Abstract
Among lepidopteran insects, the fall armyworm, Spodoptera frugiperda, deserves special attention because of its agricultural importance. Different computational approaches have been proposed to clarify the dynamics of fall armyworm populations, but most of them have not been tested in the field and do not include one of the most important variables that influence insect development: the temperature. In this study, we developed a computational model that is able to represent the spatio-temporal dynamics of fall armyworms in agricultural landscapes composed of Bt and non-Bt areas, allowing the user to define different input variables, such as the crop area, thermal requirements of S. frugiperda, migration rate, rate of larval movement, and insect resistance to transgenic crops. In order to determine the efficiency of the proposed model, we fitted it using a 4-year (2012–2015) FAW monitoring data for an area located in northern Florida, USA. Simulations were run to predict the number of adults in 2016 and examine possible scenarios involving climate change. The model satisfactorily described the main outbreaks of fall armyworms, estimating values for parameters associated with insect dynamics, i.e., resistance-allele frequency (0.15), migration rate (0.48) and rate of larval movement (0.04). A posterior sensitivity analysis indicated that the frequency of the resistance allele most influenced the model, followed by the migration rate. Our simulations indicated that an increase of 1 °C in weekly mean temperatures could almost double the levels of fall armyworm populations, drawing attention to the possible consequences of temperature rises for pest dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key Message
-
Most of the models describing the population dynamics of fall armyworms have not been tested in the field.
-
We developed a computational model that describes the dynamics of S. frugiperda, considering the spatial dynamics of the crop, temperature changes and population genetics.
-
The results were compared with insect-monitoring data and the model satisfactorily fitted the main peaks related to the population dynamics.
-
Considering the spread of fall armyworms in Africa, this model can help entomologists to design management programs.
Introduction
The fall armyworm, S. frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is a polyphagous insect pest that occurs in the Western Hemisphere (Sparks 1979), attacking several crops, including corn (maize), cotton, and soybean (Barros et al. 2010). The fall armyworm is a holometabolous insect (complete metamorphosis including egg, larva, pupa and adult). Only the larvae damage plants and the adults reproduce and migrate. This insect is highly eurytopic, capable of producing an estimated 12 generations per year in tropical climates (Busato et al. 2005). In Brazil, the fall armyworm is one of the main insect pests of corn and cotton (Martinelli et al. 2006). In the USA it is a serious crop pest, mainly in the southeast (Hogg et al. 1982). Young larvae are usually found in the whorl in corn plants and initiate injury to leaves by scraping the foliar limb, eventually destroying the leaves during the last larval instars (Vilarinho et al. 2011; Dos Santos et al. 2004). These larvae can damage up to 57% of a corn crop (Cruz et al. 1999).
One of the main insect-control strategies is the use of transgenic crops, most of which were modified to carry the Bt (Bacillus thuringiensis) gene that codes for a particular endotoxin. However, Bt toxin-resistant fall armyworm populations have been documented in Puerto Rico, Brazil and the USA (Vélez et al. 2013; Farias et al. 2014; Omoto et al. 2016). The frequency of resistance alleles is a major variable that influences the development of resistance and has been intensively studied (Farias et al. 2016). In order to manage the evolution of resistance to transgenic crops, the use of refuge areas, consisting of non-Bt crops planted to promote the survival of susceptible insects, has been recommended (Sisterson et al. 2005).
Because of its agricultural importance, the spatio-temporal dynamics of the fall armyworm has been investigated in many different studies, using computational models that have proved to be useful tools for Integrated Pest Management (IPM) programs, because they constitute a laboratory in silico where experiments can be done to predict the effect of a natural or anthropic variable on insect dynamics (Ferreira and Godoy 2014). For instance, the effects of different refuge proportions and configurations on the evolution of fall armyworm resistance have been extensively discussed, using spatial models (Carroll et al. 2012; Cerda and Wright 2004; Garcia et al. 2016). Labatte (1994) mathematically modeled the larval development, considering insect thermal requirements under various field conditions (larval damage, host plant resistance, and microbial control). More recently, Malaquias et al. (2017) used a spatial model to understand the effect of different rates of larval movement affected by a fitness cost associated with insect resistance to transgenic crops on the distribution of fall armyworm populations. Garcia and Godoy (2016) described the spatial patterns (aggregated, uniform or random) of S. frugiperda in different crops, using a computational model that represents individual insects within a grid of cells.
Although insects are poikilotherms, most models do not include the influence of temperature variation on insect development. The importance of including the effect of temperature on the development of fall armyworms can be illustrated by the migration patterns observed in the USA. Because of its tropical origin, the fall armyworm is not able to survive extended periods of low temperatures and does not have the ability to enter diapause (Luginbill 1928; Barfield et al. 1978). Therefore, the geographic distribution of the fall armyworm is closely associated with climate conditions. In Brazil, the fall armyworm is widely distributed throughout the country because of the tropical climate, whereas in the USA, insect populations must migrate northward each spring from overwintering areas in southern Florida and Texas to reinfest the central and eastern USA and parts of southern Canada (Snow and Copeland 1969; Rose et al. 1975; Young 1979). Considering the effects of different temperatures on the development of fall armyworms, a grid-based spatial model was able to reproduce the migration pattern in the USA, using degree-days accumulated by populations throughout the year (Westbrook et al. 2016).
Including the effect of temperature in modeling approaches is also important in order to respond to increased concerns regarding the impact of climate changes on the ecology of insect pests (Cannon 1998). Overwintering areas in Florida may be extending northward over the years, allowing fall armyworm populations to cover a wider area during the cold season (Wood et al. 1979; Waddill et al. 1982; Westbrook et al. 2016).
