Abstract
In the present study, the effect of nitrogen, applied as a controlled atmosphere treatment on the microbial and entomological loads, as well as on the organoleptic characteristics of stored dried currants (Corinthian raisins, Vitis vinifera L. var. Apyrena), was investigated. Trials were conducted under “real world” conditions, in the nitrogen chambers of a commercial facility, in which nitrogen was introduced by using an incorporated nitrogen generator. Prior to the initiation of the trial, chambers were filled with pallets carrying dried black currants. Subsequently, currants were artificially infected with all life stages of Tribolium confusum, eggs and larvae of Ephestia elutella and adults of Oryzaephilus surinamensis. Currants were exposed for 3 days in nitrogen (O2 concentration <1 %) at two temperature levels, 25 and 38–43 °C. After treatment, insect mortality was recorded and currant samples were collected and forwarded for microbial analysis and determination of their organoleptic characteristics. When nitrogen was applied at 25 °C, high insect mortality levels were noted; however, in most cases there were a number of insects that survived the nitrogen treatment. In contrast, complete control was achieved at 38–43 °C for all insect species and life stages tested, with the exception of T. confusum larvae. Nitrogen application at 25 °C had no effect on total microbial and yeast and mould counts, while both were reduced at 38–43 °C. Sensory attributes of Corinthian currants remained acceptable after nitrogen fumigation, although taste, odour, aroma and overall acceptance were affected by the treatments. Total phenolic as well as 5-hydroxymethylfurfural content increased after nitrogen application at 38–43 °C, while the lower temperature applied had no effect. The results of the present study suggest that nitrogen-based controlled atmosphere at elevated temperature could be a valuable tool for ensuring clean, pest-free, hygienic standards in dried Corinthian currants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Key message
-
Control was complete for most insect species after N2 fumigation for 3 days at 38–43 °C.
-
Total phenolic content was increased after N2 fumigation for 3 days at 38–43 °C.
-
N2 fumigation had no effect on Corinthian currant appearance and texture.
-
Taste, odour, aroma and general acceptance were affected by N2 fumigation.
-
N2 fumigation for 3 days at 38–43 °C reduced the total microbial and yeast and mould counts.
Introduction
The use of controlled and modified atmospheres (CA or MA, respectively) is a promising alternative to conventional chemical pesticides for the disinfection and disinfestation of agricultural products (Adler et al. 2000; Banks and Annis 1990; Fleurat-Lessard 1990; Navarro 2012). Taking into account the mounting public awareness about the adverse effects of pesticide residues in food, as well as the environmental concerns about the use of chemical pesticides, the implementation of this technology by the food industry could offer a viable means for securing foodstuff quality during storage. The term controlled atmosphere refers to a modified gas composition in the treated container, produced artificially by the introduction of gases (N2, CO2), in order to either reduce the oxygen level or increase the CO2 concentration (Navarro 2012). The idea of using controlled atmospheres to deal with post-harvest infections and infestations is based on the fact that insects, as well as many microbes, are aerobic organisms that require oxygen for their survival. Therefore, modifying the atmosphere composition within the treated enclosure has a detrimental effect on their development and survival (Butler 2013; Harrison et al. 2006; Hoback and Stanley 2001). The application of controlled atmospheres in storage environment meets several advantages, as it is a non-toxic, environmentally-friendly and residue-free process. Moreover, the mode of action of controlled atmospheres through physiological limitation of respiration renders the development of resistance to this technique by the target organisms highly improbable.
To create a low-oxygen atmosphere, usually nitrogen (N2) is used (Adler et al. 2000; Navarro 2012); however, other gases, such as helium and argon, have been also successfully tested (Ali Niazee 1972; Lindgren and Vincent 1970). Previous studies with nitrogen controlled atmosphere have showed promising results against storage insects (Bell et al. 1980; Banks and Annis 1997; Donahaye et al. 1996; Ofuya and Reichmuth 1993, 1994, 2002; Navarro 1978; Tunc and Navarro 1983; Hashem et al. 2014). For instance, Ofuya and Reichmuth (1993) evaluated the effect of pure N2 atmosphere (100 % N2) on all life stages of the cowpea seed bruchid, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae), and the bean bruchid, Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae), and reported complete control of all life stages of both bruchid species within 1–9 days of exposure. Early studies have shown that insects can tolerate low-oxygen levels for long exposure intervals (Bailey 1955, 1956, 1957); therefore, oxygen levels lower than 2 % should be achieved (Adler et al. 2000; Banks and Annis 1990; Fleurat-Lessard 1990; Navarro 1978).
Controlled atmospheres as well as modified atmosphere packaging (MAP) may extend the shelf life of several foods in terms of microbial spoilage prevention or restriction (Sandhya 2010). In MAP, inhibition of micro-organisms may be due to direct toxic effects resulting in growth and proliferation inhibition, as in the case of carbon dioxide, or due to indirect inhibitory effects, as in the case of nitrogen. Nitrogen is typically used as an oxygen displacer either alone or in combination with other gases; the gas composition modification alters the ecology of the microbial environment. On this basis, nitrogen has been shown to inhibit the growth of aerobic microbes when applied in MAP (Sandhya 2010). The potential use of nitrogen gas under CA or MAP has been investigated in the case of fresh vegetables and fruits (Ayhan and Esturk 2009; Chara et al. 2012; Koseki and Itoh 2002; Tomás-Callejas et al. 2011) as well as in the case of other food products such as milk (Munsch-Alatossava et al. 2010, 2013), with promising results in several cases. Nevertheless, atmospheres exerting a negative effect on the growth of a particular micro-organism may act as promoters for some others. For example, a high concentration of nitrogen (82 %) or argon and absence of oxygen increased the growth of Lactobacilli species in chicken breast fillets, while at the same time Pseudomonas spp. showed a stable growth (Herbert et al. 2013).
