidopteran larval assemblages associated with oak Quercus crispula: the importance of leaf quality. Ecol Entomol 30:521–531
Murakami M, Hirao T, Ichie T (2007) Comparison of lepidopteran larval communities among tree species in a temperate deciduous forest, Japan. Ecol Entomol 32:613–620
Nadkarni NM, Longino JT (1990) Invertebrates in canopy and ground organic matter in a neotropical montane forest, Costa Rica. Biotropica 22:286–289
Orians CM (2000) The effects of hybridization in plants on secondary chemistry: implications for the ecology and evolution of plant–herbivore interactions. Am J Bot 87:1749–1756
Osier TL, Lindroth RL (2001) Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J Chem Ecol 27:1289–1313
Palacios-Vargas JG, Castaño-Meneses G (2003) Seasonality and community composition of springtails in Mexican forest. In: Basset Y, Novotny V, Miller S, Kitching RL (eds) Arthropods of tropical forest. Spatio-temporal dynamics and resource use in the canopy. Chapman and Hall, London, pp 159–169
Palacios-Vargas JG, Castaño-Meneses G, Pescador A (1999) Phenology of canopy arthropods of a tropical deciduous forest in western Mexico. Pan-Pac Entomol 75:200–211
Palacios-Vargas JG, Iglesias R, Castaño-Meneses G (2003) Mites from Mexican oaks canopies. Insect Sci Appl 23:287–292
Paoletti MG, Taylor RAJ, Stinner BR, Stinner DH, Benzing DH (1991) Diversity of soil fauna in the canopy and forest floor of Venezuelan cloud forest. J Trop Ecol 7:373–383
Peeters PJ, Read J, Sanson GD (2001) Variation in the guild composition of herbivorous insect assemblages among co-occurring plant species. Aust Ecol 26:385–399
Price PW, Abrahamson WG, Hunter MD, Melika G (2004) Using gall wasps on oaks to test broad ecological concepts. Conserv Biol 18:1405–1416
Recher HF, Mayer JD, Ganesh S (1996) Eucalyptus, arthropods and birds: on the relationship between foliar nutrients and species richness. For Ecol Manag 85:177–195
Riipi M, Haukioja E, Lempa K, Ossipov V, Ossipova S, Pihlaja K (2004) Ranking of individual mountain birch trees in terms of leaf chemistry: seasonal and annual variation. Chemoecology 14:31–43
Romero S, Rojas E, Aguilar M (2002) El género Quercus (Fagaceae) en el Estado de México. Ann Misouri Bot Gard 89:551–593
Rzedowski J (1978) Vegetación de México. Limusa, México
Rzedowski J (1994) Vegetación del Pedregal de San Ángel, Distrito Federal México. In: Rojo A (ed) Reserva ecológica del pedregal de San Ángel: ecología, historia natural y manejo. UNAM, México, pp 9–65
Rzedowski J, Rzedowski GC (2001) Flora Fanerogámica del Valle de México. Instituto de Ecología, A. C., Centro Regional del Bajío. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Michoacán, México
Sarfraz M, Dosdall LM, Keddie BA (2008) Host plant genotype of the herbivore Plutella xylostela (Lepidopetera: Plutellidae) affects the performance of its parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae). Biol Control 44:42–51
Schowalter TD (1995) Canopy invertebrate community response to disturbance and consequences of herbivory intemperate and tropical forest. Selbyana 16:41–48
Schowalter TD, Zhang Y (2005) Canopy arthropod assemblages in four overstory and tree understory plant species in a mixed-conifer old growth forest in California. For Sci 51:233–242
Shuster SM, Lonsdorf EV, Wimp GM, Bailey JK, Whitham TG (2006) Community heritability measures the evolutionary consequences of indirect genetic effects on community structure. Evolution 60:991–1003
Siegismund HR, Jensen JS (2001) Intrapopulation and interpopulation genetic variation of Quercus in Denmark. Scand J For Res 16:103–116
Siemann E, Tilman D, Hassard J, Ritchie M (1998) Experimental tests of the dependence arthropod diversity on plant diversity. Am Nat 152:738–750
