Abstract
Diamide insecticides were first commercialised in 2006 by the launch of the benzenedicarboxamide derivative flubendiamide, followed by the anthranilic diamides chlorantraniliprole and cyantraniliprole. They are particularly active against a number of destructive lepidopteran pests and selectively activate insect ryanodine receptors (RyR), which are large tetrameric ryanodine-sensitive calcium release channels located in the sarco- and endoplasmic reticulum in neuromuscular tissues. Within a few years on the market, this class of insecticide chemistry gained blockbuster status by accounting for more than $1.2 billion of the 2013 global insecticide sales. On the downside, selection pressure on high-risk pests increased due to the frequent use of diamides, and high levels of field resistance to these insecticides have recently been reported in lepidopteran pests, such as diamondback moth, Plutella xylostella, and tomato leaf miner, Tuta absoluta. Here we briefly summarise cases of diamide insecticide resistance by analysing the underlying mechanisms of resistance compromising diamide efficacy in both laboratory- and field-selected strains of a number of lepidopteran pests. By far one of the most intensely investigated species, with respect to the underlying molecular mechanisms of diamide insecticide resistance, is diamondback moth. One of the major mechanisms of resistance including its underlying genetics yet identified is based on target-site mutations located in the transmembrane domain of the insect RyR. Possible fitness costs and metabolic mechanisms of resistance based on elevated levels of detoxification enzymes are not well studied yet. Finally we briefly discuss the general implications of the mechanistic findings gathered in several studies for the implementation of diamide resistance management programmes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 General
The discovery, development and registration of novel chemical classes of insecticides with new modes of action, i.e. addressing a yet unexploited/underutilised target protein, or at least interfering with a new binding site on an established insecticide target, are major challenges in modern crop protection research. A challenge, which is – after consolidation of the agrochemical industry – pursued by a rather limited number of R&D based companies, particularly because of high budget needs for insecticide development and registration, often easily exceeding $200 million (Sparks 2013). Major drivers for the discovery and development of new chemical classes of insecticides are an increasing requirement for compounds with improved environmental and toxicological profiles, as well as the global spread of pest resistance compromising field efficacy of established insecticides and thus directly influencing yield and food supply. A recent survey revealed that in 2013 approximately 70 % of the global insecticide market was based on 5 out of about 55 different chemical classes listed in the insecticide mode of action classification scheme of the Insecticide Resistance Action Committee (IRAC), including neonicotinoids acting on nicotinic acetylcholine receptors (27 % market share), pyrethroids acting on voltage-gated sodium channels (16 %), organophosphates inhibiting acetylcholinesterase (11 %), diamides acting on ryanodine receptors (8 %) and avermectins acting on ligand-gated chloride channels (7 %) (Sparks and Nauen 2015). Out of these chemical classes, diamide insecticides represent the most recent class of chemistry introduced to the market approximately 10 years ago (Nauen 2006; Jeanguenat 2013).
12.1.1 Diamide Insecticides
Three diamide insecticides, i.e. the benzenedicarboxamide (or phthalic diamide), flubendiamide (Tohnishi et al. 2005; Hirooka et al. 2007; Hamaguchi and Hirooka 2012) and anthranilic diamides chlorantraniliprole and cyantraniliprole (Lahm etal. 2005, 2007, 2009), have so far been commercialised with a global turnover of >$1.2 billion representing approx. 8 % of the insecticide market in 2013 (Sparks and Nauen 2015). However, at least three more diamide insecticides, i.e. cyclaniliprole, tetrachlorantraniliprole and tetraniliprole, are currently under development and expected to be launched to the market within the next few years (Fig. 12.1), whilst other, more recently described chemical derivatives such as diamide sulfoximines have not yet revealed development candidates (Gnamm et al. 2012). The discovery and development of diamide insecticides has been recently reviewed by Jeanguenat (2013). Whereas flubendiamide and chlorantraniliprole are particularly active at low application rates against a broad range of lepidopteran and lepidopteran/coleopteran pests, respectively, cyantraniliprole – due to its systemic properties – also targets a number of sucking pests including aphids and whiteflies (Foster et al. 2012; Li et al. 2012; Gravalos et al. 2015). However, chlorantraniliprole also exhibits root-systemic properties and can therefore be used by systemic application but mainly against foliar-feeding lepidopteran pests (Cameron et al. 2015). Diamide insecticides show low acute mammalian toxicity and a favourable environmental profile and are safe to beneficial insects and mites in many agricultural and horticultural settings investigated. When introduced to the market, diamides did not show any cross-resistance to existing chemical classes, as one would expect for a new chemical class of insecticides addressing a new binding site (mode of action) on a rather neglected molecular target, the insect ryanodine receptor (RyR). However, diamides are used to control a number of lepidopteran pests known to rapidly evolve resistance, including diamondback moth (Plutella xylostella) ranking number 2 among the globally most resistant arthropod pest species (Sparks and Nauen 2015).
Diamide insecticides acting as conformation-sensitive activators on insect ryanodine receptors. Flubendiamide (Nihon Nohyaku/Bayer), chlorantraniliprole and cyantraniliprole (DuPont) were launched in 2006, 2007 and 2012, respectively. Tetrachlorantraniliprole (Sinochem), cyclaniliprole (Ishihara) and tetraniliprole (Bayer) (ISO-proposed common names) are currently under development
12.1.2 Ryanodine Receptors and Diamide Mode of Action
Diamide insecticides were shown to act as conformation-sensitive activators of the insect ryanodine receptor (RyR), a large (homo)tetrameric calcium-channel located in the sarco- and endoplasmic reticulum in neuromuscular tissues (Ebbinghaus-Kintscher et al. 2006; Cordova et al. 2006, 2007; Lümmen et al. 2007; Sattelle etal. 2008). RyRs are endogenously activated by calcium influx, mediated by voltage-gated calcium channels upon depolarization of the cell membrane (Lümmen 2013). By addressing a new binding site of the RyR, diamides cause a calcium-dependent calcium release resulting in the depletion of internal calcium stores which leads to uncontrolled muscle contraction, paralysis and eventually death as shown in lepidopteran larvae (Tohnishi et al. 2005; Cordova et al. 2006). Due to their new biochemical mode of action (MoA), diamide insecticides were classified by IRAC as ryanodine receptor modulators and assigned to a new main MoA group 28 (Nauen 2006). Whereas mammals possess three RyR isoforms localised in different tissues (Rossi and Sorrentino 2002), insects encode a single RyR gene with an open reading frame of >15,000 nucleotides translated into a protomer with a molecular weight of more than 5,000 kDa, as first described for Drosophila melanogaster (Takeshima et al. 1994). These protomers assemble to homotetrameric membrane proteins of >2 MDa forming the largest known ion channels (Hamilton 2005). RyRs were shown to be composed of six helical transmembrane spanning domains at the C-terminal end containing the calcium ion-conducting pore and a large N-terminal cytosolic domain (Lümmen 2013). A mammalian RyR1 structure determined by single-particle electron cryomicroscopy was recently published and provided interesting insights regarding its structural features as it resolves in total 70 % of 2.2 MDa molecular mass homotetrameric channel protein (Yan et al. 2015).