Currants (Corinthian raisins) are nutrient-dense dried fruits that are produced from a grape vine variety, i.e. Vitis vinifera L. var. Apyrena. Southern Greece produces much of the world’s supply of currants, continuing a tradition of cultivation in this region for hundreds of years (Chiou et al. 2007). After harvest, grapes are naturally sun-dried in outdoor drying racks. Corinthian currants have high nutritional content, and they are a natural source of hydrophilic antioxidants; therefore, they are considered a food with health promoting properties (Chiou et al. 2007, 2014; Kaliora et al. 2008, 2009; Vasilopoulou and Trichopoulou 2014).
Although the method of controlled atmospheres is well established and its efficacy is well documented, its commercial use for the control of insects and microbes in storage facilities is still limited. Most of the studies showing the efficacy of controlled atmospheres against major stored-product insects in different commodities refer to laboratory bioassays, whereas published information on large-scale nitrogen applications against storage insects is limited. Moreover, no study has been conducted so far to evaluate the effect of nitrogen treatment on currants or Corinthian raisins. Therefore, the objectives of the present study were: (1) to determine the effect of nitrogen-based controlled atmosphere to the microbial flora of currants, (2) to study the effect of nitrogen-based controlled atmosphere on the organoleptic characteristics, antioxidant content and Maillard intermediate reaction products of currants and (3) to study the effect of nitrogen-based controlled atmosphere for the control of major stored-product insects, such as the cacao moth, Ephestia elutella Hübner (Lepidoptera: Pyralidae), the sawtoothed grain beetle, Oryzaephilus surinamensis (L.) (Coleoptera, Sylvanidae), and the confused flour beetle, Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae).
Materials and methods
Test insects and commodity
All insect species used in these trials were reared at the Laboratory of Entomology and Agricultural Zoology, Department of Agriculture, Crop Production and Rural Environment, University of Thessaly, at 26 °C, 65 % relative humidity (r.h.) and continuous darkness. From the species tested, T. confusum and O. surinamensis were reared on wheat flour and oat flakes, respectively, whereas E. elutella was reared on whole meal wheat flour with 5 % yeast (by weight). For both beetle species, adults <1 month old were used in the tests. In the case of T. confusum eggs, larvae and pupae were also used for experimentation, whereas for E. elutella, eggs and larvae were tested. All eggs were 1–4 days old, larvae were <7 days old, and pupae were <3 days old.
Untreated and clean Vostizza currants (V. vinifera L. var. Apyrena) were used in the trials. Vostizza currant is a Protected Designation of Origin (PDO) subvariety produced in the area of Aegion, Greece. Currants were packed in 10-kg carton boxes and were taken from the 2013 Greek harvest. Currants were inspected for the presence of insects before being used for the experiments and were found to be free of adult or larval stages of insects.
Nitrogen treatment
The trials were conducted in a commercial facility (AgroSpeCom L.T.D., Inofyta, Voiotia, Greece) between January and March 2015. Two sets of trials were conducted inside nitrogen chambers (237.6 m3), which were sealed and in which nitrogen was introduced by using an incorporated nitrogen generator. High purity nitrogen (99.1 % N2 and 0.9 % O2) was produced from air through a pressure swing adsorption (PSA) process and pumped inside the chambers at a maximum flow rate of 35 m3/h. Temperature and oxygen level in the nitrogen chambers were recorded throughout the trials. Each chamber was filled with eight pallets carrying boxes of packed currants (each pallet contained 64 boxes of 10 kg each).
Plastic cylindrical vials (2.5 cm in diameter, 9 cm in height) were the experimental units for the trials. The vials were perforated in the upper, lower and middle part, and the holes were covered with a US #40 fine mesh screen (0.42 mm openings). The day before nitrogen application all life stages of T. confusum (eggs, larvae, pupae and adults), adults of O. surinamensis, as well as eggs and larvae of E. elutella were taken from the cultures and ten individuals from each insect species and life stage were placed in vials (different vials for each insect species and life stage). In each vial, there were small quantities of flour to allow feeding of the exposed individuals. Vials with insects were placed in two pallets in each chamber (P1 and P2), whereas in each pallet vials were placed in five locations (Fig. 1). Briefly, vials were placed above and under the pallet (L1 and L5), as well as inside the commodity (L2, L3 and L4) (Fig. 1). In order to place the vials inside the product, carton boxes were opened, half quantity of the currants was removed and vials were placed inside the box. Afterwards, vials were covered with the removed currants and the boxes were closed again with sticky tape. There were two vial replicates for each insect species and life stage in each test location. A separate series of six vials from each insect species and life stage was placed outside of the chamber and served as control.
In the first trial, insects were exposed for 3 days to nitrogen atmosphere (O2 level <1 %) at 25 °C. Similarly, in the second trial insects were exposed for 3 days to nitrogen with temperature ranging between 38 and 43 °C. Each trial was repeated three times. After the termination of the procedure, each nitrogen chamber was opened and all vials were transferred to the laboratory for counting of the surviving individuals. Larvae and adults were counted on the next day, while pupae and eggs were placed for 7 days at 26 °C, 55 % r.h. and continuous darkness, to accelerate hatching/emergence. After these 7 days, the vials were opened and all individuals were classified as alive or dead.
At the termination of each trial, samples of currants (approximately 200 g each) were taken for determination of the organoleptic characteristics and analysis of microbial content and phenolic antioxidants. Samples were always taken in duplicate. One of the samples was forwarded for microbial content analysis and the other one for analysis of the organoleptic characteristics and phenolic content of currants. Two untreated samples of currants (approximately 200 g each) served as controls. Samples were stored at room temperature. All analyses were carried out within 1 month.