In addition to climate-related migration, i.e., insects moving to climatically suitable areas, understanding the movements of larvae and adults helps in designing refuge plantings and in understanding fall armyworm behavior and ecological interactions. Vilarinho et al. (2011), using the mark-recapture technique, reported that adult armyworms can travel up to 800 m during their life span, and therefore concluded that refuges should be located about every 800 m for large corn fields. Extensive dispersal of fall armyworm larvae between non-Bt and Bt plants could also favor the evolution of insect resistance, as reported by Malaquias et al. (2017). Larval movement can expose larvae to sublethal doses of Bt toxins, depending on the refuge configuration, i.e., the seed mixture, and this exposure increases selection for Bt resistance (Garcia et al. 2016).
The present study introduces an individual-based model that describes the dynamics of S. frugiperda based on its thermal requirements, i.e., cumulative degree-days for insect development and temperature-driven viability. It also includes the composition and spatial arrangement of the crop, adult and larval movement (proportion of insects moving per day), migration rate (mean number of adult insects migrating per day), and frequency of the resistance allele (proportion of the total number of resistant alleles in a population) to Bt corn. In order to test the model, we simulated an area located in Alachua County, Florida, composed of corn plants (Bt and non-Bt) in which the presence of S. frugiperda has been monitored for many years. Most of the parameters were obtained through laboratory experiments, and others were estimated using insect-monitoring data in the study area and an optimization technique (genetic algorithm). Then, given a set of parameters, we ran the model using a temperature database from 2012 to 2015, and determined if the results obtained (model simulations) corresponded to the insect-monitoring data obtained in the study area. Then, we made predictions (not evaluated against data), estimating the population dynamics and number of generations for 2016 and for two hypothetical situations: mean temperatures in 2016 + 1 and + 2 °C.
One novelty of the proposed model is the assumption that insect biology (development time, viability, longevity and oviposition) is driven by the temperature. This allows us to study the effect of climate changes on insect populations and the influence of seasonality on population dynamics. Additionally, the results provided by the model were compared with data from the field, increasing its reliability. We used an individual-based approach, which is appropriate to estimate the population dynamics of fall armyworms, focusing on the variability of individual characteristics among the insects in a population (allowing a more realistic representation of the population) and to represent the design, crop composition and local characteristics of a small area (Jorgensen and Chon 2009).
The model has the potential to be used for different purposes, such as estimating the population levels in a certain area, investigating the effects of temperature changes on the dynamics of fall armyworm populations, managing the evolution of insect resistance, and determining the crop calendar, i.e., a timeline that contains recommendation on planting, sowing and harvesting periods of crops, in IPM plans. Considering the rapid spread of this insect pest across the African continent since 2016 (Wild 2017), the model can play an important role in helping entomologists to understand the factors that lead to invasion and to design control strategies to manage the population dynamics of fall armyworms.
Modeling process
The overall modeling process comprised three stages. In the first stage, the structure of the model was defined, including its rules and general structure based on the biology of the insect. The second stage involved the use of the model to simulate an area in Florida and determine the reliability of the results. In this stage, data from a laboratory experiment with fall armyworm populations were used in the modeling process, and monitoring data from 2012 to 2015 were compared with the model outputs. Finally, in the third stage, after the model was validated, the population dynamics of fall armyworm adults was estimated for 2016 and for two other, hypothetical scenarios, i.e., increasing the temperatures of 2016 by 1 and 2 °C.
Model building
The proposed model was developed in C programming language, using a grid of cells to emulate the crop habitat. Each cell represents one plant in the field, which could be either empty (absence of insects) or occupied (presence of insects). Regarding the immature stage of S. frugiperda, a cell could be occupied by one or two individuals, according to Farias et al. (2001), who reported a mean number of 1–2 larvae per plant. Regarding the adult stage, we set a carrying capacity equal to 10 individuals per plant, following a similar method to Garcia et al. (2016). A cell can be occupied by multiple stages simultaneously. Each time step t corresponded to 1 day, and each cell represented 1 × 1 m of the crop system. We did not include plant dynamics in our model, and therefore we considered that the larvae had unlimited food. We also associated an energy-counter with each insect. Since insect development is determined by the cumulative energy according to temperature variation, the energy-counter represents the energy accumulated over time. An insect must be able to accumulate a certain amount of energy (degree-days) to molt to another stage. This amount of energy is called the thermal constant (Gallo et al. 2002; Padmavathi et al. 2013). The thermal constant can be defined as the number of degree-days required for a development change to occur. Knowing the thermal constant and the degree-days accumulated each day, it is possible to predict the time needed to develop to the hatching, larval and pupal stages, and adult emergence. Another important parameter related to the thermal requirements of insects is the lower temperature threshold, i.e., the lowest temperature in which an insect can develop (Dixon et al. 2008). Below the lower temperature threshold, the insect does not accumulate degree-days; therefore, it may die or enter diapause. Unlike some other insect species, the fall armyworm does not enter diapause (Sparks 1979). The energy accumulated during 1 day is calculated by subtracting the value of the lower temperature threshold from the value of the daily temperature (Gallo et al. 2002; Padmavathi et al. 2013).
The rules used in the individual-based model are summarized below and shown in Fig. 1. All parameters and equations are described in Online Resource 1 and Online Resource 2, respectively.
Scheme of the general model structure. a Eggs survive according to a factor of egg viability, and must accumulate an amount of energy equal to K1 to molt to the larval stage. Larvae survive according to a factor of larval viability and must accumulate an amount of energy equal to K2 to molt to the pupal stage. b Pupae survive according to a factor representing pupal viability and must accumulate an amount of energy equal to K3 to molt to the adult stage. Adult longevity depends on the energy accumulated over time. All adults die after accumulating an amount of energy equal to K4 and can lay eggs in a cell according to a daily oviposition probability, φ(T). At each time step, an adult female can oviposit in any cell with fewer than two immature insects inside a 5 × 5-cell neighborhood
-
(a)
Cells occupied by an immature insect. In this case, two processes can occur:
-
(a1)
A cell occupied by an immature insect (egg, larva or pupa) can become empty with a daily probability equal to \(1 - \sqrt[d]{\mu (T)}\) due to mortality, where \(\mu (T)\) is the viability corresponding to the insect stage and d is the duration of the insect stage (days) (Garcia et al. 2014, 2016). The variable d is given by \(\frac{K}{{(T - T_{b} )}}\), where T is the daily temperature mean (°C) and \(T_{b}\) (°C) and K (degree-days) are the lower threshold temperature and thermal constant corresponding to the insect stage, respectively (Gallo et al. 2002; Rao et al. 2015) (Fig. 1).