The RyR as an insecticide target-site has been utilised for decades and is named after the alkaloid insecticide ryanodine isolated from the South American plant species Ryania speciosa, known for its insecticidal properties for almost 200 years (Pepper and Carruth 1945; Rogers et al. 1948). A major problem of using ryanodine as an insecticide is its toxicity to both insects and mammals due to a lack of selective binding to RyRs (Lehmberg and Casida 1994); however, the synthesis of more selective and potent derivatives largely failed for various reasons (Waterhouse et al. 1987). The insecticidal properties of ryanodine were, however, rather limited under field conditions. Earlier work on both natural Ryania alkaloids and their semi-synthetic derivatives in order to increase their efficacy – including extensive structure activity relationship studies – failed to exploit this target to produce economically relevant insecticides (Jefferies et al. 1997, and references cited therein). Despite its limitations as an insecticide, ryanodine became a unique tool in the characterisation of RyRs owing to its binding specificity and high affinity for insect and mammalian receptors (K D 5–15 nM). However, diamide insecticides address a different binding site on insect RyRs and act as positive allosteric activators as demonstrated by the increase of [3H]ryanodine binding as a function of diamide concentration with an EC50 value in the nanomolar range to both insect thoracic microsomal membrane preparations as well as functionally expressed RyRs in insect cell lines (Ebbinghaus-Kintscher et al. 2006; Lümmen et al. 2007; Qi and Casida 2013; Steinbach et al. 2015; Troczka et al. 2015). Whereas diamides do virtually not bind to mammalian RyR isoforms (Ebbinghaus-Kintscher et al. 2006; Lahm et al. 2007), they show some species differences in terms of selectivity among insects of different orders (Qi and Casida 2013; Qi et al. 2014). When utilising a photoreactive derivative of flubendiamide against a series of Bombyx mori RyR deletion mutants recombinantly expressed in HEK293 cells, Kato et al. (2009) concluded that the diamide binding site is likely to be located in the C-terminal transmembrane spanning domain, which was confirmed by studies on diamide-resistant diamondback moth strains carrying a target-site mutation in the transmembrane domain (Troczka et al. 2012; Guo et al. 2014a, b; Steinbach et al. 2015). Further evidence for a critical role of this transmembrane region for diamide binding was provided by a study replacing a 46 amino acid segment in the Drosophila RyR C-terminal domain by that of a nematode RyR which resulted in insensitivity to diamides (Tao et al. 2013). Since the introduction of diamide insecticides, several more insect RyR genes were cloned, sequenced and compared by phylogenetic means (Fig. 12.2), including those from lepidopteran pests such as diamondback moth (Wang and Wu 2012), which subsequently allows to investigate the implications of amino acid substitutions for diamide insecticide target-site resistance first described in diamondback moth (Troczka et al. 2012; Steinbach et al. 2015).
Neighbour-joining phylogenetic analysis of the ryanodine receptor (RyR) of different insect orders and noninsect species. (A) Lepidoptera, (B) Hymenoptera, (C) Coleoptera, (D) Diptera, (E) Hemiptera. Root: Homo sapiens. The corresponding GenBank accession numbers are as follows: Coleoptera (Leptinotarsa decemlineata, AHW99830; Meligethes aeneus, unpublished (Nauen et al.); Tribolium castaneum, AIU40166.1); Diptera (Aedes aegypti, Q17EB5; Anopheles darlingi, W5JDV8; Anopheles gambiae, Q7PMK5; Anopheles sinensis, A0A084WAS3; Bactrocera dorsalis, A0A034W289; Bactrocera cucurbitae, A0A0A1WHX3; Ceratitis capitata, W8AL79; Drosophila ananassae, XP_001958793.1; Drosophila erecta, XP_001970412.1; Drosophila grimshawi, XP_001995333.1; Drosophila melanogaster, AFH07966.1; Drosophila simulans, XP_002080659.1; Drosophila willistoni, XP_002061506.1; Drosophila yakuba, XP_002089690.1; Musca domestica, XP_011296554.1); Hemiptera (Bemisia tabaci, I3VR33; Laodelphax striatellus, A0A059XRL5; Myzus persicae, A0A0A7RS32; Nilaparvata lugens, KF306296; Sogatella furcifera, KF734669); Hymenoptera (Apis mellifera, AFJ66977.1; Apis dorsata, XP_006622367.1; Bombus impatiens, XP_012250208.1; Bombus terrestris, XP_012175583.1; Camponotus floridanus, XP_011257849.1; Megachile rotundata, XP_003701507.1; Nasonia vitripennis, XP_008202582.1; Solenopsis invicta, XP_011158883.1); Lepidoptera (Bombyx mori, XP_004924916.1; Carposina sasakii, X2GG79; Chilo suppressalis, I3VR34; Cnaphalocrocis medinalis, I1XB02; Grapholita molesta, A0A089FYX0; Helicoverpa armigera, V5RE97; Heliothis virescens, DD408555.1; Ostrinia furnacalis, M4T4G3; Pieris rapae, R9R5D5; Plutella xylostella, AEI91094.1; Spodoptera exigua, A0A059XRP6; Tuta absoluta, unpublished data);Vertebrata (RyR 1) (Rattus norvegicus, F1LMY4; Homo sapiens, P21817; Oryctolagus cuniculus, P11716); others (Pediculus humanus corporis, E0VEK3; Tetranychus urticae, F5HSW9). The phylogenetic tree was generated using tree builder (Geneious 8.0) with 100 bootstrap replications. The scale bar represents 2.0 amino acid substitutions per site
12.2 Diamide Insecticide Resistance in Lepidopteran Pests
Owing to their low application rates and high insecticidal efficacy, diamide insecticides were readily used right after their launch in 2006/2007 on a rather extensive scale for the control of several lepidopteran pests, especially in Southeast Asia and China. Meanwhile diamide insecticides are globally used both solo and in mixtures by millions of farmers for foliar, drench and seed treatment applications in a broad range of agricultural and horticultural cropping systems, thus facilitating the evolution of insect resistance due to increasing selection pressure, particularly on lepidopteran pests (Teixeira and Andaloro 2013). As a result of their frequent use and due to the lack of alternatives of similar efficacy, first cases of diamide field failure were reported only 2 years after launch in the Philippines and Thailand in cabbage against diamondback moth, P. xylostella (Troczka et al. 2012), a notorious lepidopteran pest in cruciferous vegetables. Subsequently high levels of diamondback moth resistance to diamides compromising the effectiveness of field recommended rates were confirmed in China (Wang and Wu 2012; Wang et al. 2013; Gong et al. 2014), Brazil (Ribeiro et al. 2014), Taiwan, India, USA, Japan, Korea and Vietnam (Steinbach et al. 2015). Lepidopteran pests other than diamondback moth which developed high confirmed levels of diamide resistance include tomato leaf miner, Tuta absoluta (Roditakis et al. 2015), and smaller tea tortrix, Adoxophyes honmai (Uchiyama and Ozawa 2014). Whereas low to moderate resistance ratios in laboratory assays were reported for rice stem borer, Chilo suppressalis (Gao et al. 2013; He et al. 2014); beet armyworm, Spodoptera exigua (Lai et al. 2011; Che et al. 2013); oriental leafworm, Spodoptera litura (Su et al. 2012; Sang et al. 2015); rice leaffolder, Cnaphalocrocis medinalis (Zhang et al. 2014); soybean looper, Chrysodeixis includens (Owen et al. 2013); and the obliquebanded leafroller, Choristoneura rosaceana (Sial et al. 2011; Sial and Brunner 2012). Some lepidopteran pest species are known for their (geographic and intrinsic) variation in response to insecticides, and talking about resistance is misleading in those cases as one has to keep in mind that such variation is to some extent natural and not directly linked to resistance development based on selection pressure or cross-resistance issues. Such a variation in response was recently also confirmed in several baseline susceptibility studies with diamide insecticides, including high-risk pests, such as Helicoverpa armigera (Bird 2015), C. suppressalis (Su et al. 2014), S. litura (Su et al. 2012) and T. absoluta (Campos et al. 2015).