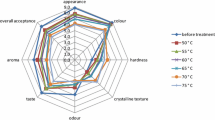
Corinthian currant organoleptic characteristics were evaluated, before and after nitrogen treatments, by ten trained panellists; appearance, texture (hardness, crystalline texture), taste, aroma, odour and general acceptance of the product were assessed. Sensory attributes were evaluated by hedonic sensory tests and rating tests, on which the sensory panel had been trained. Samples were evaluated by using a nine-point hedonic scale, including point 1 (dislike extremely), 5 (neither like nor dislike) and 9 (like extremely). The product was considered acceptable when rating score was above 5 (Mestdagh et al. 2008). Τhe intensity of sensory attributes was evaluated by a 1–9 rating scale, where 1 was defined as “very slightly/not at all detectable” and 9 as “extremely detectable”.
5-Hydroxymethylfurfural (HMF) content was determined after extracting mechanically homogenized currant samples (approximately 1 g) with methanol (2 mL) (Murkovic and Pichler 2006). For the analysis, an HPLC system (Agilent Technologies, model 1050, Waldbronn, Germany) combined with quaternary pump, auto-sampler, diode array detector (HP-1050), and data analysis software was used. RP-HPLC analysis was performed on a Purospher STAR, RP-18 endcapped (250 × 4.6 mm, 5 µm) column (Merck, Darmstadt, Germany) as previously described (Athanassiou et al. 2016).
Phenolic content was analysed as previously described (Chiou et al. 2014); mechanically homogenized currants (approx. 1.5 g) were extracted with methanol/HCl (0.1 % v/v, 4 × 5 mL). Total phenolic content was determined spectrophotometrically (Arnous et al. 2002). All data were acquired using a Specord 200 (Analytik Jena AG, Germany) UV–Vis spectrophotometer. Absorbance was read at 750 nm; results were expressed as gallic acid equivalents (GAE)/100 g currants.
Microbial analyses were performed after preparing appropriate currants dilutions (10−1, 10−2, 10−3) in peptone water; sample homogenization was carried out by Bag Mixer (Interscience, Saint Nom, France) for at least 2 min. Yeasts and moulds determination and total viable count were performed by the pour plate count on Dichloran-Glycerol Agar Base (DG18 CM729 Oxoid, Hampshire, England) and Standard Plate Count Agar (APHA Oxoid CM0463B, Hampshire, England), respectively. Plating was performed in triplicates; the enumeration was based on the average. For yeasts and moulds incubation was conducted at 25 °C for 96–144 h; for total viable counts incubation was conducted at 30 °C for 48 h. Microbial counts were expressed as total number of micro-organisms/g of sample (CFU/g = colonies forming units multiplied by dilution factor).
Data analysis
Control mortality was lower than 13 % for T. confusum adults and pupae, O. surinamensis adults and E. elutella larvae, while control mortalities for T. confusum eggs and larvae, as well as for E. elutella eggs were higher (ranged between 25 and 82 %). All mortality data from nitrogen-treated insects, with the exception of T. confusum and E. elutella eggs for which control was complete in all cases, were submitted, separately for each insect species and life stage, to a three-way analysis of variance (ANOVA), with mortality counts as the response variable and temperature, pallet and location as the main effects with the JMP 7 software (SAS Institute Inc., Cary, NC, USA) to indicate if there were differences among treatments. Student’s t test was performed to compare insect mortalities, separately for each insect species and life stage, obtained at 25 and 38–43 °C (P < 0.05) (Zar 1999). In the case of organoleptic characteristics, HMF, phenolic antioxidants and microbial content, results presented are the average of the obtained values. Data handling was carried out using Microsoft Excel and statistical analysis using SPSS (SPSS 20.0 for Windows, Chicago, IL, USA). Organoleptic characteristics were analysed with Kruskal–Wallis test (Mann–Whitney test for post hoc comparisons); statistical significance level was set at P < 0.05. For all other parameters studied, one-way ANOVA was applied; Tukey’s multiple range tests were performed post hoc to evaluate differences among groups; statistical significance level was set at P < 0.05.
Results
Insect mortality
Mortality counts of T. confusum adults were significantly affected by all main effects (temperature, pallet and location) and most associated interactions (Table 1). Specifically, for T. confusum adults complete control (100 %) was achieved at elevated temperature (38–43 °C), whereas at 25 °C average mortality did not exceed 83 % (Table 2). For the same species and stage, significant differences in mortality were recorded among the different locations tested at 25 °C (Table 3). Specifically, the higher mortality levels were observed in L1 (97 %) and L5 (98.9 %) (above and under the pallet, respectively), and were significantly higher than L3 (69.2 %) and L4 (63.3 %), which were both inside the commodity (Table 3). Similarly, for O. surinamensis adult mortality was significantly affected by temperature and location, but not by pallet and most associated interactions (Table 1), whereas mortality reached 93.6 and 100 % at 25 and 38–43 °C, respectively (Table 2). Control of O. surinamensis adults was complete in three locations (L1, L2 and L5) and significantly higher than L3 and L4 (Table 3). For the rest of the insect species and stages, mortality levels were not significantly affected by most main effects and associated interactions (Table 1). In the case of T. confusum and E. elutella eggs, complete control (100 %) was achieved in both trials (Table 2). Significant differences were recorded between the two trials for T. confusum pupae, for which complete control was achieved at elevated temperature (38–43 °C), whereas at 25 °C, mortality levels reached 89.8 % (Table 2). Finally, in the case of T. confusum and E. elutella larvae higher mortality levels were obtained at elevated temperature; however, differences between the two trials were not statistically significant (Table 2).
