Abstract
The present study assessed the phytochemicals and biological activities of Rosa persica root extracts. The phytoconstituents of R. persica root were quantified by gas chromatography-mass spectrometry (GC-MS) and ultra-performance liquid chromatography-electrospray ionization-photodiode array detection-mass spectrometry (UPLC-ESI-PDA-MSn). Concentrations of total phenolic content (TPC), total flavonoid content (TFC), total proanthocyanidin content (TPAC), and antioxidant capacity were also measured in aqueous and ethanol (EtOH) extracts. Their bioactivities in terms of antiviral (against two cell lines), antimicrobial (against eight bacteria strains), antifungal (against two fungal strains), and cytotoxic (against eight cell lines) effects were assessed. The maximum effects were found in EtOH extract of R. persica root. The main components of the R. persica essential oil (RPEO) were methyl eugenol (73.1%) and geranyl acetone (10.3%). UPLC–ESI-PDA–MSn analysis showed that gallic acid (GA) was found in the highest concentration in the EtOH extract (461.80 ± 90.3 μg/g). In the antiviral assay, the half maximal inhibitory concentrations (IC50) of EtOH extract for Coxsackievirus B3 (CV-B3) and Coxsackievirus B4 (CV-B4) were 29.26 ± 0.5 and 24.70 ± 0.2 µg/mL, respectively. The cytotoxic concentrations (CC50) calculated for EtOH extract against both cell lines were significantly lower than aqueous extract (89.13 ± 0.3 and 75.03 ± 0.1 µg/mL, respectively). The most sensitive bacteria strain in both extracts was Staphylococcus aureus. Activity in fungal strains, Candida albicans was more sensitive than Aspergillus niger. The current cytotoxicity results between the two extracts of R. persica showed that both had the highest and lowest cytotoxic activity against human glioblastoma (U-87-MG) and human breast cancer (MDA-MB-231) cell lines, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iran is one of the top ten countries that introduced 1727 endemic species of 8000 species of plants. The genus Rosa belongs to the rose family with about 100 species (Korkmaz and Dogan 2018; Sadat-Hosseini et al. 2017). These species are commonly used in the food industry (juice, jam, and herbal tea) and traditionally to treat cold and flu, infectious, and inflammatory conditions (diabetes, arthritis, and rheumatism) (Tahirović and Bašić 2017). Rosa persica, known as ‘Varak,’ is native to Iran, Afghanistan, Central Asia, and north of western Siberia. With a distinctive red eye in the center of the yellow petals, R. persica is a dwarf perennial shrub (Basaki et al. 2009). The plant protection system against insects, microorganisms, and the sun’s rays are phenolic compounds present in thorny stems (Amini et al. 2016).
R. persica is widespread in 128 genetically diverse populations due to short-distance seed dispersal and eco-geographic microscale. This species often grows as a weed in fields as well as fuel. It grows best in dry regions on more alkaline soils (Basaki et al. 2009). The flowers, fruits, leaves, and roots of wild rose have been used in traditional medicine. People used the flowers for mild inflammation of the skin or mucous membranes in the mouth and throat. The fruits were used as herbal tea, syrup, and wine. Rose hips have been used primarily for immunosuppressive, anti-inflammatory, analgesic, anti-diabetic, cardioprotective, gastric protective, and skin ameliorative effects (Koczka et al. 2018). From the perspective of Iranian traditional medicine, R. persica exerts anti-inflammatory and antimicrobial properties and has been used to treat diarrhea (Ghavi and Mazandarani 2017). A study on traditional Iranian medicine showed the inhibitory effect of R. persica on the contraction of the ileum. In this regard, the pharmacodynamic studies also showed that hydroalcoholic and hexane extracts of R. persica inhibited the effects on the isolated ileum of rats in a dependent manner due to membrane depolarization, muscarinic receptor or neuronal stimulation, and suggesting broader mechanisms of action. When the myosin light chain is phosphorylated, the smooth muscle contractile machinery is stimulated. Initiation of the enzyme myosin light chain kinase (MLCK) caused the phosphorylation process. The interaction between intracellular Ca2+ ion and calmodulin activated the enzyme (MLCK). Thus, if the extract can affect the final cell contraction pathway, its inhibitory effect will be demonstrated. The next possibility is that several active substances with different mechanisms can produce the inhibitory effect of KCl with plant extract in a concentration-dependent manner. Modern medicine has also shown that R. persica extract is more effective compared to other varieties of roses and is also useful in gastrointestinal disorders accompanied by intestinal cramps or diarrhea (Sadraei et al. 2016). R. persica has been reported to show antioxidant activity, since it is considered to be a rich source of phenolic compounds, which have been determined by reverse-phase high-performance liquid chromatography (RP-HPLC) (YASA et al. 2009). Studies on R. persica essential oil (RPEO) revealed that aliphatic hydrocarbons (heptacosane and nanocosane) are the main components (Amini et al. 2016).
