Abstract
Drosophila suzukii (Diptera: Drosophilidae) is an emerging pest of soft fruits, but in this species diapause has not been thoroughly explored. We examined the effects of different temperatures and photoperiods on diapause induction and termination under laboratory conditions. There was variation in the ovarian development and oviposition rate under different photoperiods at 10 ± 1 °C, and the percentage of adults with immature ovaries was higher during the short photoperiod (8L:16D) than other photoperiods at 10 ± 1 °C. Adults were most sensitive to photoperiod within 3 days of eclosion. The optimal combination of photoperiod and temperature for diapause termination was a long photoperiod (16L:8D) at 25 ± 1 °C. The supercooling point was significantly reduced in reproductive diapause females, and trehalase, pyruvate kinase, sorbitol dehydrogenase, hexokinase and phosphofructokinase enzyme activities were significantly reduced (36.46, 57.85, 32.64, 54.68 and 24.59 %, respectively); glycogen and triglyceride levels were significantly increased (42.17 and 120.36 %). We conclude that D. suzukii is typical of short-day diapause species within a certain photoperiod range. This information might contribute to a more fundamental understanding of adult reproductive diapause for this important pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
We report adult reproductive diapause induction and termination in D. suzukii.
-
D. suzukii was shown to be a typical of short-day diapause species.
-
The 1–3-day period after eclosion is the most sensitive stage to photoperiod in D. suzukii.
-
D. suzukii diapausing females showed higher cold tolerance than non-diapasonic ones.
-
Diapausing females had lower enzymatic activities and higher energy reserves.
Introduction
Diapause is advantageous for avoiding adverse environmental conditions, enabling females to survive through severe winter conditions and then develop when temperatures and photoperiods increase (Denlinger 2002, 2008). In addition, diapause typically occurs at a specific developmental stage for each species, such as the embryo (e.g., Bombyx mori), larvae/nymph (e.g., Loxostege sticticalis), pupae (e.g., Helicoverpa armigera) or adult (e.g., Drosophila melanogaster) stages (Allen 2007; Jiang et al. 2010; Liu et al. 2010; Kobayashi et al. 2014). Adult reproductive diapause often occurs because many insect species overwinter as adults, in which the oogenesis and vitellogenesis processes usually stop (Baker and Russell 2009).
Drosophila suzukii (Diptera: Drosophilidae) has the evolutionary advantage over other Drosophila species, that is, a serrated ovipositor to break the skin of fresh or fruits and lay eggs inside. Now, it has become an economically important pest insect for berry and some stone fruits in the world, such as in the Americas, Europe and Eastern Asia (Chabert et al. 2012; Rota-Stabelli et al. 2013; Deprá et al. 2014; Zhai et al. 2014; Lin et al. 2014a, b; Cini et al. 2014), requiring the prompt development of sustainable control tools (Asplen et al. 2015; Daane et al. 2016; Haye et al. 2016; Murphy et al. 2016). In addition, D. suzukii adults survive for 3–12 weeks, and only adult flies can overwinter in various shelters (Kimura 2004; Dalton et al. 2011; Rossi-Stacconi et al. 2016). In the winter, adult D. suzukii is darker and has longer wings than in the summer (Kanzawa 1936). Several authors found that D. suzukii adults collected in autumn were reproductively immature, suggesting overwintering adult reproductive diapause (Mitsui et al. 2010; Rossi-Stacconi et al. 2016; Wang et al. 2016). Diapause has been studied for many Drosophilids, for example, D. robusta, D. littoralis, D. montana, D. triauraria and D. melanogaster (Carson and Stalker 1948; Lumme et al. 1973; Yamada and Yamamoto 2011; Salminen and Hoikkala 2013; Kubrak et al. 2014). However, to the best of our knowledge, there are no specific studies on the mechanisms affecting D. suzukii female reproductive diapause.
In the present study, D. suzukii diapause induction and termination were investigated under laboratory and field conditions. This work could thus provide the basis for further investigation on molecular regulation of reproductive diapause in this species.
Materials and methods
Study flies
We obtained the original D. suzukii colony from cherry orchards at Tai’an (35°67′N, 116°24′E) in Shandong Province, China, in 2012. The strain was reared in a continuous laboratory culture on artificial medium. The insects were maintained in the laboratory at 25 ± 1 °C under a 16L:8D daylight cycle and 70–80 % relative humidity.