-
(a2)
An egg must accumulate energy equal to K1 (degree-days) in order to hatch. A larva must accumulate energy equal to K2 (degree-days) to pupate. A pupa must accumulate energy equal to K3 (degree-days) for the adult to emerge (Gallo et al. 2002; Rao et al. 2015) (Fig. 1).
-
(a1)
-
(b)
Cells occupied by an adult.
A cell will become empty of adults if all adults die. We assumed that an adult dies when it accumulates an amount of energy equal to K4 (degree-days). In this case, K4 is associated with metabolic acceleration, resulting in a negative correlation between temperature and longevity (Gao et al. 2013; Gunay et al. 2010). Higher temperatures, and consequently more energy, reduce adult longevity by accelerating the metabolism. Either a linear or a polynomial relationship between the amount of energy accumulated and the adult longevity has been observed in several studies of the biology of insects (Joshi 1996; Yadav and Chang 2014).
-
(c)
Empty cells.
An empty cell can become occupied by an immature insect if a female adult lays eggs in it. At each time step, a female adult can oviposit in any cell with fewer than two immature insects inside a 5 × 5-cell neighborhood. Per-capita oviposition probability per day is given by φ(T) and is dependent on the temperature T (°C) (Garcia et al. 2016).
The movement of an adult inside the simulated area at each time step had no preferential direction and was calculated as described in Garcia et al. (2016) (Eq. 3), where \(P\) is the probability that an adult will travel over a distance \(S\) (meters) per time step.
We also associated a short-scale movement component with the larva. Each larva had a probability l of moving to an adjacent plant per day, using a Moore neighborhood of radius 1, i.e., 3 × 3 cells, per day.
Genetic component
The genotype of each individual regarding its resistance to Bt corn (Cry1Fa), i.e., SS—susceptible homozygote, SR—susceptible heterozygote, or RR—resistant homozygote (Vélez et al. 2014; Huang et al. 2014), was determined using the Hardy–Weinberg equation (Hardy 1908), given by:
where \(p\) is the frequency of the S allele \(p = 1 - q\) and q is the frequency of the R allele. Knowing the frequency of the R allele in the population, the probability of an individual showing the genotype SS (\(p^{2}\)), SR (\(2pq\)) or RR (\(q^{2}\)) can be determined, using the method described by Garcia et al. (2016). Genotype-specific viability values are shown in Table 1. Regarding the fitness cost, we assumed a reduction of 20% in larval viability and a delay of 4 days in the duration of the larval stage in the absence of Bt crops, when individuals carried at least one copy of the resistance allele (RR or SR genotype) (Dangal and Huang 2015). We assumed that the adults mix sufficiently across Bt and non-Bt areas, to make the genotype probability for offspring independent of the local corn type. According to Vilarinho et al. (2011), adult females can travel up to 600 m from their release site during their life span. Therefore, using a rough approximation, we can ensure that adults are able to mix sufficiently in an area measuring up to 1200 m × 1200 m = 144 ha. Since we simulated an area much smaller than this (“Model calibration” section), we could make this assumption in our modeling process.
Model calibration
In order to determine the reliability of the model, we tested it for a real area of size 200 × 200 m (4 ha), located at Hague, Alachua County (29°46′14″N, 082°25′16″W). In recent years, this area has been monitored with two sex pheromone-baited Unitraps to assess the presence of fall armyworm adults (Meagher et al. 2013). According to Tingle and Mitchell (1979), two pheromone traps catch insects effectively within a range of attraction equal to 4 ha. Therefore, the density of insects could be calculated from the number of insects trapped, by dividing the total number of insects captured by 4 ha. Although this is an approximation based on published sources, it allowed us to compare the model output and the field data at the same scale (number of individuals per hectare). In order to grid the particular study area, we used its crop calendar. Assuming that each simulation is run during the course of 1 year (0 ≤ t ≤ 365), the simulated area could assume different spatial crop configurations: (a) from March to October (60 ≤ t ≤ 300): occupied by corn plants in a proportion of 80% transgenic plants and 20% non-transgenic plants (refuge) arranged in a block configuration; (b) from November to February (t < 60 and t > 300): no crop.
The values corresponding to the thermal constants (Table 2), the equations that define the viability of each stage, and the oviposition probability per day were chosen based on data from S. frugiperda populations reared on corn leaves in a thermal-requirement study at five different temperatures (14 °C, 18 °C, 22 °C, 26 °C and 30 °C) (Garcia et al. 2018). This study was conducted using larval populations collected from corn plants at the Plant Science Research and Education Unit, Citra, Florida (29°24′42.9″N, 082°6′35.34″W), a community located 70 km from the monitored area.
The number of degree-days accumulated on each day in our simulations was calculated using a weekly temperature database from Hague (Florida Automated Weather Network, IFAS, University of Florida 2017) from 2012 to 2015 (the same weekly T value was used for each day of the week, since the model input is daily temperatures).