Diamide resistance ratios exceeding 1000-fold were yet only reported in diamondback moth and tomato leaf miner (Table 12.1), suggesting that some insect pests carry a higher potential to develop resistance to diamides than others. Whereas high levels of diamide resistance in diamondback moth is globally on the move as demonstrated by its documented presence in more than ten countries (Steinbach et al. 2015), highly resistant tomato leaf miner populations were yet only isolated from vegetable greenhouses in southern Italy (Roditakis et al. 2015). The molecular mechanisms conferring diamide resistance in T. absoluta are largely unknown and currently under investigation by research groups in Germany, the UK, Greece, Spain and Brazil. Diamondback moth is known as a notorious candidate for rapid resistance development to almost all chemical classes of insecticide introduced for its control, particularly in (sub)tropical areas with intensive use of crop protection products (Talekar and Shelton 1993; Teixeira and Andaloro 2013). For this reason it was not surprising that diamide (cross) resistance was first described in diamondback moth. The underlying mechanisms so far investigated are largely due to target-site mutations in the transmembrane domain of the RyR and not mediated by metabolic mechanisms such as overexpressed detoxification enzymes.
12.2.1 Target-Site Resistance
Early studies on the mechanisms of diamide resistance conducted in two diamondback moth strains collected in the Philippines and Thailand revealed an amino acid substitution G4946E in the C-terminal region of the Plutella RyR (Troczka et al. 2012). The amino acid substitution was shown to have evolved independently in diamondback moth populations in the Philippines and Thailand by different non-synonymous single-nucleotide polymorphisms, i.e. GGG to GAA and GGG to GAG, respectively, both replacing a glycine by a glutamic acid residue. Subsequently other groups confirmed the presence of the G4946E mutation also in diamondback moth populations collected in China (Gong et al. 2014; Guo et al. 2014a, b; Yan et al. 2014) and other countries including India, Japan and the USA (Steinbach et al. 2015). Some studies also demonstrated that RyR transcript levels are either increased or decreased in addition to the G4946E mutation in diamide-resistant strains (Yan et al. 2014; Gong et al. 2014; Liu et al. 2015a). The fact that the G4946E mutation was found in populations from different geographies indicates once more that it evolved independently rather through migration of one population. The G4946E substitution is located in the RyR transmembrane domain approx. comprising 700 amino acids and suggested as crucial for the binding of diamides in earlier studies conducted with a photoreactive derivative of flubendiamide in RyR deletion mutants of B. mori, recombinantly expressed in human embryonic kidney cells (Kato et al. 2009). The RyR transmembrane domain is highly conserved among different insect taxa (Fig. 12.3), and homology modelling revealed that glycine 4946 is located at the interface between helix S4 and the S4–S5 linker (Steinbach et al. 2015), supposed to have a critical role in RyR gating by impacting the movement of pore-associated helices (Ramachandran et al. 2013). Phylogenetic analysis of the RyR of different insect orders reveal that lepidopteran species, which have >90 % homology in their amino acid sequence, share around 78 % homology to Coleoptera and Hymenoptera (Fig. 12.2). Other insect RyR isoforms, such as Diptera and Hemiptera, show a 75–77 % identity with Lepidoptera. As shown in Fig. 12.3, the C-terminal transmembrane part of the RyR is a highly conserved region especially in the transmembrane helices, whereas the cytoplasmic part of the protein has diverged during evolution (Lümmen 2013). The G4946E mutation was first described in 2012 and associated with a diamide-resistant phenotype of diamondback moth, but convincing functional evidence for its implications in diamide binding was only provided recently (Steinbach et al. 2015). It was shown in radioligand binding studies using thoracic microsomal membrane preparations of diamondback moth that the G4946E mutation has functional implications on both diamide-specific binding as well as on its concentration-dependent allosteric modulation of [3H]ryanodine binding (Steinbach et al. 2015). In contrast to thoracic microsomal membrane preparations of a diamide susceptible strain, a diamide-resistant Plutella strain did not show specific saturable binding of a tritiated des-methylated flubendiamide analogue, [3H]PAD1. The tritiated diamide radioligand showed nanomolar binding affinities to membrane preparations of susceptible diamondback moth (K D-value 2.7 nM), but no conclusive equilibrium kinetics with membranes isolated from a resistant strain. Thus, Steinbach et al. (2015) provided for the first time functional evidence that the G4946E mutation confers RyR target-site resistance to diamide insecticides. The importance of the G4946E mutation for diamide resistance was confirmed in another study using clonal Sf9 cell lines stably expressing either the Plutella wild type or G4946E RyR (Troczka et al. 2015). It was shown that the binding of both phthalic and anthranilic diamides was dramatically impaired by the G4946E mutation in Plutella RyR recombinantly expressed in clonal Sf9 cell lines. Apart from the functional mutation G4946E, three more mutations, E1338D, Q4594L and I4790M, were recently identified in the RyR of a highly resistant P.xylostella strain from China and supposed to be involved in diamide resistance (Guo et al. 2014b). The critical role of the transmembrane domain at the interface between helix S4 and the S4–S5 linker for diamide binding seems obvious regarding the functional implications of G4946E in diamide binding. Interestingly the mutation site I4790M described by Guo et al. (2014b) in the upper helix S2 exhibits a greater diversity among insect taxa, but is located directly opposite of the G4946E mutation as shown in homology models of the diamondback moth RyR based on rabbit RyR1 (Steinbach et al. 2015). The distance between the respective Cα atom positions of the mutation sites is approx. 13 Å (Fig. 12.4). However, functional evidence showing the impairment of diamide insecticide binding by the presence of I4790M, either alone or in combination with G4946E, is still missing. On the other hand, it is tempting to speculate that differences in chlorantraniliprole and flubendiamide binding affinity (and selectivity) recently described in Musca domestica and Apis mellifera membrane preparations (both M4790) in comparison to Lepidoptera (I4790) (Qi and Casida 2013; Qi et al. 2014) are based on such less conserved residues rather than G4946. According to the recently published closed-state cryo-EM structure of rabbit RyR1 (Yan et al. 2015), the third mutation described by Guo et al. (2014b), Q4594L, is not located within the transmembrane domains, but in a region with several predicted EF hand domains (Takeshima et al. 1989). The implication of this mutation for diamide binding in lepidopteran RyRs also needs further investigation in the future, similar to E1338D which is located towards the N-terminus of P. xylostella RyR. Therefore, it is not in proximity to the other transmembrane-linked mutations (Guo et al. 2014b) and the putative binding site of diamide insecticides (Kato et al. 2009; Steinbach et al. 2015). In summary there is compelling evidence that the substitution of amino acid residue G4946 in RyRs plays a key role in diamide insecticide resistance, albeit its role in other species than diamondback moth yet needs to be explored. On the other hand I4790 is likely to be another important RyR mutation site possibly linked to diamide species specificity (and resistance).