Females were collected within 12 h after eclosion and reared on fresh artificial medium (50 g banana, 5 g yeast, 2 g flour, 3 g sugar, 2.5 g agar, 0.5 g nipagine and 150 ml H2O) in glass jars (length: 400 mm; diameter: 200 mm) at different photoperiods and temperatures for the experiments; see below.
Field experiments
A sugar-vinegar mixture was used to trap D. suzukii adults in a cherry orchard in Tai’an region, Shandong Province. Ten field plots in this orchard were selected. Three trees were then randomly selected from each plot, and three traps with the sugar-vinegar mixture were placed in each of the selected trees. The temperature and photoperiod in the orchard were recorded. D. suzukii adults were collected from these traps and delivered to the laboratory for dissection.
Diapause induction experiments
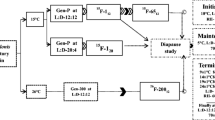
Newly emerged females were reared on artificial medium in phytotronic environments at different temperatures (5 ± 1, 10 ± 1, 15 ± 1, 20 ± 1 and 25 ± 1 °C) and photoperiods (4L:20D, 6L:18D, 8L:16D, 10L:14D, 12L:12D, 14L:10D and 16L:8D) for further observation (Williams et al. 2006). The females were dissected 15 days later, and the developmental status of the ovaries was assessed according to King (1970). The ovaries were dissected and classified into three categories: immature, developing and developed ovaries (Fig. 1). A female was scored as diapausing if the ovaries were without vitellogenin accumulation (immature ovaries); a female was scored as non-diapausing if vitellogenin was observed in the ovaries (developing and developed ovaries). The quadratic parabolic regression equation of the diapause critical photoperiod was fitted by R statistical software and used for the different photoperiod and diapause rate (arcsin square root transform) data. Approximately 40–50 individuals were observed in each of the three replicates for all treatments and placed onto fresh artificial medium in glass jars (Supplementary Table 1).
Determination of the sensitive stage to photoperiod
To determine the effect of developmental stages of D. suzukii on the diapause sensitivity to photoperiod, an experiment was conducted following Spieth (1995). Different developmental stages of D. suzukii from newly laid eggs to adults and the differently aged adults (from 1 to 15 days old at an interval of 3 days) were tested. All the insects were reared on a fresh artificial diet in glass jars initially under the standard condition (16L:8D, 25 ± 1 °C) and then transferred to photoperiods at 16L:8D and 8L:16D, respectively (see Tables 1, 2), a photoperiod at 10 ± 1 °C. The resulting females were dissected when they were 16 days old, and the number of eggs in the ovaries was counted and recorded. The oviposition and diapause rates were then calculated [oviposition rate = (number of females with oviposition/total number of females) × 100 % and the diapause rate = (number of females with immature ovaries/total number of females) × 100 %]. Approximately 40–60 individuals were tested in each treatment, and the experiment was replicated three times.
Diapause termination bioassay
Diapaused female individuals of D. suzukii were obtained after being treated for 15 days since the eclosion under the diapause-inducing photoperiod (8L:16D) at 10 ± 1 °C. The diapaused female adults (at the 16th day post-eclosion) were then treated respectively at four different temperatures (10 ± 1, 15 ± 1, 20 ± 1 and 25 ± 1 °C), each with three respective photoperiods (8L:16D, 12L:12D, 16L:8D) for 15 days. The resulting adults were then dissected and the vitellogenin observed in the counted ovaries; 50–60 individuals were tested for each of the treatments, and the experiment was replicated three times (Supplementary Table 2).
Assessment of cold tolerance
Newly emerged females were reared under different photoperiods (8L:16D, 12L:12D, 16L:8D) and temperatures (10 ± 1, 25 ± 1 °C), and the supercooling point (SCP) and freezing point (FP) of the females (15th day post-eclosion) under different treatments were measured. The abdomen of each female was fixed with plastic tape to the tip of a thermocouple attached to a multichannel automatic recorder (TOPTEST TP9024, Top Electric Co., Shenzhen, China). The thermocouple with the female was placed inside an insulating tube in a cryogenic refrigerator to ensure that the temperature of the adult decreased at a cooling rate of approximately 1 °C min−1. The SCP and FP were identified as the lowest inflexion point and peak point in the temperature, respectively (Guo et al. 2006; Formby et al. 2013). We examined 40–50 individuals in each of three replicates for all treatments and placed them on fresh artificial medium in glass jars (Supplementary Table 3).