For the viability equations, we used quadratic functions in order to define the viabilities for temperatures that were not evaluated by Garcia et al. (2018), since the relationship between insect viability levels and temperature can be satisfactorily described by quadratic expressions when viability shows a nonlinear increase or decrease with the temperature (Amarasekare and Sifuentes 2012). We also assumed that insects are not able to survive in temperatures below the lower temperature threshold. Adults die only after accumulating degree-days equal to K4. The viability functions are described in Eqs. (3), (4) and (5), corresponding, respectively, to the egg, larval and pupal stages. The domain of these functions is the range of temperatures used in our simulations, \(T \in [7, 32]\). The fits of the quadratic curves to the data from Garcia et al. (2018) are available in Online Resource 3.
The per capita oviposition probability per day by an adult female was defined in Eq. (6) using data from the study published by Garcia et al. (2018). In this case, the domain of the function is \(E_{d} \in [70,180]\), based on the energy required to initiate the oviposition period.
where E (degrees) is the total energy accumulated (indicated by the energy-counter) on days d and \(d - 1\) of adult life.
Another assumption is that the study area is located in a region where insects are not able to overwinter, and periodically receives individuals from warmer regions (Barfield et al. 1980; Westbrook et al. 2016). According to Meagher and Mitchell (2001) and Westbrook et al. (2016), this period corresponds approximately to the second half of April and the first half of May. Accordingly, in order to represent this immigration process, we included a mean migration rate higher than 0 (m > 0), occurring during the time interval given by \(105 \le t \le 135.\) Therefore, m can be interpreted as the mean number of adult insects arriving inside the lattice of cells per day (time step). The initial position of each incoming adult insect was randomly chosen by the computer inside the lattice.
Model verification
Genetic algorithm (GA)
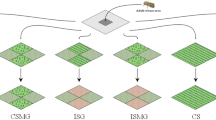
Some parameters of the model were not available in the literature and are difficult to obtain from experimental data. Therefore, in order to reproduce the monitoring data set, a genetic algorithm was used to find the appropriate set of parameters given by z = [m, f, l], corresponding, respectively, to the migration rate (number of adults migrating in each time step), the frequency of the allele for resistance to Bt corn in immigrant populations, and the rate of larval movement (proportion of larvae moving in each time step), that gave the best match between the model and the monitoring data.
GA is an optimization method based on the mechanisms of natural selection, with the goal of producing new parameter sets that are gradually fitted to the present conditions, according to some objective function (Mitchell 1999). In entomological research, Ren et al. (2016) used a GA to estimate the parameters of a model to simulate swarms of flying insects. With this in mind, \(n_{p}\) is the number of parameter sets tested. A score (or fitness) is attributed to each parameter set according to a fitness function. At each iteration of the GA algorithm, three processes may occur: reproduction, crossover, and mutation (Fig. 2) (Chambers 2000).
“Reproduction” selects the parameter sets to compose the next generation according to a fitness function. In the present study, the mean square error between the observed and the expected results (number of adults) was used as a fitness function in order to select the parameter sets that showed the smallest differences between the model results and the monitoring data. “Crossover”, which exchanges information between two parameter sets, occurred according to a probability set to 0.1. This mimics sexual reproduction; otherwise, the parents would produce an offspring identical to themselves. “Mutation” randomly modifies the value of one parameter in the set according to a probability that was set to 0.1. It allows the introduction of new values, preventing the solution from reaching a local minimum, since we were looking for a global minimum. We used a number of parameter sets equal to \(n_{p}\) = 200 and allowed them to evolve over 100 iterations. The values of the starting sets were drawn randomly from a range of values with equal probability of being selected. The range of values for m, f and l was [0, 1], [0, 0.4] and [0, 0.15], respectively, based on field and computational studies (Huang et al. 2014; Westbrook et al. 2016; Garcia et al. 2016; Farias et al. 2016; Malaquias et al. 2017).
Simulations
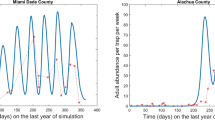
Initially we ran the model using data collected in 2012 in the study area, in order to determine the vector \(z\) = [m, f, l]. The genetic algorithm found the best match between the model prediction and the field data (mean square error = 90.35), which was z = [0.48, 0.15, 0.04]. The complete results of the genetic algorithm, indicating the global minimum and the values of the cost function for each parameter value tested, are available in Online Resource 4. Then, using the parameter values from the vector \(z\), we ran simulations for 2013, 2014 and 2015. The goodness of fit of the model to the data was measured by the relative-root-mean-square error, RRE = (r.m.s. error) / (r.m.s. count) (Fig. 3).
The RREs of the fitted curves were 17–27%. Soulsby and Thomas (2012) considered that values less than 29% correspond to a good fit.
From 2012 to 2014, five generations of fall armyworms per year were predicted by our model. The same number was found for the same region by Garcia et al. (2018), where thermal requirements and the Geographic Information System (GIS) were used to estimate the number of generations of fall armyworms from 2006 to 2016 throughout Florida. In 2015 and 2016, our model estimated six generations during the year, due to the warmer climate conditions. Garcia et al. (2018) estimated seven generations per year for 2015 and 2016.
Since the model was able to reproduce the monitoring data for the three consecutive years with good accuracy, using the combination of parameters provided by the GA for 2012, a new question was formulated: are the parameters obtained by the genetic algorithm not undergoing significant variations during the proposed time interval (2012–2015)? These questions are interesting for pest management because they allow us to detect waves of migration and/or variations in the frequency of the allele that confers resistance to Bt corn. In order to answer this question, we ran the genetic algorithm once more to find the combination of parameters that provided the best match of the model for each year from 2012 to 2015. Then, we calculated the mean values of the 20 best parameter sets obtained from simulations of the algorithm for each year (Fig. 4). We also ran a local sensitivity analysis to assess the importance of the three parameters estimated by the GA for insect dynamics. Fixing two of these parameters (using the values of the vector z = [0.48, 0.15, 0.04]) and varying the remainder, we measured how the insect population dynamics reacts to it (Saltelli et al. 2004). The conclusion was obtained from the variability measured at the end of the analysis, repeating the process for each parameter, being careful to vary \(m,f\;{\text{and}}\;l\) on the same scale and avoiding biased results, respectively, m\(\in\) [0.24, 0.96], f\(\in\) [0.075, 0.30] and \(l\)\(\in\) [0.02, 0.08] (Fig. 5).