Amino acid sequence alignment of the extended C-terminal transmembrane domain of ryanodine receptor (RyR) orthologues from mammals and arthropod species covering a broad phylogenetic range. Conserved amino acid residues across species are shaded in black. Secondary structural elements and domains are indicated above the alignment by coloured bars and based on a recently published rabbit RyR1 structure (PDB code: 3J8H) determined by single-particle cryomicroscopy (Yan et al. 2015). RyR mutation sites linked to diamide insecticide resistance in diamondback moth (P. xylostella) are located at positions Q4549L, I4790M and G4946E (numbering based on diamondback moth RyR). GenBank accession numbers are as follows: Homo sapiens, P21817; Oryctolagus cuniculus, P11716; Rattus norvegicus, F1LMY4; Myzus persicae, A0A0A7RS32; Nilaparvata lugens, KF306296; Bemisia tabaci, I3VR33; Drosophila melanogaster, AFH07966.1; Bactrocera dorsalis, A0A034W289; Musca domestica, XP_011296554.1; Anopheles gambiae, Q7PMK5; Aedes aegypti, Q17EB5; Apis mellifera, AFJ66977.1; Bombus terrestris, XP_012175583.1; Nasonia vitripennis, XP_008202582.1; Meligethes aeneus, Nauen et al. unpublished; Tribolium castaneum, AIU40166.1; Leptinotarsa decemlineata, AHW99830; Chilo suppressalis, I3VR34; Spodoptera exigua, A0A059XRP6; Plutella xylostella, AEI91094.1; Helicoverpa armigera, V5RE97
Ryanodine receptor protomer modelling based on the recently published structure of rabbit RyR1 (PDB code 3J8H; Yan et al. 2015). Two mutations conferring diamide insecticide resistance in diamondback moth (Troczka et al. 2012; Guo et al. 2014a), G4946E and I4790M, are located in transmembrane domains S4 and S2 (Steinbach et al. 2015)
See attached TIF files. The figure has been separated in two files, part 1 and 2.
12.2.2 Metabolic Resistance
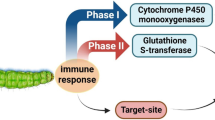
Phase I metabolism of diamide insecticides in animals depends particularly on microsomal monooxygenases, i.e. cytochrome P450s. It has been reported that flubendiamide metabolism in rats is mainly driven by multistep oxidation of methyl groups (Justus et al. 2007), and a major metabolic pathway of chlorantraniliprole and cyantraniliprole in the goat and rat, respectively, was shown to be the hydroxylation of the N-methyl and methylphenyl carbons resulting in hydroxy metabolites (Gaddamidi et al. 2011; Yoshida and McGregor 2014). Virtually nothing has been published yet regarding the metabolic fate of diamide insecticides in target organisms such as lepidopteran larvae. Metabolic resistance can be characterised by the genomic changes that lead to amplification, overexpression and coding sequence variation in the three major groups of gene superfamilies encoding for metabolic enzymes such as cytochrome P450s, carboxylesterases and glutathione S-transferases (Li et al. 2007), thus allowing the insect to overcome the toxicity of the insecticide. Studies on synergism by co-applying inhibitors of major detoxification mechanisms usually provide a first line of evidence for the presence of metabolic resistance in resistant strains.