Assessment of metabolic enzyme activities and biochemical substances
To clarify the physiological adaptation of diapause (8L:16D) or non-diapause (16L:8D) females (15th day post-eclosion) of this pest at 10 ± 1 °C, some cold tolerance-related metabolic enzymes, such as trehalase (TRE), sorbitol dehydrogenase (SDH), pyruvate kinase (PK), alkaline phosphatase (ALP), hexokinase (HK) and phosphofructokinase (PFK), were quantified. The activities of metabolic enzymes were measured by commercial kits (Suzhou Comin Biotechnology Co., Ltd., Suzhou, China), and the absorbance of TRE, SDH, PK, HK and PFK were measured at 340 nm. ALP was measured at 510 nm. Glycogen (MAK016), triglyceride (TR0100) and protein (BCA1) were measured by commercial kits (Sigma-Aldrich Co. LLC., USA). Approximately 40–50 individuals were observed in each of three replicates for all treatments and were placed onto fresh artificial medium in glass jars (Supplementary Table 4).
Statistical analyses
The statistical analyses were performed using SPSS17.0 software. The effects of photoperiod and temperature and their interactions on the induction of diapause were tested using a general linear model (GLM). Differences between treatment levels were examined using ANOVA, followed by Tukey’s analysis.
Results
Field investigation
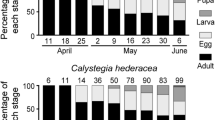
To identify the population dynamics and female ovarian development stages of D. suzukii, we investigated cherry orchards at Tai’an from 2013 to 2014, and no adults were recorded to be trapped in winter (December–February). The first and last females with immature ovaries were trapped after and before winter, respectively. In addition, the proportions of females with developing and developed ovaries increased from April to June and then gradually decreased from July to November. During this time, the photoperiod and temperature of n-valley changed, and the photoperiod was gradually increased to more than 10 h. The mean temperature was gradually increased above 10 °C (Fig. 2).
The investigation of the population dynamics and female ovarian development stages of D. suzukii in the cherry orchards at Tai’an once a month. a The photoperiod and temperature were recorded on different survey dates under various field conditions. b Proportions of the females with immature ovaries are shown in the slash and those with developing and developed ovaries in light and dark gray, respectively
Photoperiodic responses for reproductive diapause induction
Ovarian development stages of the females were measured in different photoperiod and temperature combinations, and there was variation of ovarian development stages under different temperatures and photoperiods (Fig. 3). The ovarian development was delayed with a lower temperature and short photoperiod, but development accelerateed with temperature rises and photoperiod increases. The ovarian development stage (Fig. 3a) and oviposition rate (Fig. 3b) were different at 10 ± 1 °C, during the short photoperiod (8L:16D); all the proportions of the females had immature ovaries and no sign of oviposition. The ovaries developed more rapidly under a long photoperiod (16L:8D), all the proportions of the females had developing and developed ovaries, and the oviposition rate was 100 %. The photoperiod response showed D. suzukii was typical of long-day species, with a diapause critical photoperiod of 13.35 h at 10 °C, according to the regression equation
Photoperiodic and temperature responses for diapause induction in D. suzukii. a Ovarian development stages and b oviposition rate of the females measured at different photoperiods (4L:20D, 6L:18D, 8L:16D, 10L:14D, 12L:12D, 14L:10D and 16L:8D) and temperatures (5 ± 1, 10 ± 1, 15 ± 1, 20 ± 1 and 25 ± 1 °C). a Proportions of the females with immature ovaries are shown in the slash and those with developing and developed ovaries in light and dark gray, respectively
The most sensitive stage to the photoperiod
The diapause rate increased slowly with an increase in the numbers of short-day treatment (no. 1–5), and it suddenly reached 100 % at the adult stage during the short-day photoperiod (no. 6) (Table 1). The diapause rate remained at a high level for the adult stage during the short-day photoperiod (no. 7–11), and it suddenly decreased from 83.5 to 0 % when the adult was treated with the long-day photoperiod (no. 12) (Table 1). These results suggest that the adult stage may be the most sensitive developmental stage to photoperiod in D. suzukii, while the egg may be the least sensitive stage (Table 1).
