Abstract
Polygonia c-aureum females exhibit photoperiodically induced imaginal diapause, characterized by cessation of ovarian development. Females grown at a short daylength (SD) entered imaginal diapause, whereas those grown at a long daylength (LD) produced eggs rapidly after adult emergence at 21 °C. The termination of diapause was influenced by daylength: diapause ended faster at LD than SD. Complete termination of diapause took 30 days in unchilled females reared under LD at 21 °C. On the other hand, prompt, synchronized and strong diapause termination occurred at post-chilling periods. Photoperiods at post-chilling periods affected ovarian development, when the length of pre-chilling periods or the length of chilling periods was shorter, suggesting that these treatments were not enough to complete diapause development. Ovarian development proceeded earlier in chilled and subsequent warmed females than unchilled females. Wing damage was remarkable at post-chilling periods when females were reared under an adequate length of pre-chilling and chilling periods, especially comparing with females under pre-overwintering conditions without chilling, indicating that post-diapause reproductive development was weak in unchilled females. Thus, exposure to low temperatures is necessary for a strong diapause termination in this butterfly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many insects with autumnal–hibernal imaginal diapause exhibit a gradual loss in sensitivity to various diapause-maintaining factors, and thereby diapause is terminated spontaneously in late autumn or early winter and maintain the state of quiescence under low temperatures (Tauber and Tauber 1976; Tauber et al. 1986). Temperature is one of the major stimuli affecting the termination of autumn–winter diapause in insects (Leather et al. 1993; Koštάl 2006; Koštάl et al. 2008; Jiang et al. 2010), although diapause is terminated without exposure to low temperatures in many species (Hodek and Hodkova 1988; Hodek 1999, 2002), e.g., in Coccinella septempunctata and Semiadalia undecimnotata (Hodek 1970), in Chrysopa carnea (Honěk and Hodek 1973), in Aelia acuminata (Hodek 1974, 1979), in Riptortus clavatus (Numata and Hidaka 1982), in Dolycoris baccarum (Hodkova et al. 1989), in Choristoneura fumiferana (Han and Bauce 1996), in Sesamia nonagrioides (Fantinou et al. 1998), in Euseius finlandicus (Broufas et al. 2006), in Nezara viridula (Musolin et al. 2007), and in Chilo suppressalis (Xiao et al. 2010).
Polygonia c-aureum has a distinct seasonal diphenism, in which summer and autumn forms, differ from each other in wing morphology and physiology. The autumn form butterflies enter a reproductive diapause, overwinter, and reproduce in the spring, and their progeny develop to summer forms in the summer. Summer form butterflies reproduce soon after eclosion and additional summer-form generations are produced in warm regions of Japan. Both seasonal diphenism and diapause induction of this species is controlled mainly by photoperiod and temperature experienced during the larval stage: long photoperiods and high temperatures produce the summer form without diapause, whereas short photoperiods and lower temperatures produce the autumn form with diapause (Hidaka and Aida 1963; Hidaka and Takahashi 1967). This butterfly has 4 or 5 generations per year in Tokyo, differing in the region. The diapausing autumn form adults search for the flowers before overwintering to develop the fat body. Diapause of P. c-aureum females is characterized by suppression of ovarian development (Hidaka and Aida 1963; Fukuda and Endo 1966) and accumulation of secretory substances in female accessory reproductive glands (Endo 1970a; Raabe 1986) and reduced receptivity for mates (Endo 1973). Diapause of this butterfly is controlled by corpora allata. Endo (1970b) demonstrates that transplantation of active corpora allata derived from summer form destined larvae into autumn form destined larvae promotes ovarian development in the adult stage. Application of a juvenile hormone analogue methoprene into diapausing autumn form butterflies also promotes ovarian development (Hiroyoshi et al. 2017). Fujita et al. (2009) have shown that the diapause of this butterfly was maintained for 2 months under a short photoperiod at 20 °C, whereas the diapause was terminated easily by a long photoperiod.
However, we know little about the maintenance and termination of their diapause after overwintering. The present study was undertaken to clarify the effects of photoperiod, temperature and aging on the maintenance and termination of diapause and post-diapause development in P. c-aureum. We examined ovarian development and wing damage, because they, especially the latter, reflected a weak and strong diapause termination. As the diapause terminated by long photoperiods might be artificial in this butterfly, because field examination showed no sign of diapause termination in the autumn (Hiroyoshi, unpublished data). Thus, we assessed the effects of chilling on diapause termination.