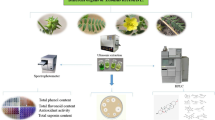
Determining the most efficient solvent for extraction and evaluating its biochemical and biological properties has always been difficult (Taghizadeh et al. 2018). The phenolic compounds with their free radical scavenging activities are known to have antioxidant potential by inducing antioxidant enzyme levels (Alcântara et al. 2019). Limited information is available to quantify individual bioactive compounds in R. persica. In general, total phenolic content (TPC) is often estimated by spectrophotometer measurements. However, the spectrophotometric assays have some limits such as the lack of separation process and accurate quantitative results of individual phenols. It is recommended to estimate the phenolic compounds with both the Folin–Ciocalteu and ultra-performance liquid chromatography-electrospray ionization-photodiode array detection-mass spectrometry (UPLC–ESI-PDA–MSn) methods. In fact, it is a powerful technique to achieve more knowledge on the metabolite profile of food plants and to link their phytochemicals with health benefits. The current study was conducted to define (I) the TPC, total flavonoid content (TFC), and total proanthocyanidin content (TPAC), (II) the antioxidant capacity, antiviral, antimicrobial, and cytotoxic effects, (III) the phytochemical profile of the RPEO by gas chromatography-mass spectrometry (GC-MS), and (IV) UPLC–ESI-PDA–MSn of the extracts of R. persica root (Fig. 1).
Materials and Methods
Reagents and Standards
Folin–Ciocalteu’s reagent (CAS: 7732-18-5), gallic acid (GA) (CAS: 149-91-7), quercetin (QE) (CAS: 117-39-5), potassium acetate (CAS: 127-08-2), aluminum (III) chloride (CAS: 7446-70-0), sodium bicarbonate (SB) (CAS: 144-55-8), and 2, 2’-diphenyl 1-picrylhydrazyl (DPPH) (CAS: 1898-66-4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Milli‑Q water (CAS: 7732-18-5) and acetonitrile (CAS: 75-05-8) (UPLC-MS grade) was purchased from Merck. Roswell Park Memorial Institute (RPMI), fetal bovine serum (FBS) (CAS: 9014-81-7), phosphate buffered saline (PBS) (CAS: 7647-14-5), trypsin-EDTA solution (170,000 U/L trypsin and 0.2 g/L EDTA) (CAS: 9002-07-7), and penicillin/streptomycin solution (10,000 U/mL penicillin and 10 mg/mL streptomycin) (CAS: 113-98-4) were obtained from Gibco. AlamarBlue® (resazurin) (CAS: 153796-08-8) was purchased from Sigma (Saint Louis, MO, USA). All the chemicals were of analytical grade.
Plant Materials
The roots of R. persica were collected in Shirvan, North Khorasan Province, Iran at 3732 N and 5754 E at 1093 meters above sea level. The average minimum and maximum temperatures of the corresponding zone were −1.1 and 25.8 °C, respectively, and the total precipitation was 279 mm (Mashhad Meteorological Organization, Iran).
Extraction Methods and Extract Preparation
The root of R. persica was washed with distilled water, air-dried at room temperature (18 °C; dark place) for 5 days, and then stored at 4 °C until use. Dried materials were grounded; 50 g of sample was extracted with ethanol (EtOH) (98% purity) and distilled water for 48 h at room temperature. The extract was filtered by using Whatman® No. 1. The solvents were evaporated under reduced pressure to afford several extracts; both extracts were then stored at −80 °C (Taghizadeh et al. 2019b).
Essential Oil (EO) Preparation
The dried roots were chopped and crushed in a mortar and pestle. Crushed roots were subjected to hydrodistillation using the Clevenger apparatus. Then, 100 g of the root was added to 800 ml of distilled water. It was placed in a balloon heater connected to a refrigerator for 3 h to ensure condensation of EO. At the end of EO extraction, both the aqueous phase and the EO phase were observed. The RPEO was dried over anhydrous sodium sulfate and stored in the sealed vials at −20 °C until use. Experiments were performed twice for each condition (Azadmanesh et al. 2021).
Extraction Yield (Content)
The extraction yield of the RPEO was calculated using Eq. 1:
Instrumentation Analysis Methods: GC-MS Analysis
The analyses were assessed by GC-MS on PAL RTC 120 sampler (Agilent 7890B series gas chromatograph and Agilent 5977A series MS spectrometer) and well-found with Wiley 7n.Llibrary. The specifications of GC-MS are given in Table 1 (Farhadi et al. 2020; Singh et al. 2022).
UPLC–ESI-PDA–MSn
MS analysis data was obtained from UPLC 1200 series (Agilent Tech—Germany) instrument using column; Gemini 3 mm C18 110 A° from phenomenex with dimensions 100 × 1 mm i. d., protected with RP C18 100 A° guard column with dimensions (5 mm × 300 mm i. d., 5 mm). Mobile phase were prepared of 2% acetic acid (A) and 90% Methanol (MeOH) (B) at flow rate of 0.5 mL/min. The sample injection volume was 10 µL, so that each sample was dissolved in 2% acetic acid and 5% MeOH. A Fourier transform ion cyclotron resonance MS analyzer used equipped with ESI system. In order to control the system, X‑calibur® software version 2.1 was used. Detection was performed in the negative ion mode by using a capillary voltage of 36 V in 275 °C. The API source voltage and the de-solvation temperature were regulated on 5 kV and 275 °C, respectively. Nitrogen gas with a flow of 15 mL/min was used as a nebulizing gas. The full MS scan included the MS range from 150 to 2000 m/z (Chakraborty et al. 2017; Handoussa et al. 2013).