Mean values of \(f\) (resistance-allele frequency in the migrant population) (a), \(m\) (migration rate) (b) and \(l\) (rate of larval movement) (c) obtained from simulations of the GA for 2012, 2013, 2014 and 2015. Error bars represent 95% confidence intervals. Different letters above the bars indicate significant differences at 5% (Tukey’s HSD, p < 0.05)
Estimation for 2016 and hypothetical scenarios
In order to investigate the possible effects of increases in temperature on the population dynamics of S. frugiperda, we tested the model using climate data from 2016, and also assumed two hypothetical scenarios: increasing the mean temperatures of 2016 by 1 °C and by 2 °C. In this case, predictions were not evaluated against data, since the model was previously tested for the period 2012–2015. Also, field data were not available for 2016. Using the weekly temperature data (Florida Automated Weather Network, IFAS, University of Florida 2017) and the combination of parameters \(z\) found by the GA for 2012, we estimated the population levels for 2016 and for each of the hypothetical scenarios (Fig. 6). All temperature data are available in Online Resource 5.
Discussion
Simulations
In general, the model satisfactorily predicted the temporal course of the adult population from 2012 to 2015, compared with the data obtained in the monitored area, and only synchronization delays of less than 5 days were observed. These delays can be explained as a response to the intervals of observation in the field data, which were longer than 1 week.
The model was able to predict two main periods of outbreak. The first (and smaller) outbreak is derived from eggs laid in mid-summer (~ July) by populations from the beginning of this season, when temperatures were highly favorable for fall armyworm development (25 < T < 30 °C) (Hogg et al. 1982; Ali et al. 1990). The second and larger outbreak would be expected in mid-autumn (~ October) from eggs laid at the end of summer, when conditions were still optimal for fall armyworm development. In addition to these two predicted outbreaks, a third occurred during December 2012, which was not predicted by the model. This outbreak was unexpected, since corn plants were not present in December. It may have occurred due to the presence of other host plants remaining in the field or an influx of moths returning from the north (Pair et al. 1987). In 2014 and 2015, only one, larger outbreak was observed, which the model was able to reproduce; however, the model also predicted another, smaller outbreak that was not observed in the monitoring data. Another reason for differences between the expected and observed results may be the assumptions in the model. The modeling approach assumed that temperature is the driving factor governing population dynamics, but other factors would also influence it, such as rainfall, corn phenology or other natural phenomena (Barfield and Ashley 1987). Considering fall armyworm biology, humidity plays an important role in the insect dynamics and could be a factor to be added (Simmons 1993) to increase the model’s predictive ability. The model’s failures can be also related to the absence of plant dynamics in the model. The model tended to overestimate the magnitude of major population peaks and failed to predict small peaks. Intra-specific competition, considering limited resources, could regulate insect population dynamics more effectively. However, many of these small peaks depend on random factors (climate and human events) and are not represented by the model because it attempts to simulate a general pattern, addressing the most important elements discussed in the specialized literature, such as the role of temperature in insect dynamics, and the importance of insect migration for overwintering areas. Adding more parameters could excessively complicate the computations and produce a very specific model, limiting its use for a diverse range of situations.
An important achievement by the model was the representation of the intensities of outbreaks. For instance, the model was able to represent the large outbreak in 2015 (exceeding 100 adults/ha). The year 2015 was one of the warmest on record in Florida, resulting in an increase in the number of fall armyworm generations throughout the state (UFWeather 2015; NOAA 2015).
The field data collected in 2012 and 2013 did not show a peak during the immigration period (105 ≤ t ≤ 135) as observed in the curves provided by the model. This difference may be related to the methodology used to model the immigration period. Because information about the specific period of immigration was sparse, we assumed that it occurred uniformly during a specific time interval based on previous observations. However, migration can be driven by different factors such as corn phenology, temperature and rainfall (Westbrook et al. 2016). The migration process could be also density-dependent, and the inclusion of this factor could correct the difference between the expected and observed number of adult insects.
Realism of parameter estimates and sensitivity analysis
Regarding the parameters found by the GA, we failed to find any published study providing rates of migration or larval movement in the field to compare with our results. Studies suggest that in case of \(l\) < 0.1 (our model estimated \(l\)= 0.04), the seed-mixture refuge strategy could be a better alternative for insect-resistance management in the field (Garcia et al. 2016).
The resistance-allele frequency found in our study ranged between 0.12 and 0.18. Different genetic studies were conducted using fall armyworm populations from counties in southern Florida, and the resistance-allele frequency was estimated: Palm Beach Co. (0.05–0.20), Hendry Co. (0.01–0.12) (Vélez et al. 2013), and Collier Co. (0.24–0.35) (Huang et al. 2014). Insects usually arrive in the study area from different southern locations during the migration period, and therefore the resistance-allele frequency (0.12–0.18) obtained in the current study can be interpreted as a mean of the allele-resistance frequency of populations from the many counties that provide fall armyworm moths to the simulation area. It also indicates that resistant individuals may be present in the area (Huang et al. 2014). This information needs to be taken into account in future management plans.