Materials and methods
Insect stock
A laboratory colony was established from larvae of P. c-aureum collected at Tokyo Metropolis and Saitama Prefecture, central region of mainland Japan in 1991. It had been maintained under long day (LD: 15 h light:9 h dark) at 21 ± 1 °C, conditions producing summer form butterflies. All experiments were done in 1992.
Rearing
To obtain autumn form butterflies, hatchlings derived from the laboratory colony were reared under short day (SD: 8 h light: 16 h dark) at 21 ± 1 °C according to the methods described previously (Hiroyoshi 1992). After adult eclosion, females were separated from males, and held in separate cages (17 cm × 16.5 cm × 46 cm). A sucrose solution (10%) absorbed in cotton balls was provided as food for adults and renewed weekly. When adults were chilled at 5 °C in darkness for the termination of diapause, they moved only little and thus no food was given to the adults.
Environmental conditions
A design of experiments was drawn in Fig. 1. After hatching, larvae were reared under SD at 21 ± 1 °C until adult eclosion. Autumn form adult females obtained under SD at 21 ± 1 °C were reared under various experimental conditions as described below (see Fig. 1): In Experiment 1, females were kept under either SD or LD at 21 °C for 0–60 days without chilling. In Experiment 2, females were pre-incubated under SD at 21 °C for 15 or 45 days, chilled at 5 °C in DD (complete darkness) for 2 months, and returned to SD or LD at 21 °C. In Experiment 3, females were pre-incubated under SD at 21 °C for a month, chilled at 5 °C in DD for 3 months, and returned to either SD or LD at 21 °C.
Ovarian development
Ovaries were dissected out in a saline solution (consisting of 8.6 g NaCl, 0.33 g CaCl2 and 0.1 g KCl per liter distilled water) under a binocular microscope every 10 days after adult eclosion or after chilling. In this study, the incidence of diapause, the frequency of females with mature oocytes, the number of eggs, and oocyte diameter were examined in the following ways. Diapausing females have small opaque or white-colored oocytes. Females with yellow, yellowish green, green oocytes or green eggs were classified as individuals in non-diapause. The number of eggs was counted. The diameter of the largest oocyte in each individual was measured with the aid of an ocular micrometer equipped with a phase-contrast microscope. The experiments were repeated at least 5 times, usually 10–40 times for each age, as described in the figures.
Our preliminary observations indicated that P. c-aureum females laid no eggs unless their host plant leaves were provided. If host plant leaves were given, even unmated sexually mature females laid eggs, although they laid much fewer eggs than mated females did. In this study, neither host plant leaves nor mates were provided for the females used in the experiments.
The incidences of diapause and females with eggs were analyzed with a χ2 square test. The number of eggs and oocyte diameter were analyzed with the Mann–Whitney’s U test.
Behavioral activity
After dissection, the length of a left or right fore-wing was measured using a vernier for each individual except for those with damaged wings. It has been reported in the monarch butterfly Danaus plexippus that the degree of wing damage increases with mating activity after the termination of reproductive diapause followed by reproductive activation (Leong et al. 1993). Thus, females of P. c-aureum with damaged both left and right fore-wings were assumed to have been reproductively active. The frequencies of SD and LD females with damaged wings was compared with a χ2 square test.
Results
Effects of photoperiod and age on diapause at constant 21 °C
To determine if age and/or photoperiod during the adult stage affect reproductive diapause of P. c-aureum, ovarian development was compared between adult females kept under SD and LD conditions at 21 °C (Experiment 1) (Fig. 2). Females kept under SD conditions continuously before and after adult eclosion did not develop the ovaries during the first 30 days of adulthood (Fig. 2a–c). The diameter of oocytes remained smaller than 200 µm and attained at most 400 µm on average by day 60 (Fig. 2a). The number of females with eggs and that of eggs increased with age over 30 or 40 days (Fig. 2b, c). Three females that had terminated diapause on day 60 had oocytes of approximately 820 µm in diameter (Fig. 2a), which corresponded to the size of eggs of this butterfly. The incidence of diapause was also high during the first 30 days and thereafter gradually decreased with time (Fig. 2d).