Phytochemical Content
TPC
Briefly, 20 µL of the extract was mixed with 100 µL Folin–Ciocalteau reagent and diluted 10 times in distilled water. Then, 1.5 mL distilled water was added to the solution and stored at room temperature for 5 min. A total of 300 µL SB (20% w/v) was added and the mixture was incubated in a dark place for 2 h. The absorbance of each sample was read using a UV-visible spectrophotometer (Cecil, UK) at 725 nm. A calibration curve was constructed by standard solution of GA (0.2–1 mg/mL) (Trandafir and Cosmulescu 2020). Results were expressed as mg GA/g dried extract (DE) (mg GA/g DE) (Fattahi et al. 2021).
TFC
For TFC assay, 0.5 mL of DE was mixed with 0.1 mL aluminum chloride (10%), 1.5 mL EtOH (95%), 0.1 mL potassium acetate (1 M), and 2.8 mL distilled water. After incubating the extract at room temperature for 30 min, the absorbance was read at 415 nm. The sample contained 5 mL extract solution and 5 mL MeOH without AlCl3. The TFC was presented as milligram of QE per gram of DE (mg QE/g DE) (Naik and Al-Khayri 2020).
TPAC
Quantitative estimation of TPAC was assessed by the vanillin-HCl method. Then, 0.5 ml was mixed with 1.5 mL 4% vanillin methanol solutions and 0.75 mL M HCl. After 20 min incubation at 30 °C, the absorbance was read at 500 nm. Based on calibration curve, TPAC was reported as milligram of catechin/g DE (Boso et al. 2019).
In Vitro Antioxidant Assays
DPPH Assay
In order to determine radical scavenging ability, 50 µL of different concentrations of the extract was added to the same value of MeOH DPPH solution (0.1 mM). The mixture was incubated at 30 °C for 20 min while being shaken. The absorbance of the samples was read at 517 nm (Zor et al. 2022). The antioxidant activity was calculated as percent inhibition by following Eq. 2 (Kamble et al. 2020):
- BA:
-
Blank absorbance
- SA:
-
Sample absorbance
A curve of percentage of inhibition was plotted against the concentrations of samples; then, the half maximal inhibitory concentration (IC50) was determined. Butylated hydroxytoluene (BHT) was used as a positive control (Xu et al. 2018).
β-Carotene Bleaching (BCB) Assay
For this assay, 20 mg linoleic acid was mixed with 100 mg Tween 40 and 1 mL β-carotene solution (0.2 mg/mL in chloroform). After chloroform was evaporated at 50 °C, 100 mL distilled water was mixed and emulsified by sonication for 1 min (emulsion A). Following, 20 mg linoleic acid was mixed with Tween 40 (200 mg) and oxygenated water (50 mL) (emulsion B). Then, 200 μL of each concentration of DE and BHT (as positive control) were added with emulsion A (5 mL). Also, a control was provided by mixing 200 mL MeOH with 5 mL of emulsion A. A mixture of 200 μL MeOH and 5 mL of emulsion B was used for calibration. The absorbance was read at 470 nm before and after 120 min of incubation. The inhibition percentage was calculated using Eq. 3 (Esmaeilzadeh Kenari et al. 2014):
- CA:
-
Control absorbance
- SA:
-
Sample absorbance
Biological Assays
Antiviral Assay
Cell Culture
The antiviral activity was estimated on the CVB3 and CVB4 cells. The cells were incubated in eagle’s minimal essential medium supplemented with 10% heat inactivated fetal calf serum (FCS, Gibco BRL), 1% (2 mM) L‑Glutamine, 1% (50 µg/mL) streptomycin, 1% (50 IU/mL), penicillin, 1% non-essential amino acids and 1% (2.5 µg/mL) Fungizone (Amphoterin B, Apothecon). The virus titer was identified as TCID50 (the 50% tissue culture infection dose) on human epithelial cell line 2 (HEp-2) and stored at −80 °C (Gore and Desai 2014; Taghizadeh et al. 2021a).
Cell Viability Assay
Briefly, HEp‑2 cells were plated at a density of 104 cells/well and cells into 96 well plates in a humidified atmosphere at 37 °C in 5% CO2. After 30 h, the medium culture was removed, cells were washed with phosphate-buffered saline (PBS). Then, 100 µL of each extract were diluted in minimum essential medium (MEM) 2% FCS. Cells were injected with 50 µL of 2% MEM (including 100 TCID50 of CVB‑3 and CVB‑4). The probable cytopathic effect was controlled daily. The percentage of viability was determined using the Eq. 4 and the selectivity index (SI) was calculated through Eq. 5 (Taghizadeh et al. 2020):
- OD:
-
Optical density
- CC50:
-
The cytotoxic concentrations
- SI:
-
Selectivity index
- IC50:
-
Half maximal inhibitory concentrations
Antimicrobial Assay
Culture Media Preparation and Bacterial Strains
The antimicrobial effects of the extracts were assessed against four Gram-negative bacterial strains including Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa, and Serratia marcescens, as well as four Gram-positive including Staphylococcus aureus, Bacillus cereus, Micrococcus luteus, and Staphylococcus epidermidis. All the strains were seeded for 24 h at 37 °C on soybean casein digest agar (SCDA) and adjusted to 106 CFU/mL with sterile normal saline (NS) (0.9%) (Majiya and Galstyan 2020).