The diapause rate increased with a decreasing short-day photoperiod, suggesting that the short day photoperiod had a cumulative effect on diapause induction in D. suzukii (Table 2). No diapause was observed during the 1–15 days in the long-day photoperiod or the 10–15 days in the short-day photoperiod (Table 2). The diapause rate remained at 53.2 % when the adults were 1–3 days old in the short-day photoperiod (no. 1), and in contrast the diapause rate decreased from 80.2 to 38.3 % when the adults were 1–3 days old in the long-day photoperiod (no. 6). These results suggest that 1–3 days after eclosion may be the most sensitive stage to photoperiod in D. suzukii. The photosensitivity decreases as the adult age increased, until the 10th day after eclosion, and the short photoperiod has no effect on the reproductive diapause.
Effect of diapause-terminating photoperiod and temperature
The photoperiod significantly affected diapause termination at low temperatures (10 ± 1 °C), and the developing and developed ovaries increased with increasing photoperiod (Fig. 4). The low temperature and short photoperiod (8L:16D) maintain the reproductive diapause state, and long photoperiods can accelerate diapause development. In addition, temperature significantly influenced the diapause. The immature ovaries were significantly reduced under different photoperiods at high temperatures. This result indicates that the optimal combination of photoperiod and temperature for diapause termination was a long photoperiod (16L:8D) at 25 ± 1 °C.
Photoperiodic and temperature responses for diapause termination in D. suzukii. Ovarian development stages at different photoperiods (8L:16D, 12L:12D and 16L:8D) and temperatures (10 ± 1, 15 ± 1, 20 ± 1 and 25 ± 1 °C). Proportions of the females with immature ovaries are shown in the grid and those with developing and developed ovaries in light and dark gray, respectively
Effect of photoperiod and temperature on cold tolerance
The SCP showed no significant differences between different photoperiods at high temperatures (25 ± 1 °C), while decreasing temperatures affected SCP or FP at different photoperiods (Fig. 5). According to previous diapause induction experiments, groups treated with different photoperiods at low temperatures were defined as diapausing or non-diapausing. A significant difference was observed between the diapausing (8L:16D) and non-diapausing (16L:8D) groups at low temperatures (10 ± 1 °C) in SCP and FP. This result suggests that the effect of photoperiod on SCP and FP decreases as the temperature increases, because the cold tolerance of diapause females was stronger compared with non-diapause females.
Photoperiodic and temperature responses for SCP and FP. The SCP and FP of females at the 15th day after eclosion were measured at different photoperiods (8L:16D, 12L:12D and 16L:8D) and temperatures (10 ± 1, 25 ± 1 °C). The data represent the mean values ± SEM (n = 3), and the values in the columns followed by different letters denote a significant difference (p < 0.05, Tukey’s post hoc test)
Physiological and biochemical changes
Diapause regulated several physiological and biochemical mechanisms, particularly modifying the activities of some metabolic enzymes and energy reserve substances. The enzymatic activities of TRE, PK, SDH, HK and PFK significantly decreased (36.46, 57.85, 32.64, 54.68 and 24.59 %, respectively). On the contrary, the glycogen and triglyceride levels significantly increased (42.17 and 120.36 %) (Fig. 6). This result showed that the metabolic rate remained at lower levels through the modulation of enzymatic activity and accumulated cold tolerance substance content, thereby improving cold resistance during diapause.
Discussion
Adult reproductive diapause is a powerful overwintering strategy for many continental insect species including Drosophila. It enables females to survive several months through harsh winter conditions and then lay eggs when the temperature and photoperiod increase (Salminen and Hoikkala 2013). Worldwide, Drosophila has more than 1500 species, and most species are generally chill-susceptible and show both rapid cold-hardening and acclimation responses (Kelty and Lee 2001; MacMillan et al. 2009). D. suzukii is mainly found in warm-temperate regions (Kondo and Kimura 2008), and there is speculation that they enter a winter reproductive diapause (Walsh et al. 2010; Zerulla et al. 2015). Stephens et al. (2015) found that winter morphs of D. suzukii were produced by placing eggs at 10 °C with a photoperiod of 12L:12D, and they conjectured that these individuals might be in diapause. Although the overwintering site is not clear, it is generally thought that D. suzukii overwinters beneath leaf litter in built structures associated with an orchard or agricultural setting (Kanzawa 1939; Jakobs et al. 2015). We found almost all of the females trapped with immature ovaries in or around winter, and there was no record of adults trapped in winter (Fig. 2). According to the results obtained in the present study and other publications (Calabria et al. 2012; Jakobs et al. 2015; Rossi-Stacconi et al. 2016; Wang et al. 2016), we proposed that the diapause stage of overwintering was adult.