The sensitivity analysis showed that the frequency of the resistance allele most influenced the population dynamics, followed by the migration rate. This result was expected, since Bt crops are widely used in agricultural systems and therefore it is essential that insect populations possess some level of resistance in order to establish in these areas. Regarding the importance of the migration rate, we are representing an overwintering area and therefore the presence of fall armyworm moths depends on a periodic influx of insects. On the other hand, the model was little affected by the rate of larval movement, which occurs on a small scale (larvae move only between neighboring plants). However, maintaining this parameter in the model is important because of resistance management. Depending on the refuge configuration, larval movement may influence the evolution of resistance. The seed-mixture configuration, for instance, adopts a random configuration instead of a structured refuge as a block. Different investigators have noted that in a seed mixture, larval movement can increase selection for Bt resistance because it can expose larvae to sublethal doses of Bt toxin as the larvae move between Bt and non-Bt plants (Head et al. 2014; Garcia et al. 2016; Malaquias et al. 2017). Since we constructed the model to allow users to change the refuge configuration, we decided to maintain this variable because it becomes important when the refuge configuration is modified.
The differences between the number of generations estimated by our model and the number estimated by Garcia et al. (2018) for 2015 and 2016 are probably related to the different approaches used in the two studies. Garcia et al. (2018) used a GIS-based analysis, including only degree-days accumulated during the egg-adult period, whereas the current study used a computational model that covered a wider range of parameters related to temperature, such as viability and fecundity. Additionally, the previous study did not include parameters such as migration rate, resistance-allele frequency and larval movement. In spite of these differences, the values were very similar in the two studies.
As indicated in Fig. 4, the frequency of the resistance allele did not differ significantly among years. We found no differences among the years because we were assessing possible changes in the population over a short time interval. We also did not observe significant variations in the rate of larval movement and migration rate (except in 2014). These results indicate that neither of these two parameters, i.e., migration rate and larval movement rate, are affected by small variations in temperature, and only drastic climate changes may affect them. They could also be more closely related to other variables that were not studied here, such as crop neighborhood, crop calendar, etc. A more-detailed study focusing on the variables that affect both parameters would be necessary to test this hypothesis.
Estimation for 2016 and hypothetical scenarios
The model result for 2016 was very similar to 2015, but it showed a larger insect outbreak that reached ~ 150 adults/ha. The year 2015 was the warmest summer in Florida’s recorded history up to that time. However, the record was broken in 2016, with temperatures reaching 30 °C on some summer days (NOAA 2016). The gradual increase in mean temperatures has raised concern that the number of insects in the field is increasing (Liebhold and Bentz 2011). The simulations for 2015 and 2016 indicated the addition of one generation compared to 2012, 2013 and 2014. Additionally, our simulations for the hypothetical scenarios indicated that an increase of only 1 °C in weekly mean temperatures could produce an outbreak 1.7 times more intense than in 2016, reaching ~ 250 adults/ha. This increase in the number of insects is not indefinitely proportional to the increase in temperatures, as observed when weekly mean temperatures were increased by 2 °C. The simulated outbreak did not show a large difference compared to the simulation corresponding to an increase of 1 °C. Insect viability is affected by extreme temperatures, and the optimal temperature for fall armyworm development is around 26 °C (Garcia et al. 2018). It is expected that fall armyworm viability will decrease as temperatures rise above the optimal temperature, but that some insects will still be able to survive. Therefore, the intensity of outbreaks is proportional to the frequency of days with mean temperatures close to 26 °C during the period evaluated (Hogg et al. 1982; Ali et al. 1990).
Final considerations
This model may constitute an important tool for integrated pest management programs, since the model can be used to predict both the effects of the implementation of a new approach for pest control, and the influence of global warming on insect dynamics. The results provided by the model also constitute a warning about the effects of a gradual increase in mean temperatures on the density of fall armyworms in the field.
African countries have recently faced severe infestations of S. frugiperda populations, with significant crop losses. The model could be used to predict the population dynamics of fall armyworms in affected areas, providing tools for researchers to design IPM programs to manage this pest. The computational code is available and explained in Online Resources 6 and 7 and can be easily modified to represent different conditions. A future study in Africa, involving the use of field data, will be a future opportunity for the use of this model.
Author contributions
AGG developed the model and analyzed data. CPF, WAC and RLM helped with model development and data analysis. RLM also provided field data for validation. All authors read, collaborated in writing and approved the manuscript.
References
Ali A, Luttrell RG, Schneider JC (1990) Effects of temperature and larval diet on development of the fall armyworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am 83:725–733