Minimum Bactericidal Concentration (MBC) and Minimum Inhibitory Concentration (MIC)
The extracts were melted in Muller Hinton broth (MHB) by dimethyl sulfoxide (DMSO). The concentration (10% v/v) was set by adding each extract (650 μL) to DMSO (320 μL) and MHB. The volume was finally adjusted to 6.5 mL. According to the serial dilution method, 200 μL of each level was inoculated in each cell culture plate. Then, 20 μL of bacterial suspension (106 CFU/ml) was added. The tests were also inoculated separately in the culture media as positive control (gentamicin and vancomycin). Moreover, MHB was used as negative control. The samples were kept at 37 °C for 24 h, then the bacterial growth of each well was measured by adding 20 µL of 2, 3, 5-triphenyltetrazolium chloride (TTC) (5 mg/mL) as a colorimetric indicator. They were incubated again at 37 °C for 1 h. The MIC value was calculated based on the lowest concentration of the extract inhibiting the main color of the culture media to red. The MBC value was also identified by content of wells not yielding any red color (24 h, 37 °C). All experiments were done in triplicate (Aziman et al. 2014).
Antifungal Assay
The antifungal assay was examined against Candida albicans and Aspergillus niger. After culture on SCDA (48 h, 25 °C), sterile 0.9% NS was used to prepare a suspension of 106 CFU/mL. The volume of extracts was adjusted to 1 mL by Sabouraud’s dextrose broth (SDB). The other concentrations of extracts were made with serial dilution technique. A total of 200 μL of cell suspension was incubated (48 h, 25 °C), and then the MIC and the minimum fungicidal concentration (MFC) values were also considered. The SDB (positive) and nystatin (negative) were used as controls (Taghizadeh et al. 2019a).
Cytotoxic Assay
Determination of Cell Viability
MCF‑7 (human breast cancer), DU-145 (human prostate cancer), PC3 (human prostate cancer), A2780 (ovarian carcinoma), C‑26 (colorectal cancer), U‑87-MG (human glioblastoma), MDA-MB-231 (human breast cancer), Hela, and NIH 3T3 (mouse embryonic fibroblast) were purchased from the Pasteur Institute, Tehran, Iran. These cells were preserved by 10% (v/v) FBS (Gibco, BRL), penicillin/streptomycin (100 IU/mL), and L‑glutamine (2 mM). Cultures were incubated at 37 °C under CO2/air (5%/95%) (Shakeri et al. 2019).
Measurement of Cytotoxic Activity of the Plant Extracts by AlamarBlue®
Cytotoxicity was assessed using AlamarBlue® proliferation assay. Cells were plated on 96-well microplates at a density of 1 × 104. After 24 h, the cells were treated with the different concentrations of extracts (50–400 μg/mL). After 48 h treatment, the cells were treated with 20 μL of AlamarBlue® reagent. After 2–4 h the absorbance was measured at 600 nm. Doxorubicin (Dox) was used as a positive control (Taghizadeh et al. 2021b).
Statistical Analysis
Statistical analysis was presented as mean ± SD. One way ANOVA and LSD test were used to determine statistical differences among groups by JMP 8 (SAS Campus Drive, Cary) (P <0.05 and n = 3).
Results
RPEO Yield and Components
Based on our findings, the ethanol and aqueous extracts were 97.11 and 79.85%, respectively (Table 4). In RPEO, the major components were methyl eugenol (73.1%) and geranyl acetone (10.3%). GC-MS analysis of RPEO chemical composition exposed 35 components in RPEO accounting for 98.4% (Table 2).
UPLC–ESI-PDA–MSn Analysis of Metabolic Extracts
A total of 32 compounds were identified in R. persica root extracts, as shown in Table 3. Based on the deprotonated molecular ions, the concentration of phenolic acids was significant differed between the solvents. The compounds GA (461.80 ± 90.30 μg/g), chlorogenic acid (302.50 ± 21.23 μg/g), and quinic acid (250.11 ± 61.70 μg/g) were found in highest concentrations in EtOH extract (Table 3).