Microbial analysis
Total microbial and yeast and mould counts before and after nitrogen-based fumigation are given in Table 4. In this table, the effect of temperature is presented, given that neither pallet nor location affected the results obtained. A 1.3-log reduction of yeasts and moulds was observed after treatment at 38–43 °C, while total viable count was less affected presenting an approximately 0.4-log reduction. Nitrogen-based controlled atmosphere at 25 °C had no statistically significant effect on either yeasts and moulds or total viable counts.
Organoleptic characteristics
Panellists evaluated the appearance, texture, i.e. hardness and crystalline texture, colour, odour, taste and aroma of currants before and after nitrogen controlled atmosphere application by using a nine-point rating scale (Fig. 2). Nitrogen-based controlled atmosphere fumigation at 25 and 38–43 °C for 3 days did not statistically affect all the sensory attributes evaluated as compared with the control, i.e. untreated currants; among the characteristics studied, taste, odour, aroma and overall acceptance were statistically lower for samples treated under modified atmospheres.
Phenolic compounds and 5-hydroxymethylfurfural
Total phenolic content of the untreated currants was 216 ± 18 mg GAE/100 g (n = 6). After nitrogen controlled atmosphere application currant phenolic content found was 196 ± 16 mg GAE/100 g (n = 6) and 249 ± 40 mg GAE/100 g (n = 12) for trials at 25 and 38–43 °C, respectively. Pair comparison before and after nitrogen application revealed statistically significant differences among trials, with samples treated at 38–43 °C having higher total phenolic content.
Untreated currant HMF content was 14.5 ± 6.9 mg/kg (n = 6). After nitrogen application at 25 and 38–43 °C the respective values were 16.9 ± 2.5 mg/kg (n = 6) and 37.9 ± 5.8 mg/kg (n = 12). The latter value was statistically significantly higher than that of untreated currants as well as of currants treated at 25 °C.
Discussion
The use of nitrogen controlled atmospheres for the control of post-harvest infestations and infections of stored agricultural products has been investigated by many researchers in the past (Banks and Annis 1997; Donahaye et al. 1996; Ofuya and Reichmuth 1993, 1994, 2002; Tunc and Navarro 1983). However, most of the studies have focused on the effect of the method on the sanitary quality of low moisture content durable commodities, such as cereals and legumes. To our knowledge, this is the first study that describes the effect of nitrogen-based controlled atmosphere on the storage insect pests of dried Corinthian currants. Based on our results, nitrogen treatment was highly effective against stored-product insects, at least for the environmental conditions, insect species and life stages tested here. In our study, we used O. surinamensis, E. elutella and T. confusum, as these species are commonly encountered in currant warehouses in Greece, but also in other similar durable commodities (Athanassiou and Eliopoulos 2003, 2004; Buchelos 1980), and high mortality levels were recorded for all three species and life stages. Although insects were kept in vials during the bioassays and were not released inside the commodity, we consider that our experimental design accurately simulates real conditions in storage facilities.
Many studies have highlighted the effect of temperature on nitrogen-based controlled atmosphere fumigation (Chiappini et al. 2009; Donahaye et al. 1994; Soderstrom et al. 1992). All studies suggest that insect mortality is higher at raised temperatures, and have attributed this effect to the speed-up of insect metabolism at higher temperatures (Banks and Fields 1995). For instance, Soderstrom et al. (1992) reported that high temperature (>38 °C) combined with nitrogen-based controlled atmosphere increased mortality of larvae of the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) and reduced treatment duration. More recently, Chiappini et al. (2009) treated T. confusum adults at various O2 percentages (1–10 %) and temperatures (23–40 °C) and found a negative correlation between the exposure interval and temperature, i.e. that the higher the temperature, the shorter the exposure interval necessary to obtain total insect mortality. A similar effect had been identified in high CO2 controlled atmospheres, where complete control of a range of storage insects has been accomplished after 1–2 days of exposure at temperatures higher than 38 °C (Jay 1986). The results of our study are in accordance with these findings. When nitrogen application was combined with high temperature levels (38–43 °C), complete control (100 %) was achieved for all insect species and life stages tested, except for T. confusum larvae. However, even in this case, mortality of T. confusum larvae was considerably high, indicating that T. confusum larvae are also highly susceptible to low-oxygen atmosphere and could have been completely controlled with longer exposure intervals. Moreover, the complete control that was achieved for T. confusum eggs and pupae, which are considered to be tolerant to stress caused by changes to oxygen level, is indicative of the high efficacy of nitrogen controlled atmosphere at raised temperature. However, what should not be overlooked when designing a disinfestation strategy that involves raised temperatures is the fact that insect thermo-tolerance varies among species, life stage, etc. (Fields et al. 2012). For instance, LT99 value for young larvae of T. castaneum reached 433 min at 50 °C (Mahroof et al. 2003), whereas 99 % mortality of old larvae of T. confusum and P. interpuncella was achieved after 90 (Boina and Subramanyam 2004) and 34 min (Mahroof and Subramanyam 2006), respectively. Therefore, depending on the range of the insect species present in a storage facility, the temperature requirements during a nitrogen application may differ.
A drawback of the nitrogen treatment at raised temperatures is the cost of heating the commodities, which can represent a considerable energy cost, especially in northern countries where low temperatures prevail. However, the increased cost will be alleviated by the reduction in treatment time needed. When treating food commodities under high temperatures, concerns may rise over the influence of temperature on the shelf life of the product and its qualitative characteristics. Nitrogen-based controlled atmosphere fumigation had no effect on Corinthian currant appearance and texture (hardness, crystalline texture). Taste, odour, aroma and general acceptance were, however, affected by the treatment. Our results are in line with those of Guarrasi et al. (2014) and Shamaila et al. (1992), where apple and strawberry sensory quality were affected under MAP as compared with air storage.