However, as major routes of detoxification in animals were shown to include oxidation, it seems appropriate to assume that cytochrome P450-driven metabolisation of diamides in pest insects may potentially mediate metabolic resistance if such enzymes are overexpressed due to prolonged selection pressure. However, even though diamides are used to control lepidopteran pests for almost 10 years, conclusive evidence of metabolic mechanisms of resistance compromising diamide efficacy at recommended field rates was not yet described. Field-collected strains of those species showing resistance ratios greater than 1000-fold, such as diamondback moth, were shown to express target-site resistance mediated by amino acid substitutions in the transmembrane domain of the RyR (Troczka et al. 2012; Guo et al. 2014b; Steinbach et al. 2015), or, such as tomato leaf miner, no concrete informations on the mechanisms of resistance were reported (Roditakis et al. 2015). Campos et al. (2015) tested both flubendiamide and anthranilic diamides against a number of field-collected strains of T. absoluta, and whilst the level of cytochrome P450 activity was significantly correlated with the variation in chlorantraniliprole and cyantraniliprole susceptibility, no such correlation was evident for the observed variation in flubendiamide efficacy. Though the observed overall variation in lethal concentration values among all tested tomato leaf miner strains against anthranilic diamides was low, it is interesting to note that those with the lowest LC50 values were also those with the lowest cytochrome P450 activity, a fact which suggests that oxidative metabolism determines at least to some extent the observed efficacy variation (Campos et al. 2015). The possible involvement of oxidative metabolism in diamide resistance was also suggested in a laboratory-selected Indian strain of S. litura exhibiting 80-fold resistance to chlorantraniliprole, but synergist studies using piperonyl butoxide (PBO) were not conclusive both in vitro and in vivo (Muthusamy et al. 2014). However, studies on Chinese S. litura strains failed to correlate low-level anthranilic diamide resistance with elevated levels of cytochrome P450 activity (Su et al. 2012; Sang et al. 2015). Another noctuid species investigated for its capacity to develop chlorantraniliprole resistance after several laboratory selection cycles was S. exigua (Lai et al. 2011). Although elevated levels of cytochrome P450 and esterase activity were measured, their inhibition by synergists did not significantly increase diamide susceptibility in the selected laboratory strain. This is in contrast to diamondback moth where Liu et al. (2015a) demonstrated high PBO-mediated synergism of chlorantraniliprole activity in a moderately resistant strain selected for 52 generations under laboratory conditions, suggesting the involvement of increased oxidative metabolism, because the carboxylesterase inhibitor S,S,S-tributyl-phosphorotrithioate (DEF) failed to significantly synergise chlorantraniliprole, thus confirming earlier studies on a field-collected diamondback moth strain (Wang et al. 2013). In another study, laboratory selection of cyantraniliprole resistance in diamondback moth resulted in an increased cross-resistance to flubendiamide and chlorantraniliprole and could be synergised to some extent by PBO and diethyl maleate (DEM) (Liu et al. 2015b). A recent RNA-seq approach to investigate the transcriptome of three diamondback moth strains exhibiting low, moderate and high levels of chlorantraniliprole resistance revealed a correlation between the level of resistance and the up-regulation of a number of detoxification genes, such as cytochrome P450s, but also downregulation of RyR contigs (Lin et al. 2013), a phenomenon also described for other diamide-resistant diamondback moth strains (Gong et al. 2014). However, this is in contrast to other studies showing up-regulation of RyR transcripts to be involved in diamide resistance (Yan et al. 2014; Liu et al. 2015a). Strong synergism of chlorantraniliprole by PBO as well as DEF was recently described in a field-collected strain of a major rice pest, C. suppressalis, suggesting a role for both monooxygenases and esterases in the detoxification of chlorantraniliprole (He et al. 2014). Interestingly increased esterase activity was also found in a chlorantraniliprole-selected strain of Choristoneura rosaceana (Sial et al. 2011), and subsequent synergist studies principally supported the role of hydrolytic enzymes in chlorantraniliprole detoxification (Sial and Brunner 2012). In conclusion it seems fair to claim that most if not all studies on lepidopteran pests so far published failed to clearly demonstrate strong implications of metabolic mechanisms of diamide resistance causing field failure at recommended rates, but this may (will) change in the future. However, the growing tendency to utilise technologies such as RNA-seq for transcriptome assembly and expression analysis will for sure facilitate the identification of specific biochemical mechanisms and candidate genes to be principally capable to confer metabolic resistance to diamide insecticides in pest species under continuous selection pressure.
12.2.3 Genetics of Diamide Resistance
Among the few studies published to date of either field- or laboratory-selected diamide resistance high enough to compromise field efficacy, only some of those done on diamondback moth have examined the genetics of resistance to diamide insecticides. To date, there have been a few different but highly resistant diamondback moth strains examined in these studies, and of these, all have suggested an autosomal incomplete to almost recessive mode of inheritance (degree of dominance D ranges from −0.13 to −0.81) based on reciprocal crosses of diamide-resistant and susceptible individuals (Wang et al. 2013; Guo et al. 2014; Steinbach et al. 2015; Liu et al. 2015a, b). Two of these studies tested the level of diamide resistance of backcrosses of the F1 progeny with the resistant parental strain and investigated whether the observed diamide resistance is conferred by a single or multiple genes (Steinbach et al. 2015; Liu et al. 2015a, b). For example, flubendiamide resistance (RR >10,000) in a field-collected Philippine strain of P. xylostella was found to be almost recessive (D −0.81) and near monogenic, based on the presence of a homozygous target-site mutation (G4946E) in the transmembrane domain of the diamondback moth RyR (Steinbach et al. 2015). The authors have shown that the frequency of the resistance allele is likely to be 100 % in their strain, which was maintained without selection pressure under laboratory conditions for more than 4 years. A second diamondback moth study found that cyantraniliprole resistance (RR >3000) in a field-collected Chinese strain selected for three generations under laboratory conditions was autosomal and incompletely recessive (D < −0.2), but controlled by multiple genes as shown by differences between expected and observed mortality figures in dose-response tests of the backcross of F1 progeny with the parental strain (Liu et al. 2015a, b). The authors have not analysed the molecular mechanisms conferring the high levels of cyantraniliprole resistance in their strain, but earlier studies on diamondback moth populations collected in the very same region, i.e. Zengcheng, Guangdong Province (southern China), revealed a high frequency of individuals showing a G4946E RyR target-site mutation (including heterozygotes) and diamide resistance levels greater than 2000-fold (Gong et al. 2014; Yan et al. 2014). However, one can only speculate that possibly a mix of diamide-resistant genotypes present in the cyantraniliprole-resistant strain (ZC, i.e. Zengcheng) investigated by Liu et al. (2015b) may have prevented to find a near monogenic resistance as well as an almost recessive mode of inheritance resulting in a heterozygously susceptible phenotype, as shown for a near-isogenic strain from the Philippines (Steinbach et al. 2015). Most of the available information on diamide resistance in diamondback moth seems to suggest a single, recessive gene, which is consistent with the presence of a target-site-based mechanism of resistance. Even when other (detoxification) genes may be involved, the known and well-described target-site-based resistance mechanism seems most important for diamide resistance in diamondback moth and possibly other pest insects exhibiting high levels of diamide resistance such as T. absoluta (Roditakis et al. 2015).