Phytochemical Content and Antioxidant Activity
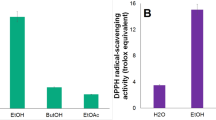
Table 4 revealed that the use of EtOH resulted in extraction of higher TPC, TFC, and TPAC from R. persica root (Table 4). The content of TPC varied from 53.05 ± 1.7 mg GA/g DE in aqueous extract to 98.73 ± 2.5 mg GA/g DE in EtOH extracts. The EtOH extracts of R. persica root had the highest contents of TFC (85.33 ± 2.2 mg of QE/g DE) and TPAC (90.05 ± 2.3 mg of catechin/g DE). As can be seen, among the R. persica root extracts in the DPPH assay, the EtOH extract exhibited the IC50 value of 19.22 ± 0.8 µg/mL, which is weaker than BHT (IC50 = 6.41 ± 0.3 µg/mL). Results of the BCB assay were also expressed as IC50. The IC50 values of R. persica root extracts in the BCB assay were in the range 46.02 ± 1.2 µg/mL to 93.44 ± 2.1 µg/mL. The EtOH extract showed the highest percentage of BCB inhibition. It was interesting to note that a high correlation was detected between phenolic compounds and antioxidant activities in both antioxidant assays (Table 4).
Biological Effects
Antiviral Effects
The antiviral activity of R. persica root extracts was evaluated against Coxsackievirus B3 (CV-B3) and Coxsackievirus B4 (CV-B4). To evaluate the potential use of R. persica root extracts, we tested the inhibition of virus-induced pathogenicity on HEp‑2 cells. The mean IC50, CC50, and SI values for each extract are shown in Table 5. The IC50 values of R. persica EtOH extracts for CV-B3 and CV-B4 were 29.26 ± 0.5 and 24.70 ± 0.2 µg/ml, respectively. Higher IC50 values indicate the lowest percentage of inhibition. The aqueous extracts were less potent (106.05 ± 0.3 and 86.10 ± 0.2 µg/mL). Based on our results, CC50 values calculated for EtOH extract against both CV-B3 and CV-B4 cell lines were significantly less than these values of the aqueous extract (89.13 ± 0.3 and 75.03 ± 0.1 µg/mL, respectively). The SI of various extracts for both cell lines were above 3.
Antimicrobial Effects
As shown in Table 6, S. aureus was the most sensitive strain in both extracts. The aqueous and EtOH extracts had different MIC values against S. aureus (20.20 and 7.00 µg/mL, respectively). Moreover, the MBC values against relevant bacteria were reported in aqueous and EtOH extracts (25.90 and 10.00 µg/mL, respectively). Moreover, in fungal strains, C. albicans was more sensitive than A. niger. The MIC and MFC values against C. albicans for aqueous and EtOH extracts were 55.20 and 67.00 µg/mL, as well as 22.00 and 29.00 µg/mL, respectively. Furthermore, the MIC and MFC values for aqueous extract was 81.10 and 90.22 µg/mL against A. niger, and these values were 43.00 and 58.00 µg/mL, respectively against A. niger with respect to EtOH extract. Both samples presented antifungal and antibacterial efficacy at significantly different levels (Table 6).
Cytotoxicity Effects
After 48 h of treatment, we found that both extracts had the highest cytotoxic activity against U‑87-MG. The IC50 values were determined for aqueous and EtOH extracts, against U‑87-MG were 51.22 ± 3.41 and 10.10 ± 1.02 µg/mL, respectively. The aqueous and EtOH extracts showed the lowest cytotoxic activity against MDA-MB-231cell (IC50 = 100.00 ± 4.85 and 80.55 ± 3.23 µg/mL, respectively) (Table 7).
Discussion
The current study evaluated the phytochemicals (e.g., TPC, TFC, TPAC, and phenolic acids), antioxidant capacity, and biological activities of R. persica root extracts. Our results showed that the EtOH extract of R. persica had significantly different phytochemicals and biological effects. This led us to realize that polar protic solvents such as EtOH are the most efficient. Based on the current results, there were significant differences between water and EtOH used for extraction in terms of TPC, TFC, and TPAC. These results agreed with those reported by Yu et al. (Yu et al. 2005). In another experiment performed on Pistacia vera, the EtOH extract was more efficient in terms of phenolic compound extraction and antioxidant capacity (Taghizadeh et al. 2018). Our results were also consistent with a previous study showing that EtOH extract had a higher concentration of TFC than aqueous extract (Wang and Helliwell 2001). Li et al. expressed that TPC was up to 10-fold higher in polar extract than non-polar extract (Li et al. 2006). Among the secondary metabolites present in R. persica, phenolic acids are considered to be the main group associated with numerous pharmacological properties including antioxidant, antiviral, antimicrobial, and cytotoxic activities. It is evident that phenolic compounds are responsible for antioxidant capacity. Several studies indicate that there is a positive correlation between TPC, TFC, TPAC, and antioxidant capacity of various extracts (Llorent-Martínez et al. 2017; Rocchetti et al. 2019; Sarikurkcu et al. 2019). This correlation is due to the presence of hydrogen donor bands in phenolic compounds (Messi et al. 2016). In addition, the differences in the main components have been related to different raw materials, the type of solvent, and the extraction methods in relevant studies. The major component in the UPLC–ESI-PDA–MSn analysis of R. persica root extracts was GA, which is consistent with the results reported by Ambigaipalan et al. (Ambigaipalan et al. 2017). Besides, another study showed that different geographic conditions significantly affect the concentration of polyphenolic compounds in some Rosa species (Koczka et al. 2018). Based on the DPPH assay, the R. persica root extract was shown to have potential antioxidant capacity and moderately scavenged DPPH radical compared to the positive control. The associated evidence showed that the phenolic compounds of R. persica were remarkably higher than those of R. pimpinellifolia (Mavi et al. 2004).