HMF is an intermediate Maillard reaction product that is formed by the dehydration of sugars under acidic conditions (Capuano and Fogliano 2011). Although HMF can be formed at ambient temperature, thermal processes are known to drastically increase HMF content (Capuano and Fogliano 2011). In this context, Corinthian currant HMF content was assessed before and after N2 fumigation treatments. Untreated currant HMF content found in the present study was 14.5 ± 6.9 mg/kg, being in the rather lower limit of dried fruit HMF content reported, i.e. 1–2900 mg/kg (Capuano and Fogliano 2011; Karadeniz et al. 2000; Murkovic and Pichler 2006). Corinthian currant HMF value was also within the ranges (3.6–55.0 mg/kg) reported for dried vine products such as sultanas or Thompson Seedless raisins (Çaglarirmak 2006; Karadeniz et al. 2000; Şevik et al. 2014). Nitrogen application at 25 °C had no effect on the product HMF content, while the content significantly increased at 38–43 °C. In the study of Frank et al. (2004), a significant increase in HMF concentration was observed after storage of V. vinifera L. cv. Sultana (Thompson Seedless) raisins for 14 months at 30 °C as compared with storage at 10 °C, hence supporting the present findings. Noteworthy, the HMF value at 38–43 °C, i.e. 37.9 ± 5.8 mg/kg, still remained under the reported ranges for raisin HMF content cited above. Controlled atmosphere treatment for 3 days did not seem to affect HMF formation given that at ambient temperature (treatment at 25 °C) HMF content was practically the same as that of the untreated currants. Therefore, the increase in HMF content observed at 38–43 °C could be attributed rather solely to the temperature increase.
Polar phenolic compounds are ubiquitously present in the plant kingdom. The in vitro ability of phenolics to scavenge free radicals is well established, while evidence from epidemiological and clinical intervention studies is emerging with respect to their protective effects against degenerative diseases, including cancer and cardiovascular diseases (Del Rio et al. 2013). Corinthian currants have been found to contain several simple phenol species and anthocyanins, their total phenolic content being in the range of 150–395 mg GAE/100 g (Chiou et al. 2007, 2014). An increase in the total phenolic content was found after N2 fumigation at 38–43 °C; this finding is in accordance with the findings of Frank et al. (2004) that also reported an apparent increase in sultana total phenolic content after storage at 30 °C. Such an increase has been attributed to reductones formed via Maillard reactions that together with other species may interfere with the Folin–Ciocalteu assay (Singleton et al. 1985). Additionally, Lee et al. (2003) reported that heat treatment may liberate and activate several low molecular weight natural antioxidants, found in bound forms.
Corinthian currants are usually consumed raw, as snacks; alongside, they are used in bakery and confectionary formulations. In this context, the microbial load is rather of insignificance; however, it becomes crucial under the perspective of expanding currant uses in other edible products, such as dairy products. Nitrogen-based fumigation for 3 days at 25 °C did not affect total microbial and yeast and mould counts. Data on the effect of N2 atmosphere on the microbial load of dried fruits are scarce. N2 has been used under MAP for fresh fruits and vegetables preservation with rather contradictory results. The aerobic mesophilic growth on fresh-cut lettuce and cabbage for 5 days at 5 °C and on red chard baby leaves for 6 days at 5 °C was delayed under N2-enriched MAP, however, analogously to that of passive MAP (Koseki and Itoh 2002; Tomás-Callejas et al. 2011). In ready-to-eat pomegranate arils, the aerobic mesophilic bacteria remained unaltered after 9 days of storage under N2 atmosphere, while an increase was observed at the end of storage, i.e. 18 days (Ayhan and Esturk 2009). In ready-to-eat arugula salads, N2-enriched atmospheres combined with H2O2 resulted in a reduction in psychrotrophic bacterial load, total mesophilic count and Enterobacteriaceae population after 8 days of storage (Chara et al. 2012). On the contrary, nitrogen was proved ineffective in extending carrot juice shelf life under MAP (Alklint et al. 2004). In our study, a reduction of yeasts and moulds together with a weak effect on total viable counts was observed after treatment at higher temperature, i.e. 38–43 °C. Dried fruits, including raisins, are frequently affected by fungi from Aspergillus and Penicillium genera (El Halouat and Debevere 1997; Hakobyan 2014) while the osmotolerant Zygosaccharomyces species are the main spoilage yeasts in food with low aw (El Halouat and Debevere 1996). Most yeasts and moulds are heat-sensitive, being destroyed by treatments at temperatures higher than 60 °C (Jermini and Schimidt-Lorenz 1987). In fact, few Penicillium spp. have been reported to grow above 37 °C (Kushner et al. 1979). El Halouat and Debevere (1997) evaluated the effect of water activity, MAP and temperature on the conidial germination of Aspergillus niger, Eurotium amstelodami, Penicillium chrysogenum and Fusarium oxysporum isolated from prunes; P. chrysogenum and F. oxysporum were found incapable to germinate and grow at 40 °C, while A. niger germinated and grew better at 30 and 40 °C. On this basis, perhaps the present finding is attributed to the combination of temperatures higher than the ambient with the N2 environment, although this is a matter of further investigation.