Amarasekate P, Sifuentes R (2012) Elucidating the temperature response of survivorship in insects. Funct Ecol 26:959–968
Barfield CS, Ashley TR (1987) Effects of corn phenology and temperature on the life cycle of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Fla Entomol 70:110–117
Barfield CS, Mitchell ER, Poe SL (1978) A temperature-dependent model for fall armyworm development. Ann Entomol Soc Am 71:70–74
Barfield CS, Stimac JL, Keller MA (1980) State-of-the-art for predicting damaging infestations of fall armyworm. Fla Entomol 63:364–375
Barros EM, Torres JB, Bueno AF (2010) Oviposition, development, and reproduction of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) fed on different hosts of economic importance. Neotrop Entomol 39:996–1001
Busato GR, Grützmacher AD, Garcia MS, Giolo FP, Zotti MJ, Bandeira JM (2005) Exigências térmicas e estimativa do número de gerações dos biótipos “milho” e “arroz” de Spodoptera frugiperda. Pesqui Agropec Bras 40:329–335
Cannon RJC (1998) The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Glob Change Biol 4:785–796
Carroll MW, Head G, Caprio M (2012) When and where a seed mix refuge makes sense for managing insect resistance to Bt plants. Crop Prot 38:74–79
Cerda H, Wright DJ (2004) Modeling the spatial and temporal location of refugia to manage resistance in Bt transgenic crops. Agric Ecosyst Environ 102:163–174
Chambers LD (2000) The practical handbook of genetic algorithms, 2nd edn. Chapman and Hall, Boca Raton
Cruz I, Figueiredo MLC, Oliveira AC, Vasconcelos CA (1999) Damage of Spodoptera frugiperda (Smith) in different maize genotypes cultivated in soil under three levels of aluminum saturation. Int J Pest Manag 45:293–296
Dangal V, Huang F (2015) Fitness costs of Cry1F resistance in two populations of fall armyworm, Spodoptera frugiperda (J.E. Smith), collected from Puerto Rico and Florida. J Invertebr Pathol 127:81–86
Dixon AFG, Honek A, Keil P, Kotela ALS, Jarosik V (2008) Relationship between the minimum and maximum temperature thresholds for development in insects. Funct Ecol 23:257–264
Dos Santos LM, Redaelli LR, Diefenbach LMG, Efrom CFS (2004) Fertilidade e longevidade de Spodoptera frugiperda (J.E. Smith) (Lepidoptera:Noctuidae) em genótipos de milho. Ciênc Rural 34:345–350
Farias PRS, Barbosa JC, Busoli AC (2001) Spatial distribution of the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), on corn crop. Neotrop Entomol 30:681–689
Farias JR, Andow DA, Horikoshi RJ, Sorgatto RJ, Fresia P, Santos AC, Omoto C (2014) Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot 64:150–158
Farias JR, Andow DA, Horikoshi RJ, Bernardi D, Ribeiro RS, Nascimento ARB, Santos AC, Omoto C (2016) Frequency of Cry1F resistance alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Pest Manag Sci 72:2295–2302
Ferreira CP, Godoy WAC (2014) Ecological modelling applied to entomology. Springer, Switzerland
Florida Automated Weather Network, IFAS, University of Florida (2017) Archived Weather Data. https://fawn.ifas.ufl.edu/. Accessed 19 Aug 2017
Gallo D, Nakano O, Silveira Neto S, Carvalho RPL, Batista GC, Berti Filho E, Parra JRP, Zucchi RA, Alves SB, Vendramin JD, Marchini LC, Lopes JRS, Omoto C (2002) Entomologia agrícola. FEALQ, Piracicaba
Gao G-Z, Perkins LE, Zalucki MP, Lu Z-Z, Ma J-H (2013) Effect of temperature on the biology of Acyrthosiphon gossypii Mordvilko (Homoptera: Aphididae) on cotton. J Pest Sci 86:167–172
Garcia AG, Godoy WAC (2016) A theoretical approach to analyze the parametric influence on spatial patterns of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) populations. Neotrop Entomol 46:283–288
Garcia A, Cônsoli FL, Godoy WAC, Ferreira CP (2014) A mathematical approach to simulate spatio-temporal patterns of an insect-pest, the corn rootworm Diabrotica speciosa (Coleoptera: Chrysomelidae) in intercropping systems. Land Ecol 29:1531–1540
Garcia AG, Cônsoli FL, Ferreira CP, Godoy WAC (2016) Predicting evolution of insect resistance to transgenic crops in within-field refuge configurations, based on larval movement. Ecol Complex 28:94–103
Garcia AG, Godoy WAC, Thomas JMG, Nagoshi RN, Meagher RL (2018) Delimiting strategic zones for the development of fall armyworm (Lepidoptera: Noctuidae) on corn in the State of Florida. J Econ Entomol 111:120–126
Gunay F, Alten B, Ozsoy ED (2010) Estimating reaction norms for predictive population parameters, age specific mortality, and mean longevity in temperature-dependent cohorts of Culex quinquefasciatus Say (Diptera: Culicidae). J Vector Ecol 35:354–362
Hardy GH (1908) Mendelian proportions in a mixed population. Science 28:49–50
Head G, Campbell LA, Carroll M, Clark T, Galvan T, Hendrix WM, Prasifka PL, Price P, Storer NP, Stork L (2014) Movement and survival of corn rootworm in seed mixtures of SmartStax® insect-protected corn. Crop Prot 58:14–24
Hogg DB, Pitre HN, Anderson RE (1982) Assessment of early-season phenology of the fall armyworm (Lepidoptera: Noctuidae) in Mississippi. Environ Entomol 11:705–710
Huang F, Qureshi JA, Meagher RL, Reisig DD, Head GP, Andow DA, Ni X, Kerns D, Buntin GD, Niu Y, Yang F, Dangal V (2014) Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize. PLoS One 9(11):e112958
Jorgensen SE, Chon TS (2009) Model types available for ecological modelling. In: Jorgensen SE, Chon TS, Friedrich R (eds) Handbook of ecological modelling and informatics. WIT Press, Southampton, pp 9–40
Joshi DS (1996) Effect of fluctuating and constant temperatures on development, adult longevity and fecundity in the mosquito Aedes kombeini. J Therm Biol 21:151–154
Labatte JM (1994) Modelling the larval development of Spodoptera frugiperda (J. E. Smith), (Lepidoptera: Noctuidae) on corn. J Appl Entomol 118:172–176
Liebhold A, Bentz B (2011) Insect disturbance and climate change. U.S. Department of Agriculture, Forest Service, Climate Change Resource Center. www.fs.usda.gov/ccrc/topics/insect-disturbance/insect-disturbance. Accessed 14 May 2016