In warm-temperate regions, the photoperiod and temperature are the two key determinants of diapause-inducing environmental factors. Decades of research on insect diapause suggest that the relative importance of photoperiod and temperature in diapause regulation is widely variable and highly species-specific, and temperature often enhances or inhibits the induction of diapause through the photoperiod (Hodek and Hodkov 1988). In the present study, the ovarian development stages and oviposition rate were different at 10 ± 1 °C (Fig. 3). All of the females with immature ovaries at a short photoperiod (8L:16D) and reproductive diapause phenomenon gradually weaken with increasing or decreasing temperatures. The ovaries developed quickly under a long photoperiod (16L:8D) at different temperatures for all of the females with developing and developed ovaries, and the oviposition rate was 100 % above 10 ± 1 °C. This showed that D. suzukii was typical of short-day diapause species within a certain photoperiod range. The reproductive diapause induction condition of a low temperature and short-day photoperiod agreed with the late autumn and early winter climatic conditions in warm-temperate regions, and this is consistent with other research on Drosophila species' reproductive diapause (Schmidt et al. 2008; Baker and Russell 2009; Kankare et al. 2010; Yamada and Yamamoto 2011; Rossi-Stacconi et al. 2016; Wang et al. 2016).
Different insect species exhibit different sensitivities to photoperiod, but the sensitive stage was not basically changed for a particular species (Beck 1980), such as the sensitive stage in maternity for most embryo diapause (Kobayashi et al. 2014), previous instars for most larvae/nymph diapause (Xiao et al. 2013), larvae stage for most pupae diapause (Liu et al. 2010) and preliminary stage of eclosion for most reproductive diapause (Xue et al. 2002). These results suggested that 1–3 days after eclosion is the most sensitive stage in D. suzukii, and the photosensitivity decreased with increasing days of adult stages (Table 1). A short photoperiod had a cumulative effect on diapause induction in D. suzukii (Table 2).
Insects' cold tolerance is influenced by various factors, and different insect species adjust their cold tolerance in different ways, such as the reduction of SCP, FP and water content, accumulation of cold tolerance substances, export of ice nucleating substances, regulation of metabolic enzyme activity, etc. Stephens et al. (2015) found that D. suzukii was a chill-intolerant insect, and winter-morph adults are the most cold-tolerant life stage. The lower lethal temperature of D. suzukii winter-morph adults was significantly colder than that of summer-morph adults, while the SCP of winter-morph adults was actually warmer than that of summer-morph adults and pupae. The SCP of D. suzukii was between −16 and −23 °C and was chill-susceptible (Jakobs et al. 2015). The SCP of adult D. melanogaster is approximately −20 °C and its lower lethal temperature above −10 °C (Chen and Walker 1994). Other Drosophila species may be tolerant to colder temperatures, such as when the lower lethal temperature is approximately −13 °C in D. borealis (Nyamukondiwa et al. 2011). The SCP was below −20 °C (Fig. 5), reflecting the fact that D. suzukii had some cold tolerance and could cope with the cold environment, thereby possibly explaining the D. suzukii outbreaks in recent years around the world.
The diapause stages accumulate cold tolerance substances, such as trehalose, sorbitol and glucose, glycerinum, etc., to improve the cold tolerance and overcome severe winter environments (Rozsypal et al. 2013). TRE is a key enzyme in trehalose hydrolysis, and changes in the activity of this enzyme directly affect energy metabolism (Kamei et al. 2011). Sorbitol, through SDH, is converted to glycogen and subsequently utilized as energy for embryonic development (Rubio et al. 2011). PK, HK and PFK are the key enzymes in the glycolytic pathway, and PK mediates the conversion of phosphoenolpyruvic acid and ADP into pyruvic acid and ATP. HK catalyzes glucose from a steady state into an active state (Rider et al. 2011). In the present study, TRE, PK, SDH, HK and PFK enzyme activity levels significantly decreased, indicating that the diapause female improves cold tolerance through the accumulation of cold tolerance substances.