Effects of photoperiod on ovarian development and the diapause incidence in autumn-form adults of Polygonia c-aureum reared under SD or LD at 21 °C. Open circles and triangles indicate the females kept under LD and SD, respectively. a Mean diameter of oocyte; b mean number of ovarian eggs; c females with eggs; d the incidence of diapause. The numerical number in parentheses indicates the number of animals used under LD (thick letters) and SD (thin letters). Asterisk indicates the significant difference
Under LD photoperiodic conditions, ovarian development proceeded rapidly with age (Fig. 2a–c). The average number of eggs was less than 50 on day 30 (Fig. 2c). The incidence of diapause decreased with age in the females, and all individuals terminated diapause within 30 days (Fig. 2d).
Effects of lengths of pre-incubation conditions and chilling on diapause
Whether the length of pre-chilling periods (15 or 45 days) affects diapause was examined, because pre-chilling periods affect the survival rate during the chilling period (Hiroyoshi, unpublished data). First, females were kept under SD at 21 °C for the first 15 days of adulthood, chilled at 5 °C in DD for 2 months, and then returned to either SD or LD at 21 °C (Fig. 3: Experiment 2). Ovarian development of LD females tended to show higher values than those of SD females at any time examined (Fig. 3a–c). Although the incidence of diapause was significantly lower in the LD females than in the SD females, 10 days after post-chilling (p < 0.05, by Mann Whitney’s U test), there were no significant differences between the two photoperiodic treatments thereafter (p > 0.05, by Mann–Whiteny’s U test) (Fig. 3).
Effects of photoperiod on ovarian development and the diapause incidence in autumn-form adults of Polygonia c-aureum reared under SD at 21 °C for 15 days after adult eclosion, chilled under DD at 5 °C for 2 months, and then transferred to SD or LD at 21 °C. a–d Show mean diameter of oocyte, mean number of ovarian eggs, females with mature eggs, and diapause rate, respectively. Open circles and triangles indicate the females kept under LD and SD, respectively. The numerical number of parentheses indicates the number of animals used under LD (thick letters) and SD (thin letters). Asterisk indicates the significant difference
On the other hand, when females were kept under SD at 21 °C for the first 45 days of adulthood followed by chilling at 5 °C in DD, and then returned to 21 °C under either SD or LD, photoperiods during the post-chilling period did not affect ovarian development (Fig. 4a–c: Experiment 3), diapause termination and subsequent reproductive development significantly (Fig. 4d). Three out of 15 females did not show diapause characteristics before transfer from chilling conditions to SD or LD condition.
Effects of photoperiod on ovarian development and the diapause incidence of Polygonia c-aureum in autumn-form adults reared under SD at 21 °C for 45 days after adult eclosion, chilled under DD at 5 °C for 2 months, and then transferred to an SD or LD condition at 21 °C. a–d Show mean diameter of oocyte, mean number of ovarian eggs, females with mature eggs, and diapause rate, respectively. Open circles and triangles indicate the females kept under LD and SD, respectively. The numerical number indicates the number of animals used under LD (thick letters) and SD (thin letters). Asterisk indicates the significant difference
Effects of long-term chilling on diapause
To determine the effects of extension of chilling period on diapause, newly emerged females were pre-incubated under SD at 21 °C for a month, chilled at 5 °C in DD for 3 months, and then returned to either SD or LD at 21 °C (Fig. 5: Experiment 4).
Effects of photoperiod on ovarian development and the diapause incidence of Polygonia c-aureum in autumn-form adults reared under SD condition at 21 °C for 30 days after adult eclosion, chilled under DD at 5 °C for 3 months, and then transferred to SD or LD at 21 °C. a–d Show mean diameter of oocyte, mean number of ovarian eggs, females with mature eggs, and diapause rate, respectively. Open circles and triangles indicate the females kept under LD and SD, respectively. The numerical number indicates the number of animals used under LD (thick letters) and SD (thin letters). Asterisk indicates the significant difference
Photoperiods after chilling did not affect ovarian development, diapause termination and subsequently reproductive development. Under both photoperiods, ovarian development increased to maximum levels 10 days after chilling (Fig. 5a–c). The incidence of diapause decreased to 0% during the same period (Fig. 5d).
Role of females with damaged fore-wings
To determine if physiological state of females affects the intensity of behavioral activity after diapause and subsequent reproductive activation, the rate of females with damaged fore-wings used in the Experiments 1–4 was examined (Fig. 6).