Moreover, 35 compounds, representing 98.4% of RPEO, were identified in the present study. The main components were methyl eugenol (73.1%) and geranyl acetone (10.3%), respectively. R. persica is a rich source of valuable components and bioactive compounds such as heptacosane, isobutyl phthalate, nonacosane, dibutyl phthalate, pentacosane, hexadecanoic acid, linalool, ethyl linoleolate, hexyl hexoate, and octacosane (Amini et al. 2016).
Our results indicated that EtOH was better than water for extraction of the antiviral compounds from R. persica root extracts. This study supports the previous findings that natural compounds with multiple inhibitory activities may provide effective therapeutic approaches to treating several diseases. Various TFCs including apigenin, QE, kaempferol, genistein, and galangin showed antiviral activity against HSV‑1 and HSV‑2 (Kumar and Pandey 2013). It was also demonstrated that flavones and flavonols have a synergistic effect against some viruses. It is believed that the main mechanism of antiviral activity of secondary compounds is mediated by the inhibition of enzymes involved in the virus life cycle (Čulenová et al. 2019).
The present study showed that the most sensitive bacteria strain in both extracts was S. aureus. Moreover, in fungal strains, C. albicans was more sensitive than A. niger. In the previous study, the antimicrobial activity among wild British Columbia roses including R. woodsii, R. nutkana, and R. pisocarpa was investigated. These three different roses exhibited antimicrobial activity (Yi et al. 2007). Another study showed that the petals of R. canina exhibited an antimicrobial effect against S. aureus (Shiota et al. 2004).
The current cytotoxicity results between the two extracts of R. persica showed that both extracts had the highest and the lowest cytotoxic activity against U‑87-MG and MDA-MB-231cell lines, respectively. Several studies reported that the TPC and TFC of Rosa species can inhibit hepatotoxicity (Liu et al. 2011; Tao et al. 2016; Zhang et al. 2013). Jassbi et al. found that the hydroalcoholic extract of R. persica reduced the oxidative stress and balanced between intracellular antioxidants and free radicals (Jassbi et al. 2003). A study assessing the aerial parts extract of R. persica against cadmium-hepatotoxicity found that the serum hepatic enzyme levels decreased and oxidative hepatic damage improved with R. persica hydroalcoholic extract (Moradkhani et al. 2020). The inhibitory effects of citronellol (0.8–6.4 µg/mL) and geraniol (0.2–3.2 µg/mL) isolated from R. damascene EO was observed on rat ileum contraction (Sadraei et al. 2013).
Conclusion
In this study, UPLC–ESI-PDA–MSn and GC-MS profiling, phytochemical, and biological activities of R. persica root extracts were assessed. In RPEO, the major components were methyl eugenol (73.1%) and geranyl acetone (10.3%). GC-MS analysis of RPEO chemical composition exposed 35 components in RPEO accounting for 98.4%. Moreover, a total of 32 compounds were identified in R. persica root extracts. The phenolic acids, GA, chlorogenic acid, and quinic acid occurred in high amounts in EtOH extract. The EtOH extract was found to possess remarkable antioxidant activity in the DPPH and BCB assays. The EtOH extract isolated from R. persica root presented considerable effects against biofilm-related infections, CV-B3 and CV-B4, and several cell lines. In terms of antimicrobial and antifungal results, the most sensitive strains were S. aureus and C. albicans, respectively. As EtOH extract presented considerable effects against biofilm-related infections, and several cell lines, it might be employed as an alternative/complementary treatment after further preclinical and clinical studies. To optimize pharmaceutical applications, it is necessary to identify the EtOH extract in vivo assay. Meanwhile, ethanol was an efficient solvent for extraction of polyphenol compounds from R. persica root, which is safe for human consumption.