12.2.4 Fitness Costs of Diamide Resistance
The process of natural selection favours genes of phenotypes that show the highest fitness within a population (Holloway et al. 1990). As a result of the selection pressure on insects, caused by extensive use of insecticides, the selection of alleles that confer an adaptation to this environmental stress factor is facilitated. Therefore, most insecticide resistance mechanisms are associated with fitness costs as these mutational changes often have deleterious effects on the overall fitness of a resistant insect compared to a susceptible counterpart. However, the costs caused by resistance are not fixed and are more or less dependent on environmental factors, such as temperature (Li et al. 2007), food quality (Janmaat and Myers 2005; Golizadeh et al. 2009; Farahni et al. 2011) and parasitism (Raymond et al. 2007). Furthermore, negative genetic trade-offs are often shown in the absence of the insecticide or in the presence of sublethal doses (Hoffmann and Parsons 1991; Ribeiro et al. 2014). When selecting for diamide resistance in P. xylostella, fitness costs were identified as a consequence of diamide resistance (Han et al. 2012; Yan et al. 2014), e.g. lower fertility in a cyantraniliprole-selected laboratory strain (Liu et al. 2015b). The overall fitness was strongly affected, showing a longer developmental time of larva as well as a decreased rate of pupation and adult emergence with a low relative fitness. When applying a sublethal concentration of chlorantraniliprole to a Brazilian field-evolved chlorantraniliprole-resistant diamondback moth strain (RR >27,000) and a susceptible reference strain, both strains were significantly affected in their fitness (Ribeiro et al. 2014). Moreover, the resistant strain had shown negative trade-offs, such as significantly reduced larval weight and fecundity, when chlorantraniliprole was absent. In other studies there was no significant effect on the longevity in P. xylostella and S. exigua when the insects were treated with a sublethal concentration of chlorantraniliprole (Lai et al. 2011; Han et al. 2012). In Cydia pomonella, it was shown that chlorantraniliprole exposure affected males more than females in terms of mating behaviour (Knight and Flexner 2007). Despite the fitness costs involved in diamide resistance, positive traits could be observed in diamondback moth, such as an increased larval survival, egg hatchability and male longevity (Ribeiro et al. 2014). This suggests that a physiological mechanism is present in order to compensate for associated fitness costs. However, Ribeiro et al. (2014) associated the reduced fitness in diamondback moth with the reversion of resistance to chlorantraniliprole as the resistant strain had shown a rapid decline in resistance without selection pressure. Most studies on fitness costs were yet conducted with diamide-resistant diamondback moth strains due to the fact that in most other lepidopteran targeted by diamides, resistance ratios so far reported are quite low and in most cases not compromising field efficacy.
12.3 Diamide Resistance Management
The development of field resistance depends on several factors including the genetic variability already present in a population of pests treated by consecutive applications with the same mode of action, thus facilitating the survival and reproduction of genotypes with a heritable ability to resist such applications at manufacturer recommended label rates. However, steadily increasing but still low levels of resistance are often less obvious under field conditions in terms of initial efficacy, but can result in an incremental reduction of residual activity due to the capacity of selected genotypes to resist declining quantities of active substance which would still provide reasonable control of completely susceptible individuals. In order to prevent or – realistically spoken – delay such a process, resistance management strategies need to be implemented in order to sustain the efficacy of a mode of action or chemical class of insecticide. The introduction of diamide insecticides into global markets was accompanied by communication and educational activities mainly driven by an IRAC International Diamide Working Group as well as more than 20 different IRAC Diamide Country Groups, tying together knowledge including baseline studies on high-risk pests and suitable (regional) IRM strategies in a diverse range of cropping systems (Teixeira and Andaloro 2013). The main objectives of the established regional IRAC Country Teams were (a) the identification and prioritisation of high resistance risk pests and cropping systems; (b) the adaptation of the global IRM guidelines into appropriate regional resistance management strategies; (c) the development of communication strategies particularly facilitating product labelling (IRAC Group 28 insecticides), advertising and education; (d) the communication of IRM recommendations, rotation strategies and optimal number of applications per cropping cycle by a so-called window approach (Fig. 12.5); (e)the development of an extensive education and knowledge transfer programme to train influencers and growers utilising local industry and IRM experts; and, last but by no means least, (f) the implementation of IRM strategies through education and training programmes, both on a global and regional scales (Teixeira and Andaloro 2013; refer also to www.irac-online.org for continuous updates on general IRM strategies, including diamides). A key point of established IRM strategies is the rotation of diamide insecticides between pest generations with other modes of action and to limit the number of applications throughout the cropping cycle by an IRM window approach (Fig. 12.5). In addition diamides exhibit some favourable application characteristics, such as low effects on populations of most beneficial insects, known to facilitate IRM within integrated pest management programmes. As diamides are distinct from all other chemical classes of insecticides (Sparks and Nauen 2015), they can principally be rotated with all those classes in IRM strategies. Currently, there is no metabolic detoxification mechanism described in any diamide-targeted pest conferring field-relevant cross-resistance to other Lepidoptera-active insecticides, rendering them highly valuable tools for both combining and alternating insecticide modes of action. The predominance and global spread of a target-site-based resistance mechanism in diamondback moth (Steinbach et al. 2015) – though recessive – should serve as a warning that resistance development may also easily extend to other pests if these are continuously selected by the treatment of consecutive generations, such as what recently happened for T. absoluta in southern Europe (Roditakis et al. 2015). However, cases of significant resistance to diamides under applied field conditions are so far regionally restricted to a few Lepidoptera species (Table 12.1), with the notable exception of P. xylostella (Troczka et al. 2012; Wang and Wu 2012; Gong et al. 2014; Ribeiro et al. 2014; Steinbach et al. 2015).
Recommended insecticide mode of action rotation practice for resistance management by an application window approach to avoid exposure of consecutive pest generations to the same mode of action such as diamides acting on insect ryanodine receptors (IRAC MoA group 28; www.irac-online.org)
12.4 Conclusions
Diamide insecticides show a remarkable overall activity against lepidopteran pest species, and after 10 years on the market, this chemical class gained blockbuster status economically and considering its global impact in many agricultural and horticultural cropping systems. However, despite their widespread use, diamide resistance development compromising field efficacy is yet restricted to a few, mostly regional cases, except for diamondback moth. Investigations into the molecular mechanisms of diamide resistance in this pest revealed RyR target-site mutations with strong functional implications for diamide binding. This also facilitated fundamental research on the genetics of diamide resistance and associated fitness costs. However, the evolution of target-site resistance is definitely an unpleasant event from an applied perspective, but it also offers opportunities to extend our knowledge on the biochemistry of insect RyRs as insecticide targets, e.g. by contributing to the understanding of diamide selectivity (insects vs. mammals) and by mapping the elusive diamide binding site, possibly allowing the design of novel ligands overcoming target-site resistance. The fairly rapid evolution of this target-site resistance mechanism in diamondback moth, due to high treatment frequency in tropical conditions, suggests that other pests with a lower number of generations per year and thus less frequently treated are likely to follow soon, if no appropriate IRM strategies as outlined above are implemented, helping to conserve diamide insecticides as a valuable chemical tool for sustainable agriculture.