Frequencies of wing damage of female Polygonia c-aureum under various conditions. a–d Are based on the individuals presented in Figs. 2, 3, 4 and 5, respectively. Open circles and triangles indicate the females kept under LD and SD, respectively. The numerical number indicates the number of animals used under LD (thick letters) and SD (thin letters). Asterisk indicates the significant difference
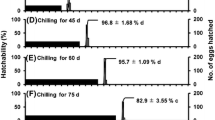
Among autumn form females kept under LD at 21 °C for 60 days after adult eclosion (Experiment 1: Fig. 6a), a few individuals (8.3%) had damaged fore-wings. The rate of damaged fore-wing was much higher in chilled females than in unchilled females as mentioned below. Unchilled females almost not lost the scales of wings, while chilled females lost them considerably 30 days after chilling (Hiroyoshi, unpublished observation). The flying speed of butterflies experiencing chilling and then transferred to 21 °C was much faster than that of unchilled ones. When females were kept under SD at 21 °C for 15 or 45 days after adult eclosion, chilled at 5 °C for 2 months, and returned to 21 °C (Experiment 2 and 3), the damaged fore-wing rates increased slightly with time after chilling irrespective of photoperiod (Fig. 6b, c). When females were kept under SD at 21 °C for a month, chilled at 5 °C for 3 months, and then returned to 21 °C (Experiment 4), the damaged fore-wing rates were higher in LD butterflies than in SD butterflies (Fig. 6d), and these values were much higher than those obtained from the other experiments (Fig. 6a–c). It is noted that photoperiod after chilling had almost no significant influence on either ovarian development and diapause termination (Fig. 5).
For comparison, wing damage of males was examined. As a result, the results on males were similar to those on females (Fig. 7). Male butterflies that did not receive overwintering conditions showed low wing damage rates.
Frequencies of wing damage of male Polygonia c-aureum under various conditions. a–d Are based on the individuals under the rearing conditions as the same with experiments of females presented in Fig. 2, 3, 4 and 5, respectively. Open circles and triangles indicate the males kept under LD and SD, respectively. The numerical number indicates the number of animals used under LD (thick letters) and SD (thin letters). Asterisk indicates the significant difference
Discussion
Role of photoperiod
In many insects, diapause is controlled by abiotic factors, especially, photoperiod and temperature. Recent studies on lepidopteran insects indicate that photoperiods appear to have an influence on diapause development during the early phase of diapause (Milonas and Savopoulou-Soultani 2004; Yang et al. 2014). Short daylength may have an important role in the maintenance of winter diapause during autumn and early winter. Photoperiod during the early stage of adult life affected diapause of P. c-aureum females. Females kept under SD at 21 °C in the adult stage had suppressed ovarian development for the first 30 days and then gradually developed ovaries with time. On the other hand, females kept under LD at 21 °C during the adult stage showed rapid ovarian development with age. These results indicate that diapause of this butterfly was maintained by SD for a certain period of time but rapidly terminated by LD. Interestingly, diapause can be terminated even under SD spontaneously, without being exposed to low temperatures. High temperatures such as over 25 °C also terminated diapause quickly in this species (Hiroyoshi, unpublished observation). These results might suggest a possibility that some adults terminate diapause before winter in the field. However, all autumn-form females (n = 27) collected in the autumn (September–November), which represented the diapausing generation in Japan, were found in a state of reproductive diapause (Hiroyoshi, unpublished data). Therefore, it seems that this butterfly maintains diapause in the autumn in nature.
This discrepancy seems to be caused from a rearing temperature. The constant temperature (21 °C) at adult stage used in the present study could be too high to maintain the diapause of this butterfly, because diapause is maintained for about 3–4 months, when females were kept under SD at 15 °C (Hiroyoshi, unpublished data). Decrease of temperature may be necessary for their diapause maintenance and/or subsequent quiescence, since outdoor temperatures in autumn decrease with the advance of the season.
Role of low temperature
Many studies have shown that low temperatures during overwintering are important for diapause development to take place. In this study, a rise in temperature after simulated overwintering conditions terminate diapause quickly in chilled P. c-aureum, compared with unchilled females. This suggests that low temperature during the winter is effective in accelerating diapause development, resulting in the synchronization of reproduction in the spring. Since P. c-aureum females examined after chilling were old (3–5-month-old), aging might also be involved in diapause development. After chilling period, a pair of autumn form mated and females laid viable eggs (Hiroyoshi, unpublished observation).