References
Alcântara MA et al (2019) Effect of the solvent composition on the profile of phenolic compounds extracted from chia seeds. Food Chem 275:489–496
Ambigaipalan P, de Camargo AC, Shahidi F (2017) Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem 221:1883–1894
Amini M, Rineh MK, Yazdani M (2016) Study of the chemical compositions of Iranian rose flower essence oil (Rosa persica). Bulg Chem Commun 48:27–29
Azadmanesh R, Tatari M, Asgharzade A, Taghizadeh SF, Shakeri A (2021) GC/MS profiling and biological traits of eucalyptus globulus L. essential oil exposed to solid lipid nanoparticle (SLN). J Essent Oil Bear Plants 24:863–878
Aziman N, Abdullah N, Noor ZM, Kamarudin WSSW, Zulkifli KS (2014) Phytochemical profiles and antimicrobial activity of aromatic Malaysian herb extracts against food-borne pathogenic and food spoilage microorganisms. J Food Science 79:M583–M592
Basaki T et al (2009) Assessing Rosa persica genetic diversity using amplified fragment length polymorphisms analysis. Sci Hortic 120:538–543
Boso S, Gago P, Santiago JL, Álvarez-Acero I, Martínez MC (2019) Concentration of flavanols in red and white winemaking wastes (grape skins, seeds and bunch stems), musts, and final wines. Erwerbs-Obstbau 61:75–84
Chakraborty N, Chandra S, Acharya K (2017) Biochemical basis of improvement of defense in tomato plant against Fusarium wilt by CaCl2. Physiol Mol Biol Plants 23:581–596
Čulenová M et al (2019) Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. J Ethnopharmacol 248:112296
Esmaeilzadeh Kenari R, Mohsenzadeh F, Amiri ZR (2014) Antioxidant activity and total phenolic compounds of Dezful sesame cake extracts obtained by classical and ultrasound-assisted extraction methods. Food Sci Nutr 2:426–435
Farhadi F, Iranshahi M, Taghizadeh SF, Asili J (2020) Volatile sulfur compounds: The possible metabolite pattern to identify the sources and types of asafoetida by headspace GC/MS analysis. Ind Crops Prod 155:112827
Fattahi A et al (2021) UPLC–PDA-ESI–QTOF–MS/MS and GC-MS analysis of Iranian Dracocephalum moldavica L. Food Sci Nutr 9(8):4278–4286
Ghavi ABA, Mazandarani M (2017) Aut ecology and phytochemical survey of ziziphora clinopodioides lam. With ethnopharmacology and floristic spectrum of medicinal plants in bovanlou region (northern khorasan province)
Gore M, Desai N (2014) Characterization of phytochemicals and evaluation of anti-cancer potential of Blumea eriantha DC. Physiol Mol Biol Plants 20:475–486
Handoussa H et al (2013) Anti-inflammatory and cytotoxic activities of dietary phenolics isolated from Corchorus olitorius and Vitis vinifera. J Funct Foods 5:1204–1216
Jassbi A, Zamanizadehnajarib S, Tahara S (2003) Polyphenolic antioxidant constituents of Rosa persica. J Chem Soc Pak 25:323–327
Kamble V, Attar U, Umdale S, Nimbalkar M, Ghane S, Gaikwad N (2020) Phytochemical analysis, antioxidant activities and optimized extraction of embelin from different genotypes of Embelia ribes Burm f.: a woody medicinal climber from Western Ghats of India. Physiol Mol Biol Plants 26:1855–1865
Koczka N, Stefanovits-Bányai É, Ombódi A (2018) Total polyphenol content and antioxidant capacity of rosehips of some rosa species. Medicines 5:84
Korkmaz M, Dogan NY (2018) Analysis of genetic relationships between wild roses (Rosa L. Spp.) growing in Turkey. Erwerbs-Obstbau 60:305–310
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:1–16
Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S (2006) Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 96:254–260
Liu Y, Lu B, Peng J (2011) Hepatoprotective activity of the total flavonoids from Rosa laevigata Michx fruit in mice treated by paracetamol. Food Chem 125:719–725
Llorent-Martínez E et al (2017) Evaluation of antioxidant potential, enzyme inhibition activity and phenolic profile of Lathyrus cicera and Lathyrus digitatus: Potential sources of bioactive compounds for the food industry. Food Chem Toxicol 107:609–619
Majiya H, Galstyan A (2020) Dye extract of calyces of Hibiscus sabdariffa has photodynamic antibacterial activity: A prospect for sunlight-driven fresh produce sanitation. Food Sci Nutr 8:3200–3211
Mavi A, Terzi Z, Özgen U, Yildirim A, Coşkun M (2004) Antioxidant properties of some medicinal plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), malva neglecta (malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), urtica dioica (urticaceae). Biol Pharm Bull 27:702–705
Messi AN et al (2016) Phenolic compounds from the roots of Ochna schweinfurthiana and their antioxidant and antiplasmodial activities. Phytochem Lett 17:119–125
Moradkhani S, Rezaei-Dehghanzadeh T, Nili-Ahmadabadi A (2020) Rosa persica hydroalcoholic extract improves cadmium-hepatotoxicity by modulating oxidative damage and tumor necrosis factor-alpha status. Environ Sci Pollut Res Int 27:31259–31268. https://doi.org/10.1007/s11356-020-09450-4
Naik PM, Al-Khayri JM (2020) Influence of culture parameters on phenolics, flavonoids and antioxidant activity in cell culture extracts of date palm (Phoenix dactylifera L.). Erwerbs-Obstbau 62:181–188