Soberón J, De la Maza R, Hernández A, Bonfil C, Careaga S (1991) Reporte Técnico final del primer año del proyecto “Restauración ecológica de lomas del seminario”. Centro de Ecología UNAM y Coordinación de reordenación urbana y protección ecológica, DF, México
Solow RA (1993) A simple test for change in community structure. J Anim Ecol 62:191–193
Southwood TRE (1978) The components of diversity. In: Mound LA, Waloff N (eds) Diversity of insects fauna. Blackwell, Oxford, London, pp 19–40
Southwood TRE, Moran VC, Kennedy CEJ (1982) The richness, abundance and biomass of the arthropod communities on trees. J Anim Ecol 51:635–649
Southwood TRE, Wint GRW, Kennedy CEJ, Greenwood SR (2004) Seasonality, abundance, species richness and specificity of the phytophagous guild of insects on oak (Quercus) canopies. Eur J Entomol 101:43–50
Southwood TRE, Wint GRW, Kennedy CEJ, Greenwood SR (2005) The composition of the arthropod fauna of the canopies of some species of oak (Quercus). Eur J Entomol 102:65–72
Statsoft INC (1998) STATISTICA for Windows. Tulsa, USA
Steinkellner H, Fluch S, Turetschek E, Lexer C, Streiff R, Kremer A, Burg K, Glössl J (1997) Identification and characterization of (GA/CT)n—microsatellite loci form Quercus petraea. Plant Mol Biol 33:1093–1096
Stork NE, Hammond PM (1997) Sampling arthropods from tree-crowns by fogging with knockdown insecticides: lessons from studies of oak tree beetle assemblages in Richmond Park. In: Stork NE, Adis J, Didham RK (eds) Canopy arthropods. Chapman and Hall, London, pp 3–26
Strong DR, Lawton JH, Southwood SR (1984) Insects on plants. Community patterns and mechanisms. Harvard University Press, Cambridge
Stuntz S, Simon U, Zotz G (2003) Arthropod seasonality in tree crowns with different epiphyte loads. In: Basset Y, Novotny V, Miller S, Kitching RL (eds) Arthropods of tropical forest. Spatio-temporal dynamics and resource use in the canopy. Chapman and Hall, London, pp 176–185
Summerville KS, Crist TO (2002) Effects of timber harvest on forest Lepidoptera: community, guild, and species responses. Ecol Appl 12:820–835
Summerville KS, Crist TO (2003) Determinants of lepidopteran species diversity and composition in eastern deciduos forest:roles of season, region and patch size. Oikos 100:134–148
Summerville KS, Boulware MJ, Veech JA, Crist TO (2003) Spatial variation in species diversity and composition of forest Lepidoptera in eastern deciduous forest of North America. Conserv Biol 17:1045–1057
Tassone RA, Majer JD (1997) Abundance of arthropods of tree canopies of Banksia woodland on the Swan Coastal Plain. J R Soc West Aus 80:281–286
Templeton AR, Routman E, Phillips CA (1995) Separating population structure from population history: a cladistic analysis of the geographical distribution of mitochondrial DNA haplotypes in the tiger salamander, Ambystoma tigrinum. Genetics 140:767–782
Thunes KH, Skartveit J, Gjerde I, Fennica E (2004) The arthropod community of Scots Pine (Pinus sylvestris L.) canopies in Norway. Entomol Fenn 15:65–90
Tovar-Sánchez E (2009) Canopy arthropods community within and among oak species in Central Mexico. Curr Zool 55:132–144
Tovar-Sánchez E, Oyama K (2004) Natural hybridization and hybrid zones between Quercus crassifolia and Quercus crassipes (Fagaceae) in Mexico: morphological and molecular evidence. Am J Bot 91:1352–1363
Tovar-Sánchez E, Oyama K (2006a) Community structure of canopy arthropods associated en Quercus crassifolia × Quercus crassipes complex. Oikos 112:370–381
Tovar-Sánchez E, Oyama K (2006b) Effect of hybridization of the Quercus crassifolia × Quercus crassipes complex on the community structure on endophagous insects. Oecologia 147:702–713