The length of pre-chilling period affected the maintenance of diapause (Figs. 3 and 4). Early timing of temperature shift affected photoperiodism after chilling (Fig. 3), while late timing of temperature shift did not (Fig. 4). This seems to show that early timing of chilling did not complete diapause development and thus the sensitivity for photoperiods was still maintained after chilling (Fig. 3). On the other hand, late timing of chilling completed diapause development and photoperiod no longer affected post-chilling development (Fig. 4). Thus, it appears that females that completed diapause development lost the sensitivity to photoperiod, as known in many insects, e.g., in Chrysopa oculata (Propp et al. 1969), in C. Mohave (Tauber and Tauber 1973), in C. harrisii (Tauber and Tauber 1974), in Semiadalia undecimnotata (Hodek and Ruzicka 1977), in Aelia acuminata (Hodek 1979), in Coccinella septempunctata (Hodek et al. 1989), and in Leptinotarsa decemlineata (de Kort 1990). This finding is supported by the fact that when females of P. c-aureum were pre-incubated at 21 °C and chilled at 5 °C for 3 months, photoperiods during the post-chilling period at 21 °C did not affect diapause termination (Fig. 5d). From these lines of evidence, it seems likely that not only the periods from adult eclosion to overwintering but also the duration of overwintering periods affect diapause development of this butterfly.
When P. c-aureum females were chilled at 5 °C from 45 days of adult age for 2 months, 20% females were not in a state of diapause just after chilling. As it seems unlikely that ovarian development progressed during chilling at 5 °C, the diapause of these non-diapaused females could be terminated before chilling (Fig. 4d). Although why these females could survive during the chilling is uncertain, they did not die probably because these females prechilled at 21 °C for 30 or 45 days had well-developed fat body just after chilling (Hiroyoshi, unpublished observation), supporting their survival. As these females immediately after chilling had mature eggs, they seemed to complete diapause termination. This explains that a proportion of weak diapause-terminated females could survive during the chilling, although diapause development finished. Estimated from the results on Fig. 2a, non-diapause rate was much lower than approximately 50% of non-diapause rate in unchilled females 45 days after adult eclosion. Thus, it appears that a proportion of females, which broke diapause completely before chilling, did not survive during the course of the chilling period (Hiroyoshi, unpublished data). We assumed here that this butterfly have a weak and strong diapause termination for unchilled and chilled females, respectively. We defined that a weak diapause is terminated by factors such as long photoperiods, which do not occur in the autumn, and subsequent reproductive development including flight behavior is slow, whereas a strong diapause is terminated by low temperatures and aging, which occur in the winter, and subsequent reproductive development is fast. Since unchilled females did not experience enough diapause development, they showed a weak diapause termination. On the other hand, chilled females completed diapause development and are ready for a strong diapause termination.
Wing damage
Wing damage or wing wear is often used to estimate the age of the butterfly and moth, for example, Colias sp., Cnaphalocrocis medinalis (Guenée), Pieris rapae (L.), and Colias erate Esper (Watt et al. 1977; Wada and Kobayashi 1985; Watanabe and Ando 1993; Watanabe and Nakanishi 1996), because daily activity reflects the accumulation of wing damage. Percentage of P. c-aureum females with damaged forewings which experienced chilling at 5 °C for 3 months was much higher than that of unchilled females and that of females chilled for 2 months. This suggests the possibility that exposure to low temperatures for larger term followed by rising temperature and aging provided a subsequent enhancement of behavioral activity of females, which would be likely to damage the wings. Different from the ovarian development, the reason of why photoperiods affected their behavioral activity is unclear. One may argue that the increase of females with damaged wings is due to the difference of photophase length, because this butterfly is diurnal, and therefore, females under LD might engage in foraging and flight behavior for a longer time than those under SD. However, this cannot explain why unchilled females showed extremely low percentages of damaged fore-wings irrespective of photoperiods and no differences were found between SD and LD in females chilled for 2 months. Thus, it is evident that chilling treatment and a subsequent rise of temperature, but not the solely difference of photophase, caused the increase of the wing damage. It is noted that if wing damage is related to the degree of sexual maturity, it may be used as an indicator in addition to the ovarian development to elucidate post-diapause reproductive development. Although the discrepancy between diapause rate and wing damage seen in the present study is unclear, it is probably due to the difference of the mechanism underlying the mating behavior and diapause (Endo 1973). If we could measure the amount of scales or changes of fringe of wings in this butterfly, wing damage would reflect the strength (weak or strong) of the diapause termination more precisely.