A matter of concern when working with gases is the ability of the gas to overcome any packaging material and penetrate deep enough in the commodity, in order to efficiently kill insects and microbes. For example, ozone, a powerful oxidant that is also used to control insects and fungi in stored grains, rapidly degrades as it moves through the grains and interacts with the grain surface for the first time (Kells et al. 2001; Mendez et al. 2003). At the same time, ozone has poor ovicidal effect (Isikber and Athanassiou 2015). Similar reports have been published for other gases, such as sulfuryl fluoride (Athanassiou et al. 2012; Jagadeesan et al. 2015). The results of the current study showed that nitrogen has a very good ovicidal effect and that, for the species tested, egg was also susceptible to nitrogen. In our study, in order to investigate the penetration ability of nitrogen in the dried currants, vials with insects were placed in various locations inside the nitrogen chamber and in the pallet, i.e. outside but also inside the commodity. At elevated temperature, insect mortality was high in all locations, even in vials placed inside the commodity, where air flow is restricted and oxygen can be easily trapped and create localized limited “oxygen nests”. This finding indicates that the reduction in oxygen concentration at the aforementioned conditions was adequate for insect control also inside the commodity and the effect of nitrogen is not location-specific. Similar results, for various exposure intervals have been reported by other researchers, as well as with different experimental protocols (Banks and Annis 1990, 1997; Jay 1986; Navarro 2006). In contrast, at 25 °C, location significantly affected the mortality levels in the case of T. confusum and O. surinamensis adults, for which control was high in locations outside the treated commodity, but was significantly reduced in locations inside the commodity.
To conclude, nitrogen treatment was highly effective for the range of species examined in this study, at least for the conditions tested. Temperature was identified as one of the key elements that affects the performance of the method, as the increase in temperature resulted in the increase in mortality and the complete control of the insects in most cases. In general, the application of nitrogen against insects was considered as a slow-acting method that usually required long exposure intervals (Navarro et al. 2012). Our study illustrates that by using elevated temperatures, nitrogen can be effective at short intervals (3 days), without any effect on the commodity. This is particularly important, as efficacy at these intervals is usually not achievable by other gases, such as phosphine. Finally, it was shown that nitrogen is remarkably penetrative inside the commodity, i.e. currants, as the efficacy of the method was similar against insects placed inside or outside the commodity.
Author contributions
CGA, AC and VK conceived and designed research. CGA, AC, CIR, SV, MS, EKN, EAP, AK and EK conducted experiments. CGA, AC, CIR and VK analysed data. CGA, AC, CIR and VK contributed to writing the paper. All authors reviewed and approved the final manuscript.
References
Adler C, Corinth HG, Reichmuth C (2000) Modified atmospheres. In: Subramanyam B, Hagstrum DW (eds) Alternatives to pesticides in stored-product IPM. Kluwer, Boston, pp 105–146
Ali Niazee MT (1972) Susceptibility of the confused and red flour beetles to anoxia produced by helium and nitrogen in various temperatures. J Econ Entomol 65:60–64
Alklint C, Wadso L, Sjoholm I (2004) Effects of modified atmosphere on shelf-life of carrot juice. Food Control 15:131–137
Arnous A, Makris DP, Kefalas P (2002) Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J Food Compos Anal 15:655–665
Athanassiou CG, Eliopoulos PA (2003) Seasonal abundance of insect pests and their parasitoids in stored currants. IOBC WPRS Bull 26:283–291
Athanassiou CG, Eliopoulos PA (2004) Occurrence of stored-product pyralid moths and their parasitoids in stored currant and in vineyards. IOBC WPRS Bull 27:181–190
Athanassiou CG, Phillips TW, Aikins MJ, Hasan MM, Throne JE (2012) Effectiveness of sulfuryl fluoride for control of different life stages of stored-product psocids (Psocoptera). J Econ Entomol 105:282–287
Athanassiou CG, Chiou A, Rumbos CI, Karagiannis A, Nikolidaki E, Panagopoulou E, Kouvelas A, Karathanos VT (2016) Effects of electric infrared heating with light source penetration on microbial and entomological loads on dried currants and their organoleptic characteristics. J Pest Sci. doi:10.1007/s10340-015-0727-2
Ayhan Z, Esturk O (2009) Overall quality and shelf life of minimally processed and modified atmosphere packaged “ready-to-eat” pomegranate arils. J Food Sci 74:399–405
Bailey SW (1955) Air-tight storage of grain: its effect on insect pests. I. Calandra granaria L. (Coleoptera, Curculionidae). Aust J Agric Res 6:33–51
Bailey SW (1956) Airtight storage of grain—its effects on insect pests. II. Calandra oryzae (small strain). Aust J Agric Res 7:7–19
Bailey SW (1957) Airtight storage of grain—its effects on insect pests. III. Calandra oryzae. Aust J Agric Res 8:595–603
Banks HJ, Annis PC (1990) Comparative advantages of high CO2 and low O2 types of controlled atmospheres for grain storage. In: Calderon M, Barkai-Golan R (eds) Food preservation by modified atmospheres. CRC Press, Boca Raton, pp 93–122
Banks HJ, Annis PC (1997) Purging grain bulks with nitrogen. In: Donahaye EJ, Navarro S, Varnava A (eds) Proceedings of the international conference on controlled atmospheres and fumigation in stored products. Printco, Nicosia, pp 273–285