Tovar-Sánchez E, Cano-Santana Z, Oyama K (2003) Canopy arthropod on Mexican oaks at sites with different disturbance regimes. Biol Conserv 115:79–87
Vakkari P, Blom A, Rusanen M, Raiso J, Toivonen H (2006) Genetic variability of fragmented stands of pedunculate oak (Quercus robur) in Finalnd. Genetica 127:231–241
Valencia S (2004) Diversidad del género Quercus (Fagaceae) en México. Bol Soc Bot Mex 75:33–53
Vu QM, Nguyen TT (2000) Microarthropod community structures (Oribatei and Collembola) in Tam Dao National Park, Vietnam. J Biosci 25:379–386
Wagner T (2003) Seasonality of canopy beetles in Uganda. In: Basset Y, Novotny V, Miller S, Kitching RL (eds) Arthropods of tropical forest. Spatio-temporal dynamics and resource use in the canopy. Chapman and Hall, London, pp 146–158
Walker M, Hartley SE, Jones TH (2008) The relative importance of resources and natural enemies in determining herbivore abundance: thistles, tephritids and parasitoid. J Anim Ecol 77:1063–1071
Warwick RM, Clarke KR, Suharsono A (1990) A statistical analysis of coral community response to the 1982–1983 El Niño in the Thousand Island, Indonesia. Coral Reefs 8:171–179
White ES, Reyes CM, Ortega RJ, Valastro S (1990) El Ajusco: Geomorfología volcánica y acontecimientos glaciales durante el pleistoceno superior y comparación con las series glaciales mexicanas y de las montañas Rocallosas. INAH, México
Whitham TG, Martinsen GD, Floate KD, Dungey HS, Potts BM, Keym P (1999) Plant hybrid zones affect biodiversity: tools for a genetic based understanding of community structure. Ecology 80:416–428
Whitham TG, Young WP, Martinsen GD, Gebring CA et al (2003) Community and ecosystem genetics: consequence of the extended phenotype. Ecology 84:559–573
Whitham TG, Bailey JK, Scheweitzer JA, Shuster SM et al (2006) Community and ecosystem genetics: a framework for integrating from genes to ecosystems, Invited paper. Net Rev Gen 7:510–523
Wimp GM, Young PW, Woolbright SA, Martinsen GD, Keim P, Whitham TG (2004) Conserving plant genetic diversity for dependent animal communities. Ecol Lett 7:776–780
Wimp GM, Martinsen GD, Floate KD, Bangert RK, Whitham TG (2005) Conserving plant genetic diversity for dependent animal community structure and diversity. Evolution 59:61–69
Wimp GM, Wooley S, Bangert K, Young WP, Martinsen GD, Keim P, Rehill B, Lindroth RL, Whitham TG (2007) Plant genetics intra-annual variation in phytochemistry and arthropod community structure. Mol Ecol 16:5057–5069
Wolda H (1979) Abundance and diversity of Homoptera in the canopy of a tropical forest. Ecol Entomol 4:181–190
Yarnes CT, Boecklen WJ (2005) Abiotic factors promote plant heterogeneity and influence herbivore performance and mortality in Gambel’s oak (Quercus gambelii). Entomol Exp Appl 114:87–95
Zar JH (2010) Biostatistical analysis. Prentice-Hall, Inc., Upper Saddle River
Acknowledgments
We thank the specialists on arthropod and plant taxonomy for their interest and assistance in this project: Susana Valencia-Avalos (Oaks), Mauricio Mora-Jarvio (Oaks), Cristina Mayorga (Hemiptera), Guillermina Ortega León (Hemiptera), Blanca Mejía (Collembola and Acari), A. Godínez (Diptera), A. Ibarra Vázquez (Lepidoptera), Guadalupe Rangel Altamirano, Laura Márquez Valdelamar, and Maribel Paniagua for their technical assistance. This research was supported by grants from CONACYT-Mexico (61275) to E. T. S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Müller.
Rights and permissions
About this article
Cite this article
Tovar-Sánchez, E., Valencia-Cuevas, L., Castillo-Mendoza, E. et al. Association between individual genetic diversity of two oak host species and canopy arthropod community structure. Eur J Forest Res 132, 165–179 (2013). https://doi.org/10.1007/s10342-012-0665-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-012-0665-y