References
Broufas GD, Pappas ML, Koveos DS (2006) Effect of cold exposure and photoperiod on diapause termination of the predatory mite Euseius finlandicus (Acari: Phytoseiidae). Environ Entomol 35:1216–1221
de Kort CAD (1990) Thirty-five years of diapause research with the Colorado potato beetle. Entomol Exp Appl 56:1–13
Endo K (1970a) Relation between accumulation of secretory fluid in the accessory gland of the female genital organ activity of the corpus allatum in Polygonia c-aureum L. Zool Mag 82:53–58 (in Japanese with English summary)
Endo K (1970b) Relation between ovarian maturation and activity of the corpora allata in seasonal forms of the butterfly, Polygonia c-aureum L. Dev Growth Differ 11:297–304
Endo K (1973) Hormonal regulation of mating in the butterfly, Polygonia c-aureum L. Dev Growth Differ 15:1–10
Fantinou AA, Tsitsipisk JA, Karandinos MG (1998) Diapause termination in Sesamia nonagrioides (Lepidoptera: Noctuidae) under laboratory and field conditions. Environ Entomol 27:53–58
Fujita KM. Inoue M, Watanabe A, Islam TMF, Shahjahan RM, Endo K, Yamanaka A (2009) Photoperiodic regulation of reproductive activity in summer- and autumn-morph butterflies of Polygonia c-aureum L. Zool Stud 48:291–297
Fukuda S, Endo K (1966) Hormonal control for the development of seasonal forms in the butterfly, Polygonia c-aureum L. Proc Jpn Acad 42:1082–1087
Han E-N, Bauce E (1996) Diapause development of spruce budworm larvae, Choristoneura fumiferana (Clem) (Lepidoptera: Tortricidae), at temperatures favoring post-diapause development. Can Entomol 128:167–169
Hidaka T, Aida S (1963) Day length as the main factor of seasonal form determination in Polygonia c-aureum (Lepidoptera, Nymphalidae). Zool Mag 72:77–83
Hidaka T, Takahashi H (1967) Temperature conditions and maternal effect as modifying factors in photoperiodic control of the seasonal form in Polygonia c-aureum (Lepidoptera: Nymphalidae). Annot Zool Japon 40:200–204
Hiroyoshi S (1992) Effects of photoperiod and temperature on several pupal characters associated with imaginal polyphenism in Polygonia c-aureum (Lepidoptera, Nymphalidae). Appl Entomol Zool 27:155–159
Hiroyoshi S, Reddy GVP, Mitsuhashi J (2017) Effects of juvenile hormone analogue (methoprene) and 20-hydroxyecdysone on reproduction in Polygonia c-aureum (Lepidoptera: Nymphalidae) in relation to adult diapause. J Comp Physiol A 203:635–647
Hodek I (1970) Termination of diapause in two Coccinellids (Coleoptera). Acta Entomol Bohemoslov 67:218–222
Hodek I (1974) Reactivation of diapausing Aelia acuminate adults before hibernation (Heteroptera). Acta Entomol Bohemoslov 71:65–71
Hodek I (1979) Intermittent character of adult diapause in Aelia acuninata (Heteroptera). J Insect Physiol 25:867–871
Hodek I (1999) Environmental regulation and some neglected aspects of insect diapause. Entomol Sci 2:533–537
Hodek I (2002) Controversial aspects of diapause development. Eur J Entomol 99:163–173
Hodek I, Hodkova M (1988) Multiple role of temperature during insect diapause: a review. Entomol Exp Appl 49:153–165
Hodek I, Ruzicka Z (1977) Insensitivity to photoperiod after diapause in Semiadalia undecimnotata (Col.: Coceinellidae). Entomophaga 22:169–174
Hodek I, Hodkova M, Semyanov VP (1989) Physiological state of Coccinella septempunctata adults from northern Greece sampled in mid-hibernation. Acta Entomol Bohemoslov 86:241–251
Hodkova M, Hodek I, Sommer L (1989) Cold in not prerequisite for the completion of photoperiodically induced diapause in Dolycoris baccarum from Norway. Entomol Exp Appl 52:185–188
Honěk A, Hodek I (1973) Diapause of Chrysopa carnea (Chrysopidae: Neuroptera) females in the field. Vestnik Ceskosl Spol Zool 37:95–100
Jiang XF, Huang SH, Luo LZ, Zhang L (2010) Diapause termination, post-diapause development and reproduction in the beet webworm, Loxostege sticticalis (Lepidoptera: Pyralidae). J Insect Physiol 56:1325–1331