Luginbill P (1928) The fall army worm. U.S. Department of agriculture technical bulletin, vol 34, Washington, pp 1–92
Malaquias JB, Godoy WAC, Garcia AG, Ramalho FS, Omoto C (2017) Larval Dispersal of Spodoptera frugiperda strains on Bt cotton: A model for understanding resistance evolution and consequences for its management. Sci Rep 7:16109. https://doi.org/10.1038/s41598-017-16094-x
Martinelli S, Barata RM, Zucchi MI, Silva-Filho MC, Omoto C (2006) Molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations associated to maize and cotton crops in Brazil. J Econ Entomol 99:519–526
Meagher RL, Mitchell ER (2001) Collection of fall armyworm (Lepidoptera: Noctuidae) using selected pheromone lures and trap designs. J Entomol Sci 36:135–142
Meagher RL, Nagoshi RN, Armstrong JS, Niogret J, Epsky ND, Flanders KL (2013) Captures and host strains of fall armyworm (Lepidoptera: Noctuidae) males in traps baited with different commercial pheromone blends. Fla Entomol 96:729–740
Mitchell M (1999) An introduction to genetic algorithms. MIT Press, Cambridge
NOAA. National Climate Report—2015. https://www.ncdc.noaa.gov. Accessed 14 May 2015
NOAA. National Climate Report—2016. https://www.ncdc.noaa.gov. Accessed 14 May 2016
Omoto C, Bernardi O, Salmeron E, Sorgatto RJ, Dourado PM, Crivellari A, Carvalho RA, Willse A, Martinelli S, Head GP (2016) Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag Sci 72:1727–1736
Padmavathi C, Katti G, Sailaja V, Padmakumari AP, Jhansilakshmi V, Prabhakar M, Prasad YG (2013) Temperature thresholds and thermal requirements for the development of the rice leaf folder, Cnaphalocrocis medinalis. J Insect Sci 13:96
Pair SD, Raulston JR, Rummel DL, Westbrook JK, Wolf WW, Sparks AN, Schuster MF (1987) Development and production of corn earworm and fall armyworm in the Texas high plains: evidence for reverse fall migration. Southwest Entomol 12:89–100
Rao MS, Swathi P, Rao CAR, Rao KV, Raju BMK, Srinivas K, Manimanjari D, Maheswari M (2015) Model and scenario variations in predicted number of generations of Spodoptera litura Fab. on peanut during future climate change scenario. PLoS ONE 10(2):e0116762
Ren J, Wang X, Jin X, Manocha D (2016) Simulating flying insects using dynamics and data-driven noise modeling to generate diverse collective behaviors. PLoS One 11(5):e0155698
Rose AH, Silversides RH, Lindquist OH (1975) Migration fight by an aphid, Rhopalosiphum maidis (Hemiptera: Aphididae) and a noctuid, Spodoptera frugiperda (Lep.: Noctuidae). Can Entomol 107:567–576
Saltelli A, Tarantola S, Campolongo F, Ratto M (2004) Sensitivity analysis in practice: a guide to assessing scientific methods. Wiley, Chichester
Simmons AM (1993) Effects of constant and fluctuating temperatures and humidities on the survival of Spodoptera frugiperda pupae (Lepidoptera: Noctuidae). Fla Entomol 72:333–340
Sisterson MS, Carrière Y, Dennehy TJ, Tabashnik BE (2005) Evolution of resistance to transgenic crops: interaction between insect movement and field distribution. J Econ Entomol 98:1751–1762
Snow JW, Copeland WW (1969) Fall Armyworm: Use of virgin female traps to detect males and to determine seasonal distribution. U.S. Department of agriculture production research report, vol 110, Washington, DC, pp 1–9
Soulsby RL, Thomas JA (2012) Insect population curves: modelling and application to butterfly transect data. Methods Ecol Evol 3:832–841
Sparks AN (1979) A review of the biology of the fall armyworm. Fla Entomol 62:82–87
Tingle FC, Mitchell ER (1979) Spodoptera frugiperda factor affecting pheromone trap catches in corn and peanuts. Environ Entomol 8:989–992
UFWEATHER (2015) It wasn’t just the warmest year EVER in Gainesville. http://ufweather.org. Accessed 13 May 2015
Vélez AM, Spencer TA, Alves AP, Moellenbeck D, Meagher RL, Chirakkal H, Siegfried BD (2013) Inheritance of Cry1F resistance, cross-resistance and frequency of resistant alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae). Bull Entomol Res 103:700–713
Vélez AM, Spencer TA, Alves AP, Crespo ALB, Siegfried BD (2014) Fitness costs of Cry1F resistance in fall armyworm, Spodoptera frugiperda. J Appl Entomol 128:315–325
Vilarinho EC, Fernandes OA, Hunt TE, Caixeta DF (2011) Movement of Spodoptera frugiperda adults (Lepidoptera: Noctuidae) in maize in Brazil. Fla Entomol 94:480–488
Waddill VH, Mitchell ER, Denton WH, Poe SL, Schuster DJ (1982) Seasonal abundance of fall armyworm and velvetbean caterpillar (Lepidoptera: Noctuidae) at four locations in Florida. Fla Entomol 65:350–354
Westbrook JK, Nagoshi RN, Meagher RL, Fleischer SJ, Jairam S (2016) Modeling seasonal migration of fall armyworm moths. Int J Biometeorol 60:255–267
Wild S (2017) African countries mobilize to battle invasive caterpillar. Nature, vol 543, p 13. http://www.nature.com/news/african-countries-mobilize-to-battle-invasive-caterpillar1.21527. Accessed 17 Sept 2017
Wood JR, Poe SL, Leppla NC (1979) Winter survival of fall armyworm pupae in Florida. Environ Entomol 8:249–252
Yadav R, Chang N-T (2014) Effects of temperature on the development and population growth of the melon thrips, Thrips palmi, on eggplant, Solanum melongena. J Insect Sci 14:78
Young JR (1979) Fall armyworm: control with insecticides. Fla Entomol 62:130–133
Funding
AGG holds a fellowship awarded by FAPESP (2015/10640-2, 2014/16609-7) and held a Foreign Research Fellowship awarded by FAPESP (2016/00831-8) during the project execution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals.
Additional information
Communicated by M. Jonsson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garcia, A.G., Ferreira, C.P., Godoy, W.A.C. et al. A computational model to predict the population dynamics of Spodoptera frugiperda. J Pest Sci 92, 429–441 (2019). https://doi.org/10.1007/s10340-018-1051-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-018-1051-4