Diapause regulation has been shown as a pest control method, and several strong candidate genes/proteins can terminate diapause, causing them to fail to overwinter successfully. Zhou et al. (2015) found treatment with a recombinant TAT-caDH protein can affect larval development in H. armigera. Using RNAseq technology, several diapause candidate genes code for upstream regulators of a complex change of gene expression, which leads to a phenotypic switch from direct ontogeny to larval of the drosophilid fly Chymomyza costata (Poupardin et al. 2015). In a future study, several strong candidate genes/proteins of diapause will be investigated for functional studies, which may be useful for controlling the pest.
To our knowledge, this is the first study to ever report the adult reproductive diapause induction and termination in D. suzukii. Furthermore, our results emphasize the importance of understanding how D. suzukii is induced to initiate and terminate diapause under laboratory conditions of changing photoperiod and temperature, and we performed a preliminary examination of the physiological and biochemical mechanism. Follow-up studies will reveal the molecular mechanisms of reproductive diapause at the level of omics in D. suzukii females and provide instructions for pest control.
Author contribution
YZ and QL conceived and designed the research. YZ, QL and JZ conducted experiments. FZ and LZ analyzed the data. YZ and YY wrote the manuscript. All authors read and approved the manuscript.
References
Allen MJ (2007) What makes a fly enter diapause? Fly (Austin) 1(6):307–310
Asplen MK, Anfora G, Biondi A, Choi DS, Chu D, Daane KM et al (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494
Baker DA, Russell S (2009) Gene expression during Drosophila melanogaster egg development before and after reproductive diapause. BMC Genomics 24:242
Beck SD (1980) Insect photoperiodism, 2nd edn. Academic Press, New York, pp 119–185
Calabria G, Maca J, Bachli G, Serra L, Pascual M (2012) First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol 136:139–147
Carson HL, Stalker HD (1948) Reproductive diapause in Drosophila Robusta. Proc Natl Acad Sci USA 34(3):124–129
Chabert S, Allemand R, Poyet M, Eslin P, Gibert P (2012) Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol Control 63:40–47
Chen CP, Walker VK (1994) Cold-shock and chilling tolerance in Drosophila. J Insect Physiol 40:661–669
Cini A, Anfora G, Escudero-Colomar LA, Grassi A, Santosuosso U, Seljak G, Papini A (2014) Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J Pest Sci 87:559–566
Daane KM, Wang XG, Biondi A, Miller B, Miller JC et al (2016) First exploration of parasitoids of Drosophila suzukii in South Korea as potential classical biological agents. J Pest Sci. doi:10.1007/s10340-016-0740-0
Dalton DT, Walton VM, Shearer PW, Walsh DB, Caprile J, Isaacs R (2011) Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag Sci 67(11):1368–1374
Denlinger DL (2002) Regulation of diapause. Annu Rev Entomol 47:93–122
Denlinger DL (2008) Why study diapause? Entomol Res 38:1–9
Deprá M, Poppe JL, Schmitz HJ, De Toni DC, Valente VLS (2014) The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci 87(3):379–383
Formby JP, Krishnan N, Riggins JJ (2013) Supercooling in the Redbay Ambrosia Beetle (Coleoptera: Curculionidae). Fla Entomol 96(4):1530–1540
Guo HB, Xu YY, Ju Z, Li MG (2006) Seasonal changes of cold hardiness of the green lacewing, Chrysoperla sinica (Tjeder) (Neuroptera:Chrysopidae). Acta Ecol Sin 26(10):3238–3244
Haye T, Girod P, Cuthbertson AGS, Wang XG, Daane KM, Hoelmer KA et al (2016) Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J Pest Sci. doi:10.1007/s10340-016-0737-8
Hodek I, Hodkov M (1988) Multiple role of temperature during insect diapause. A review. Entomol Exp Appl 49:153–166