Koštάl V (2006) Eco-physiological phases of insect diapause. J Insect Physiol 52:113–127
Koštάl V, Tollarova M, Doležel D (2008) Dynamism in physiology and gene transcription during reproductive diapause in a heteropteran bug, Pyrrhocoris apterus. J Insect Physiol 54:77–88
Leather SR, Walters KFA, Bale JS (1993) The ecology of insect overwintering. Cambridge University Press, Cambridge
Leong KLH, Frey D, Hamaoka D, Honma K (1993) Wing damage in overwintering populations of monarch butterfly at two California sites. Ann Entomol Soc Am 86:728–733
Milonas PG, Savopoulou-Soultani M (2004) Diapause termination in overwintering larvae of a Greek strain of Adoxophyes orana (Lepidoptera: Tortricidae). Environ Entomol 33:513–519
Musolin DL, Fujisaki K, Numata H (2007) Photoperiodic control of diapause termination, colour change and postdiapause reproduction in the southern green stink bug, Nezara viridula. Physiol Entomol 32:64–72
Numata H, Hidaka T (1982) Photoperiodic control of adult diapause in the bean bug, Riptortus clavatus Thunberg (Heteroptera: Coreidae). I. Reversible induction and termination of diapause. Appl Entomol Zool 17:530–538
Propp GD, Tauber MJ, Tauber CA (1969) Diapause in the neuropteran Chrysopa oculata. J Insect Physiol 15:1749–1757
Raabe M (1986) Insect reproduction of successive steps. Advan Insect Physiol 19:29–154
Tauber MJ, Tauber CA (1973) Nutritional and photoperiodic control of the seasonal reproductive cycle in Chrysopa Mohave (Neuroptera). J Insect Physiol 19:729–736
Tauber MJ, Tauber CA (1974) Thermal accumulations, diapause and oviposition in a conifer-inhabiting predator, Chrysopa harrisii (Neuroptera). Can Entomol 106:969–978
Tauber MJ, Tauber CA (1976) Insect seasonality. Diapause maintenance, termination and post-diapause development. Ann Rev Entomol 21:81–107
Tauber MJ,. Tauber CA, Masaki S (1986) Seasonal adaptation of insects. Oxford University Press, New York
Wada T, Kobayashi M (1985) Seasonal changes of wing wear of Cnaphalocrocis medinalis Guenee in paddy fields. Jpn J Appl Entomol Zool 29:41–44 (In Japanese.with English summary)
Watanabe M, Ando S (1993) Influence of mating frequency on lifetime fecundity I in wild females the small white Pieris rapae (Lepidoptera: Pieridae). Jpn J Entomol 61:691–696 (In Japanese with English summary)
Watanabe M, Nakanishi Y (1996) Population structure and dispersals of the sulfur butterfly Colias erate (Lepidoptera: Pieridae) in an isolated plain located in a cool temperate zone of Japan. Jpn J Entomol 64:17–29 (In Japanese with English summary)
Watt WB, Chew FS, Snyder LRG, Watt AG, Rothshild DE (1977) Population structure of pierid butterflies. I. Numbers and movements of some montane Colias species. Oecologia 27:1–22
Xiao H-J, Mou F-C, Zhu X-F, Xue F-S (2010) Diapause induction, maintenance and termination in the rice stem borer Chilo suppressalis (Walker). J Insect Physiol 56:1558–1564
Yang H-Z, Tu X-Y, Xia Q-W, He H-M, Chen C, Xue F-S (2014) Photoperiodism of diapause induction and diapause termination in Ostrinia furnacalis. Entomol Exp Appl 153:34–46
Acknowledgements
We thank for Dr. S. Tanaka of the National Institute of Agrobiological Sciences (NIAS) for critical reading of the manuscript. Thanks are also due to Dr. S. Moriya, Dr. K. Tateishi and Mr. K. Takashino for their help.
Author information
Authors and Affiliations
Ethics declarations
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animal were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Hiroyoshi, S., Reddy, G.V.P. & Mitsuhashi, J. Effects of photoperiod, temperature and aging on adult diapause termination and post-diapause development in female Asian comma butterflies, Polygonia c-aureum Linnaeus (Lepidoptera: Nymphalidae). J Comp Physiol A 204, 849–858 (2018). https://doi.org/10.1007/s00359-018-1284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-018-1284-y