References
Bird LJ (2015) Baseline susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to indoxacarb, emamectin benzoate, and chlorantraniliprole in Australia. J Econ Entomol 108:294–300
Cameron RA, Williams CJ, Portillo HE et al (2015) Systemic application of chlorantraniliprole to cabbage transplants for control of foliar-feeding lepidopteran pests. Crop Prot 67:13–19
Campos MR, Silva TBM, Silva WM et al (2015) Susceptibility of Tuta absoluta (Lepidoptera: Gelechiidae) Brazilian populations to ryanodine receptor modulators. Pest Manag Sci 71(4):537–544
Che W, Shi T, Wu Y et al (2013) Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J Econ Entomol 106(4):1855–1862
Cordova D, Benner EA, Sacher MD et al (2006) Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic Biochem Physiol 84:196–214
Cordova D, Benner EA, Sacher MD et al (2007) The novel mode of action of anthranilic diamide insecticides: ryanodine receptor activation. In: Synthesis and chemistry of agrochemicals VII, ACS symposium series 948. American Chemical Society, Washington DC, pp 223–234
Ebbinghaus-Kintscher U, Luemmen P, Lobitz N et al (2006) Phthalic acid diamides activate ryanodine sensitive Ca2+ release channels in insects. Cell Calcium 39:21–33
Farahni S, Naseri B, Talebi AA (2011) Comparative life table parameters of beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera, Noctuidae) on five host plants. J Entomol Res Soc 13(1):91–101
Foster SP, Denholm I, Rison JL et al (2012) Susceptibility of standard clones and European field populations of the green peach aphid, Myzus persicae, and the cotton aphid, Aphis gossypii (Hemiptera: Aphididae), to the novel anthranilic diamide insecticide cyantraniliprole. Pest Manag Sci 68:629–633
Gaddamidi V, Scott MT, Swain RS et al (2011) Metabolism of [14C]chlorantraniliprole in the lactating goat. J Agric Food Chem 59:1316–1323
Gao C, Yao R, Zhang Z et al (2013) Susceptibility baseline and chlorantraniliprole resistance monitoring in Chilo suppressalis (Lepidoptera: Pyralidae). J Econ Entomol 106(5):2190–2194
Gnamm C, Jeanguenat A, Dutton AC et al (2012) Novel diamide insecticides: sulfoximines, sulfonimidamides and other new sulfonimidoyl derivatives. Bioorg Med Chem Lett 22:3800–3806
Golizadeh A, Kamali K, Fathipour Y et al (2009) Life table of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) on five cultivated Brassicaceous host plants. J Agric Sci Technol 11:115–124
Gong W, Yan HH, Gao L et al (2014) Chlorantraniliprole resistance in the diamondback moth (Lepidoptera Plutellidae). J Econ Entomol 107(2):806–814
Gravalos C, Fernández E, Belando A et al (2015) Cross-resistance and baseline susceptibility of Mediterranean strains of Bemisia tabaci to cyantraniliprole. Pest Manag Sci 71:1030–1036
Guo L, Liang P, Zhou X et al (2014a) Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci Rep 4:6924
Guo L, Wang Y, Zhou X et al (2014b) Functional analysis of a point mutation in the ryanodine receptor of Plutella xylostella (L.) associated with resistance to chlorantraniliprole. Pest Manag Sci 70:1083–1089
Hamaguchi H, Hirooka T (2012) Insecticides affecting calcium homeostasis – flubendiamide. In: Krämer W, Schirmer U, Jeschke P, Witschel M (eds) Modern crop protection compounds, vol 3. Wiley-VCH, Weinheim, pp 1396–1409
Hamilton S (2005) Ryanodine receptors. Cell Calcium 38:253–260
Han W, Zhang S, Shen F et al (2012) Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag Sci 68:1184–1190
He Y, Zhang J, Chen J (2014) Effect of synergists on susceptibility to chlorantraniliprole in field populations of Chilo suppressalis (Lepidoptera: Pyralidae). J Econ Entomol 107:791–796
Hirooka T, Nishimatsu T, Kodama H et al (2007) The biological profile of flubendiamide: a new benzenedicarboxamide insecticide. Pflanzenschutz NachrBayer 60:183–202
Hoffmann AA, Parsons PA (1991) Evolutionary genetics and environmental stress. Oxford University Press, New York
Holloway GJ, Povey SR, Sibly RM (1990) The effect of new environment on adapted genetic architecture. Heredity 64:323–330
Janmaat AF, Myers J (2005) The cost of resistance to Bacillus thuringiensis varies with the host plant of Trichoplusia ni. Proc R Soc Lond Ser B 272:1031–1038
Jeanguenat A (2013) The story of a new insecticidal chemistry class: the diamides. Pest Manag Sci 69:7–14
Jefferies PR, Yu P, Casida JE (1997) Structural modifications increase the insecticidal activity of ryanodine. Pestic Sci 51:33–38
Justus K, Motoba K, Reiner H (2007) Metabolism of flubendiamide in animals and plants. Pflanzenschutz Nachr Bayer 60:141–166
Kato K, Kiyonaka S, Sawaguchi Y et al (2009) Molecular characterization of flubendiamide sensitivity in the lepidopterous ryanodine receptor Ca2+ release channel. Biochemistry 48:10342–10352
Knight AL, Flexner L (2007) Disruption of mating in codling moth (Lepidoptera: Tortricidae) by chlorantranilipole, an anthranilic diamide insecticide. Pest Manag Sci 63:180–189
Lahm GP, Selby TP, Freudenberger JH et al (2005) Insecticidal anthranilic diamides: a new class of potent ryanodine receptor activators. Bioorg Med Chem Lett 15:4898–4906
Lahm GP, Stevenson TM, Selby TP et al (2007) Rynaxypyr: a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg Med Chem Lett 17:6274–6279
Lahm GP, Cordova D, Barry JD (2009) New and selective ryanodine receptor activators for insect control. Bioorg Med Chem Lett 17:4127–4133
Lai T, Li J, Su J (2011) Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic Biochem Physiol 101:198–205
Lehmberg E, Casida JE (1994) Similarity of insect and mammalian ryanodine binding sites. Pestic Biochem Physiol 48:145–152
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52:231–253
Li X, Degain BA, Harpold VS et al (2012) Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Manag Sci 68:83–91
Lin Q, Jin F, Hu Z et al (2013) Transcriptome analysis of chlorantraniliprole resistance development in the diamondback moth Plutella xylostella. PLoS One 8(8):e72314
Liu X, Wang H, Ning YB et al (2015a) Resistance selection and characterization of chlorantraniliprole resistance in Plutella xylostella (Lepidoptera: Plutellidae). J Econ Entomol 108(4):1978–1985
Liu X, Ning Y, Wang H et al (2015b) Cross-resistance, mode of inheritance, synergism, and fitness effects of cyantraniliprole resistance in Plutella xylostella. Entomol Exp Appl 157:271–278
Lümmen P (2013) Calcium channels as molecular target sites of novel insecticides. In: Advances in insect physiology, vol 44. Elsevier, Amsterdam, pp 287–347
Lümmen P, Ebbinghaus-Kintscher U, Funke C et al (2007) Phthalic acid diamides activate insect ryanodine receptors. In: Synthesis and chemistry of agrochemicals VII, ACS symposium series 948. American Chemical Society, Washington, DC, pp 235–248