Jakobs R, Gariepy TD, Sinclair BJ (2015) Adult plasticity of cold tolerance in a continental-temperate population of Drosophila suzukii. J Insect Physiol 79:1–9
Jiang XF, Huang SH, Luo LZ, Liu Y, Zhang L (2010) Diapause termination, post-diapause development and reproduction in the beet webworm, Loxostege sticticalis (Lepidoptera: Pyralidae). J Insect Physiol 56:1325–1331
Kamei Y, Hasegawa Y, Niimi T, Yamashita O, Yaginuma T (2011) Trehalase-2 protein contributes to trehalase activity enhanced by diapause hormone in developing ovaries of the silkworm, Bombyx mori. J Insect Physiol 57:608–613
Kankare M, Salminen T, Laiho A, Vesala L, Hoikkala A (2010) Changes in gene expression linked with adult reproductive diapause in a northern malt fly species: a candidate gene microarray study. BMC Ecol 10:3
Kanzawa T (1936) Studies on Drosophila suzukii mats. J Plant Prot (Tokyo) 23:66–70
Kanzawa T (1939) Studies on Drosophila suzukii mats. Kofu. Yamanashi Agricultural Experimental Station, Yamanashi, pp 1–49
Kelty JD, Lee RE Jr (2001) Rapid cold-hardening of Drosophila melanogaster (Diptera: Drosophilidae) during ecologically based thermoperiodic cycles. J Exp Biol 204:1659–1666
Kimura MT (2004) Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 140:442–449
King RC (1970) Ovarian development in Drosophila melanogaster. Academic, New York
Kobayashi N, Takahashi M, Kihara S, Niimi T, Yamashita O, Yaginuma T (2014) Cloning of cDNA encoding a Bombyx mori homolog of human oxidation resistance 1 (OXR1) protein from diapause eggs, and analyses of its expression and function. J Insect Physiol 68:58–68
Kondo M, Kimura MT (2008) Diversity of drosophilid Flies on Kume-jima, a subtropical island: comparison with diversity on Iriomote-jima. Entomol Sci 11:7–15
Kubrak OI, Kučerová L, Theopold U, Nässel DR (2014) The sleeping beauty: how reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS ONE 9(11):e113051
Lin QC, Zhai YF, Zhou CG, Li LL, Zhuang QY, Zhang XY et al (2014a) Behavioral rhythms of Drosophila suzukii and Drosoplia melanogaster. Fla Entomol 97(4):1424–1433
Lin QC, Zhai YF, Zhang AS, Men XY, Zhang XY, Zalom FG et al (2014b) Comparative developmental times and laboratory life tables for Drosophila suzukii and Drosophila melanogaster. Fla Entomol 97(4):1434–1442
Liu Z, Gong P, Li D, Wei W (2010) Pupal diapause of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) mediated by larval host plants: pupal weight is important. J Insect Physiol 56:1863–1870
Lumme J, Oikarinen A, Lakovaara S, Alatalo R (1973) The environmental regulation of adult diapause in Drosophila littoralis. J Insect Physiol 20(10):2023–2033
MacMillan HA, Guglielmo CG, Sinclair BJ (2009) Membrane remodeling and glucose in Drosophila melanogaster: a test of rapid cold-hardening and chilling tolerance hypotheses. J Insect Physiol 55:243–249
Mitsui H, Beppu K, Kimura MT (2010) Seasonal life cycles and resource uses of flower- and fruit-feeding drosophilid flies (Diptera: Drosophilidae) in central Japan. Entomol Sci 13:60–67
Murphy KA, West JD, Kwok RS, Chiu JC (2016) Accelerating research on Spotted Wing Drosophila management using genomic technologies. J Pest Sci. doi:10.1007/s10340-016-0741-z
Nyamukondiwa C, Terblanche JS, Marshall KE, Sinclair BJ (2011) Basal cold but not heat tolerance constrains plasticity among Drosophila species (Diptera: Drosophilidae). J Evol Biol 24:1927–1938
Poupardin R, Schöttner K, Korbelová J, Provazník J, Doležel D, Pavlinic D et al (2015) Early transcriptional events linked to induction of diapause revealed by RNAseq in larvae of drosophilid fly Chymomyza costata. BMC Genomics 16(1):720
Rider MH, Hussain N, Dilworth SM, Storey JM, Storey KB (2011) AMP-activated protein kinase and metabolic regulation in cold-hardy insects. J Insect Physiol 57:1453–1462