Rocchetti G, Senizza B, Zengin G, Senkardes I, Sadeer NB, Mahomoodally MF, Lucini L (2019) Metabolomics-based profiling with chemometric approach to delineate the bio-pharmaceutical properties of fruit extracts from Ligustrum vulgare L. Ind Crops Prod 140:111635
Sadat-Hosseini M, Farajpour M, Boroomand N, Solaimani-Sardou F (2017) Ethnopharmacological studies of indigenous medicinal plants in the south of Kerman, Iran. J Ethnopharmacol 199:194–204
Sadraei H, Asghari G, Emami S (2013) Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Res Pharma Sci 8:17
Sadraei H, Asghari G, Jalali F (2016) Assessment of hydroalcoholic and hexane extracts of Rosa persica Mich. flower on rat ileum spasm. Res Pharma Sci 11:160
Sarikurkcu C, Ozer MS, Tlili N (2019) LC–ESI–MS/MS characterization of phytochemical and enzyme inhibitory effects of different solvent extract of Symphytum anatolicum. Ind Crops Prod 140:111666
Shakeri A et al (2019) LC-ESI/LTQOrbitrap/MS/MS and GC–MS profiling of Stachys parviflora L. and evaluation of its biological activities. J Pharm Biomed Anal 168:209–216
Shiota S, Shimizu M, Sugiyama J, Morita Y, Mizushima T, Tsuchiya T (2004) Mechanisms of action of corilagin and tellimagrandin I that remarkably potentiate the activity of beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol Immunol 48:67–73. https://doi.org/10.1111/j.1348-0421.2004.tb03489.x
Singh C, Upadhyay R, Tiwari KN (2022) Comparative analysis of the seasonal influence on polyphenolic content, antioxidant capacity, identification of bioactive constituents and hepatoprotective biomarkers by in silico docking analysis in Premna integrifolia L. Physiol Mol Biol Plants 28(1):223–249
Taghizadeh SF, Rezaee R, Davarynejad G, Karimi G, Nemati SH, Asili J (2018) Phenolic profile and antioxidant activity of Pistacia vera var. Sarakhs hull and kernel extracts: the influence of different solvents. J Food Meas Charact 12:2138–2144
Taghizadeh SF, Rezaee R, Badiebostan H, Giesy JP, Karimi G (2019a) Occurrence of mycotoxins in rice consumed by Iranians: a probabilistic assessment of risk to health. Food Addit Contam Part A 37(2):342–354
Taghizadeh SF, Rezaee R, Mehmandoust M, Madarshahi FS, Tsatsakis A, Karimi G (2019b) Coronatine elicitation alters chemical composition and biological properties of cumin seed essential oil. Microb Pathog 130:253–258
Taghizadeh SF, Rezaee R, Mehmandoust M, Badibostan H, Karimi G (2020) Assessment of in vitro bioactivities of Pis v 1 (2S albumin) and Pis v 2.0101 (11S globulin) proteins derived from pistachio (Pistacia vera L.). J Food Meas Charact 14:1054–1063
Taghizadeh SF, Azizi M, Asili J, Madarshahi FS, Rakhshandeh H, Fujii Y (2021a) Therapeutic peptides of Mucuna pruriens L.: Anti-genotoxic molecules against human hepatocellular carcinoma and hepatitis C virus. Food Sci Nutr 9(6):2908–2914
Taghizadeh SF, Azizi M, Rezaee R, Madarshahi FS, Mehmandoust M, Karimi G, Asili J (2021b) Cytotoxic activity of cis-(E)-and trans-(Z)-spiroethers isolated from various Arnebia species. S Afr J Bot 142:114–123
Tahirović A, Bašić N (2017) Determination of phenolic content and antioxidant activity of Rosa canina L. fruits in different extraction systems. Work Fac For Univ Sarajevo 1:47–59
Tao X et al (2016) Total flavonoids from Rosa laevigata Michx fruit ameliorates hepatic ischemia/reperfusion injury through inhibition of oxidative stress and inflammation in rats. Nutrients 8:418
Trandafir I, Cosmulescu S (2020) Total phenolic content, antioxidant capacity and individual phenolic compounds of defatted kernel from different cultivars of walnut. Erwerbs-Obstbau 62:309–314
Wang H, Helliwell K (2001) Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res Int 34:223–227
Xu X, Guo S, Hao X, Ma H, Bai Y, Huang Y (2018) Improving antioxidant and antiproliferative activities of colla corii asini hydrolysates using ginkgo biloba extracts. Food Sci Nutr 6:765–772
Yasa N, Masoumi F, Rouhani RS, Haji AA (2009) Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan
Yi O, Jovel EM, Towers GH, Wahbe TR, Cho D (2007) Antioxidant and antimicrobial activities of native Rosa sp. from British Columbia, Canada. Int J Food Sci Nutr 58:178–189. https://doi.org/10.1080/09637480601121318
Yu J, Ahmedna M, Goktepe I (2005) Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem 90:199–206
Zhang S et al (2013) Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol 55:60–69
Zor M, Şengül M, Topdaş EF, Yılmaz B (2022) Physicochemical properties, antioxidant activities, and chemical compositions of extracts obtained from Rosa pimpinellifolia L. flesh and seeds using different methods and solvents. Erwerbs-Obstbau. https://doi.org/10.1007/s10341-022-00676-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Koohestanian, M. Tatari, M. Samadi Kazemi, A. Asgharzade, and S.F. Taghizadeh declare that they have no competing interests.
Rights and permissions
Springer Nature oder sein Lizenzgeber hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Koohestanian, A., Tatari, M., Samadi Kazemi, M. et al. Phytochemicals, Antioxidant Activity, and Biological Activities of Rosa persica Root. Erwerbs-Obstbau 65, 1127–1138 (2023). https://doi.org/10.1007/s10341-022-00740-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-022-00740-4