Banks J, Fields P (1995) Physical methods for insect control in stored-grain ecosystems. In: Jayas DS, White NDG, Muir WE (eds) Stored Grain ecosystems. Marcel Dekker, New York, pp 353–409
Bell CH, Spratt EC, Mitchell DJ (1980) The effect of nitrogen and carbon dioxide on eggs of Ephestia cautella (Walker) and E. kuehniella Zeller (Lepidoptera: Pyralidae). Bull Entomol Res 70:293–298
Boina D, Subramanyam Bh (2004) Relative susceptibility of Tribolium confusum (Jacquelin du Val) life stages to elevated temperatures. J Econ Entomol 97:2168–2173
Buchelos CT (1980) Moth populations at a typical flour mill. Ann Benaki Phytopathol Inst 12:188–197
Butler G (2013) Hypoxia and gene expression in eukaryotic microbes. Annu Rev Microbiol 67:291–312
Çaglarirmak N (2006) Ochratoxin A, hydroxymethylfurfural and vitamin C levels of sun-dried grapes and sultanas. J Food Process Preserv 30:549–562
Capuano E, Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci Technol 44:793–810
Chara C, Silveira AC, Inestroza-Lizardo C, Hinojosa A, Machucaa A, Escalona VH (2012) Effect of noble gas-enriched atmospheres on the overall quality of ready-to-eat arugula salads. Postharvest Biol Technol 73:50–55
Chiappini E, Molinari P, Cravedi P (2009) Mortality of Tribolium confusum J. du Val (Coleoptera: Tenebrionidae) in controlled atmospheres at different oxygen percentages. J Stored Prod Res 45:10–13
Chiou A, Karathanos VT, Mylona A, Salta FN, Preventi F, Andrikopoulos NK (2007) Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem 102:516–522
Chiou A, Panagopoulou EA, Gatzali F, De Marchi S, Karathanos VT (2014) Anthocyanins content and antioxidant capacity of Corinthian currants (Vitis vinifera L., var. Apyrena). Food Chem 146:157–165
Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A (2013) Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18:1818–1892
Donahaye E, Navarro S, Rindner M (1994) The influence of temperature on the sensitivity of two nitidulid beetles to low oxygen concentrations. In: Highley HE, Wright EJ, Banks HJ, Champ BR (eds) Proceedings of 6th international working conference on stored-product protection. CAB International, Wallingford, pp 88–90
Donahaye EJ, Navarro S, Rindner M, Azrieli A (1996) The combined influence of temperature and modified atmospheres on Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Stored Prod Res 32:225–232
El Halouat A, Debevere JM (1996) Influence of modified atmosphere and preservatives on the growth of Zygosaccharomyces rouxii isolated from dried fruits. Int J Food Microbiol 33:219–229
El Halouat A, Debevere JM (1997) Effect of water activity, modified atmosphere packaging and storage temperature on spore germination of moulds isolated and from prunes. Int J Food Microbiol 35:41–48
Fields PG, Subramanyam B, Hulasare R (2012) Extreme temperatures. In: Hagstrum DW, Phillips TW, Cuperus G (eds) Stored product protection. Kansas State University, Manhattan, p 351
Fleurat-Lessard F (1990) Effect of modified atmospheres on insects and mites infesting stored products. In: Calderon M, Barkai-Golan R (eds) Food preservation by modified atmospheres. CRC Press, Boca Raton, pp 21–38
Frank D, Gould I, Millikan M (2004) Browning reactions during storage of low-moisture Australian sultanas: evidence for arginine-mediated Maillard reactions. Aust J Grape Wine Res 10:151–163
Guarrasi V, Giacomazza D, Germanà MA, Amenta M, San Biagio PL (2014) Monitoring the shelf-life of minimally processed fresh-cut apple slices by physical–chemical analysis and electronic nose. Agrotechnology 3:126–129
Hakobyan LL (2014) Fungi species from penicillium genera in raisins consumed in Armenia. Biol J Armen 3:49–53
Harrison J, Frazier MR, Henry JR, Kaiser A, Klok CJ, Rascón B (2006) Responses of terrestrial insects to hypoxia or hyperoxia. Resp Physiol Neurobiol 154:4–17
Hashem MY, Ahmed SS, El-Mohandes MA, Hussain ARE, Ghazy SM (2014) Comparative effectiveness of different modified atmospheres enriched with carbon dioxide and nitrogen on larval instars of almond moth Ephestia cautella (Walker) (Lepidoptera: Pyralidae). J Stored Prod Res 59:314–319
Herbert U, Rossaint S, Khanna M-A, Kreyenschmidt J (2013) Comparison of argon-based and nitrogen-based modified atmosphere packaging on bacterial growth and product quality of chicken breast fillets. Poult Sci 92:1348–1356
Hoback WW, Stanley DW (2001) Insects in hypoxia. J Insect Physiol 47:533–542
Isikber AA, Athanassiou CG (2015) The use of ozone gas for the control of insects and micro-organisms in stored products. J Stored Prod Res 64:139–145
Jagadeesan R, Nayak MK, Pavic H, Chandra K, Collins PJ (2015) Susceptibility to sulfuryl fluoride and lack of cross-resistance to phosphine in developmental stages of the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Pest Manag Sci 71:1379–1386
Jay EG (1986) Factors affecting the use of carbon dioxide for treating raw and processed agricultural products. In: GASGA seminar on fumigation technology in developing countries. Tropical Development and Research Institute, London, pp 173–189
Jermini M, Schimidt-Lorenz W (1987) Heat resistance of vegetative cells and asci of two Zygosaccharomyces yeasts in broths at different water activity values. J Food Prot 50:835–841
Kaliora AC, Kountouri AM, Karathanos VT, Koumbi L, Papadopoulos NG, Andrikopoulos NK (2008) Effect of Greek raisins (Vitis vinifera L.) from different origins on gastric cancer cell growth. Nutr Cancer 60:792–799
Kaliora AC, Kountouri AM, Karathanos VT (2009) Antioxidant properties of raisins (Vitis vinifera L.). J Med Food 12:1302–1309