Rossi-Stacconi MV, Kaur R, Mazzoni V, Ometto L, Grassi A, Gottardello A et al (2016) Multiple lines of evidence for reproductive winter diapause in the invasive pest Drosophila suzukii: useful clues or control strategies. J Pest Sci. doi:10.1007/s10340-016-0753-8
Rota-Stabelli O, Blaxter M, Anfora G (2013) Drosophila suzukii. Curr Biol 23:R8–R9
Rozsypal J, Koštál V, Zahradníčková H, Šimek P (2013) Overwintering strategy and mechanisms of cold tolerance in the codling moth (Cydia pomonella). PLoS ONE 8(4):e61745
Rubio RO, Suzuki A, Mitsumasu K, Homma T, Niimi T, Yamashita O, Yaginuma T (2011) Cloning of cDNAs encoding sorbitol dehydrogenase-2a and b, enzymatic characterization, and up-regulated expression of the genes in Bombyx mori diapause eggs exposed to 5°C. Insect Biochem Mol Biol 41:378–387
Salminen TS, Hoikkala A (2013) Effect of temperature on the duration of sensitive period and on the number of photoperiodic cycles required for the induction of reproductive diapause in Drosophila montana. J Insect Physiol 59(4):450–457
Schmidt PS, Zhu CT, Das J, Batavia M, Yang L, Eanes WF (2008) An amino acid polymorphism in the couch potato gene forms the basis for climatic adaptation in Drosophila melanogaster. Proc Natl Acad Sci USA 105(42):16207–16211
Spieth HR (1995) Change in photoperiodic sensitivity during larval development of Pieris brassicae. J Insect Physiol 41:77–83
Stephens AR, Asplen MK, Hutchison WD, Venette RC (2015) Cold hardiness of winter-acclimated Drosophila suzukii (Diptera: Drosophilidae) adults. Environ Entomol 44(6):1619–1626
Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee JC (2010) Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integr Pest Manag 2:G1–G7
Wang XG, Stewart TJ, Biondi A, Chavez BA, Ingels C, Caprile J et al (2016) Population dynamics and ecology of Drosophila suzukii in Central California. J Pest Sci. doi:10.1007/s10340-016-0747-6
Williams KD, Busto M, Suster ML, So AK, Ben-Shahar Y, Leevers SJ, Sokolowski MB (2006) Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc Natl Acad Sci USA 103(43):15911–15915
Xiao L, Fu S, Xue FS (2013) Characters of insect diapause stage and photoperiod sensitive stage. Biol Disaster Sci 36:1–8 (in Chinese)
Xue FS, Spieth HR, Li AQ, Hua A (2002) The role of photoperiod and temperature in determination of summer and winter diapause in the cabbage beetle, Colaphellus bowringi (Coleoptera: Chrysomelidae). J Insect Physiol 48:279–2861
Yamada H, Yamamoto MT (2011) Association between circadian clock genes and diapause incidence in Drosophila triauraria. PLoS ONE 6(12):e27493
Zerulla FN, Schmidt S, Streitberger M, Zebitz CPW, Zelger R (2015) On the overwintering ability of Drosophila suzukii in South Tyrol. J Berry Res 5:41–48
Zhai YF, Lin QC, Zhou XH, Zhang XY, Liu TL, Yu Y (2014) Identification and validation of reference genes for quantitative real-time PCR in Drosophila suzukii (Diptera: Drosophilidae). PLoS ONE 9(9):e106800
Zhou Z, Li Y, Yuan C, Zhang Y, Qu L (2015) Oral administration of TAT-PTD-DIAPAUSE hormone fusion protein interferes with Helicoverpa armigera (Lepidoptera: Noctuidae) development. J Insect Sci 28:15
Acknowledgments
This work was financially supported through a grant from the Shandong Provincial Natural Science Foundation, China (ZR2014CQ014), and the National Natural Science Foundation of China (31401803). The authors wish to thanks Jianlong Bi for the manuscript language revision.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Biondi.
Special Issue: Spotted Wing Drosophila.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhai, Y., Lin, Q., Zhang, J. et al. Adult reproductive diapause in Drosophila suzukii females. J Pest Sci 89, 679–688 (2016). https://doi.org/10.1007/s10340-016-0760-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-016-0760-9