Muthusamy R, Vishnupriya M, Shivakumar MS (2014) Biochemical mechanism of chlorantraniliprole resistance in Spodoptera litura (Fab) (Lepidoptera: Noctuidae). J Asia-Pac Entomol 17(4):865–869
Nauen R (2006) Insecticide mode of action: return of the ryanodine receptor. Pest Manag Sci 62:690–692
Pepper BP, Carruth LA (1945) A new plant insecticide for control of the European corn borer. J Econ Entomol 38:59–66
Qi S, Casida JE (2013) Species differences in chlorantraniliprole and flubendiamide insecticide binding sites in the ryanodine receptor. Pestic Biochem Physiol 107:321–326
Qi S, Lümmen P, Nauen R et al (2014) Diamide insecticide target site specificity in the Heliothis and Musca ryanodine receptors relative to toxicity. J Agric Food Chem 62(18):4077–4082
Ramachandran S, Chakraborty A, Xu L et al (2013) Structural determinants of skeletal muscle ryanodine receptor gating. J Biol Chem 288:6154–6165
Raymond B, Sayyed AH, Hails RS et al (2007) Exploiting pathogens and their impact on fitness costs to manage the evolution of resistance to Bacillus thuringiensis. J Appl Ecol 44:768–780
Ribeiro LMS, Wanderley-Teixeira V, Ferreira HN et al (2014) Fitness costs associated with field-evolved resistance to chlorantraniliprole in Plutella xylostella (Lepidoptera: Plutellidae). Bull Entomol Res 104:88–96
Roditakis E, Vasakis E, Grispou M et al (2015) First report of Tuta absoluta resistance to diamide insecticides. J Pest Sci 88:9–16
Rogers EF, Koniuszy FR, Shavel J et al (1948) Plant insecticides. I. Ryanodine, a new alkaloid from Ryania speciosa Vahl. Am Chem Soc 70:3086
Rossi D, Sorrentino V (2002) Molecular genetics of ryanodine receptors Ca -release channels. Cell Calcium 32:307–319
Sang S, Shu B, Yi X et al (2015) Cross-resistance and baseline susceptibility of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Manag Sci. doi:10.1002/ps.4068
Sattelle DB, Cordova D, Cheek TR (2008) Insect ryanodine receptors: molecular targets for novel pest control chemicals. Invert Neurosci 8:107–119
Sial AA, Brunner JF (2012) Selection for resistance, reversion towards susceptibility and synergism of chlorantraniliprole and spinetoram in obliquebanded leafroller, Choristoneura rosaceana (Lepidoptera: Tortricidae). Pest Manag Sci 68:462–468
Sial AA, Brunner JF, Doerr MD (2010) Susceptibility of Choristoneura rosaceana (Lepidoptera: Tortricidae) to two new reduced-risk insecticides. J Econ Entomol 103:140–146
Sial AA, Brunner JF, Garczynski SF (2011) Biochemical characterization of chlorantraniliprole and spinetoram resistance in laboratory-selected obliquebanded leafroller, Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae). Pestic Biochem Physiol 99:274–279
Sparks TC (2013) Insecticide discovery: an evaluation and analysis. Pestic Biochem Physiol 107:8–17
Sparks TC, Nauen R (2015) IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121:122–128
Steinbach D, Gutbrod O, Lümmen P et al (2015) Geographic spread, genetics and functional characteristics of ryanodine receptor based target-site resistance to diamide insecticides in diamondback moth, Plutella xylostella. Insect Biochem Mol Biol 63:14–22
Su J, Lai T, Li J (2012) Susceptibility of field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in China to chlorantraniliprole and the activities of detoxification enzymes. Crop Prot 42:217–222
Su J, Zhang Z, Wu M et al (2014) Geographic susceptibility of Chilo suppressalis Walker (Lepidoptera: Crambidae), to chlorantraniliprole in China. Pest Manag Sci 70:989–995
Takeshima H, Nishimura S, Matsumoto T et al (1989) Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339:439–445
Takeshima H, Nishi M, Iwabe N et al (1994) Isolation and characterization of a gene for a ryanodine receptor/calcium release channel in Drosophila melanogaster. FEBS Lett 337:81–87
Talekar NS, Shelton AM (1993) Biology, ecology and management of the diamondback moth. Annu Rev Entomol 38:275–301
Tao Y, Gutteridge S, Benner EA (2013) Identification of a critical region in the Drosophila ryanodine receptor that confers sensitivity to diamide insecticides. Insect Biochem Mol Biol 43:820–828
Teixeira LA, Andaloro JT (2013) Diamide insecticides: global efforts to address insect resistance stewardship challenges. Pestic Biochem Physiol 106:76–78
Tohnishi M, Nakao H, Furuya T et al (2005) Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J Pestic Sci 30:354–360
Troczka B, Zimmer CT, Elias J et al (2012) Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem Mol Biol 42:873–880
Troczka BJ, Williams AJ, Williamson MS et al (2015) Stable expression and functional characterisation of the diamondback moth ryanodine receptor G4946E variant conferring resistance to diamide insecticides. Sci Rep 5:14680
Uchiyama T, Ozawa A (2014) Rapid development of resistance to diamide insecticides in the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae), in the tea fields of Shizuoka Prefecture, Japan. Appl Entomol Zool 49:529–534
Wang X, Wu Y (2012) High levels of resistance to chlorantraniliprole evolved in field populations of Plutella xylostella. J Econ Entomol 105(3):1019–1023
Wang X, Khakame SK, Ye C et al (2013) Characterisation of field-evolved resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella, from China. Pest Manag Sci 69:661–665
Waterhouse AL, Pessah IN, Francini AO et al (1987) Structural aspects of ryanodine action and selectivity. J Med Chem 30:710–716
Yan HH, Xue CB, Li GY et al (2014) Flubendiamide resistance and Bi-PASA detection of ryanodine receptor G4946E mutation in the diamondback moth (Plutella xylostella L.). Pestic Biochem Physiol 115:73–77
Yan Z, Bai XC, Yan C et al (2015) Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517:50–66
Yoshida M, McGregor D (2014) Cyantraniliprole. In: Pesticide residues in food – 2013 Part 2: Toxicological evaluations. WHO Press, World Health Organization, Geneva, pp 131–176
Zhang SK, Ren XB, Wang YC et al (2014) Resistance in Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) to new chemistry insecticides. J Econ Entomol 107(2):815–820
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Nauen, R., Steinbach, D. (2016). Resistance to Diamide Insecticides in Lepidopteran Pests. In: Horowitz, A., Ishaaya, I. (eds) Advances in Insect Control and Resistance Management. Springer, Cham. https://doi.org/10.1007/978-3-319-31800-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-31800-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31798-4
Online ISBN: 978-3-319-31800-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)