Karadeniz F, Durst RW, Wrolstad RE (2000) Polyphenolic composition of raisins. J Agric Food Chem 48:5343–5350
Kells S, Mason LJ, Maier DE, Woloshuk CP (2001) Efficacy and fumigation characteristics of ozone in stored maize. J Stored Prod Res 37:371–382
Koseki S, Itoh K (2002) Effect of nitrogen gas packaging on the quality and microbial growth of fresh-cut vegetables under low temperatures. J Food Prot 65:326–332
Kushner L, Rosenzweig WD, Stotzky G (1979) Effects of salts, sugars, and salt-sugar combinations on growth and sporulation of isolates of Eurotium rubrum from pan-cake syrup. J Food Prot 41:706–711
Lee S-C, Kim J-H, Jeong S-M, Kim D-R, Ha J-U, Nam KC, Ahn DU (2003) Effect of far-infrared radiation on the antioxidant activity of rice hulls. J Agric Food Chem 51:4400–4403
Lindgren DL, Vincent LE (1970) Effect of atmospheric gases alone or in combination on the mortality of granary and rice weevils. J Econ Entomol 63:1926–1929
Mahroof R, Subramanyam Bh (2006) Susceptibility of Plodia interpunctella (Lepidoptera: Pyralidae) developmental stages to high temperatures used during structural heat treatments. Bull Entomol Res 96:539–545
Mahroof R, Subramanyam Bh, Throne JE, Menon A (2003) Time-mortality relationships for Tribolium castaneum (Coleoptera: Tenebrionidae) life stages exposed to elevated temperatures. J Econ Entomol 96:1345–1351
Mendez F, Maier DE, Mason LJ, Woloshuk CP (2003) Penetration of ozone into columns of stored grains and effects on chemical composition and processing performance. J Stored Prod Res 39:33–44
Mestdagh F, De Wilde T, Delporte K, Van Peteghem C, De Meulenaer B (2008) Impact of chemical pre-treatments on the acrylamide formation and sensorial quality of potato crisps. Food Chem 106:914–922
Munsch-Alatossava P, Gursoy O, Alatossava T (2010) Potential of nitrogen gas (N2) to control psychrotrophs and mesophiles in raw milk. Microbiol Res 165:122–132
Munsch-Alatossava P, Ghafar A, Alatossava T (2013) Potential of nitrogen gas (N2) flushing to extend the shelf life of cold stored pasteurised milk. Int J Mol Sci 14:5668–5685
Murkovic M, Pichler N (2006) Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine. Mol Nutr Food Res 50:842–846
Navarro S (1978) The effects of low oxygen tensions on three stored-product insect pests. Phytoparasitica 6:51–58
Navarro S (2006) Modified atmospheres for the control of stored-product insects and mites. In: Heaps JW (ed) Insect management for food storage and processing. AACC International, St. Paul, pp 105–146
Navarro S (2012) The use of modified and controlled atmospheres for the disinfestation of stored products. J Pest Sci 85:301–322
Navarro S, Timlick B, Demianyk CJ, White NDG (2012) Controlled or modified atmospheres. In: Hagstrum DW, Phillips TW, Cuperus G (eds) Stored product protection. Kansas State Research and Extension, Publication No. S156, Manhattan, p 16
Ofuya TI, Reichmuth C (1993) Control of two bruchid pests of stored grain legumes in a nitrogen atmosphere. Crop Prot 12:394–396
Ofuya TI, Reichmuth C (1994) Effect of level of seed infestation on mortality of larvae and pupae of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in some controlled atmospheres. J Stored Prod Res 30:75–78
Ofuya TI, Reichmuth C (2002) Effect of relative humidity on the susceptibility of Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) to two modified atmospheres. J Stored Prod Res 38:139–146
Sandhya (2010) Modified atmosphere packaging of fresh produce: current status and future needs. LWT Food Sci Technol 43:381–392
Şevik R, Şen L, Nas S (2014) Determination of color quality and HMF content of unprocessed sultanas obtained from different vineyards. Int J Res Agric Food Sci 2:32–42
Shamaila M, Powrie WD, Skura BJ (1992) Sensory evaluation of strawberry fruit stored under modified atmosphere packaging (MAP) by quantitative descriptive analysis. J Food Sci 57(1168):1184
Singleton V, Trousdale E, Zaya J (1985) One reason sun-dried raisins brown so much. Am J Enol Vitic 36:111–113
Soderstrom EL, Brandl DG, Mackey B (1992) High temperature combined with carbon dioxide enriched or reduced oxygen atmospheres for control of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Stored Prod Res 28:235–238
Tomás-Callejas A, Boluda M, Robles PA, Artés F, Artés-Hernández F (2011) Innovative active modified atmosphere packaging improves overall quality of fresh-cut red chard baby leaves. LWT Food Sci Technol 44:1422–1428
Tunc I, Navarro S (1983) Sensitivity of Tribolium castaneum eggs to modified atmospheres. Entomol Exp Appl 34:221–226
Vasilopoulou E, Trichopoulou A (2014) Greek raisins: a traditional nutritious delicacy. J Berry Res 4:117–125
Zar HJ (1999) Biostatistical analysis. Prentice-Hall, Upper Saddle River
Acknowledgments
This study was funded by the research grant “Integrated management of insect pests and microbial infestation during processing, storage and transportation of currants by using non-chemical, ecologically compatible methods: sustainability in practice” (Grant Number 1422-BET-2013, Greek General Secretariat for Research and Technology).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors of this research declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. This article does not contain any studies with animals performed by any of the authors.
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Athanassiou, C.G., Chiou, A., Rumbos, C.I. et al. Effect of nitrogen in combination with elevated temperatures on insects, microbes and organoleptic characteristics of stored currants. J Pest Sci 90, 557–567 (2017). https://doi.org/10.1007/s10340-016-0806-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-016-0806-z