Abstract
The larvae of Curculio elephas (Coleoptera: Curculionidae) and Polyphylla fullo (Coleoptera: Scarabaeidae) are major agricultural pests of chestnut and roots of cultivated crops, respectively. Previous research showed that they are relatively resistant to nematode infection. Accordingly, we evaluated the efficacy of Steinernema glaseri, S. weiseri or Heterorhabditis bacteriophora alone or in combination against these two insect pests. A nematode concentration of 50 or 100 infective juveniles (IJs)/larva for C. elephas or 50 or 100 IJs/cm2 for P. fullo at 25 °C was used. The highest (81 %) and the lowest (21 %) larval mortalities for C. elephas were obtained with S. weiseri+H. bacteriophora combined application and S. glaseri alone, respectively. The nature of the interactions (antagonism, additive, or synergy) for the larval mortality was evaluated. For C. elephas, S. weiseri combined with S. glaseri or H. bacteriophora was additive, whereas the combination of S. glaseri+H. bacteriophora was antagonistic. For P. fullo, the efficiency of nematodes used alone or combinations was very low, and there were no significant differences among the treatments at 50 or 100 IJs/cm2. The interaction was additive with the combinations of S. glaseri+H. bacteriophora against P. fullo larvae. No synergistic effect was observed for any combination against C. elephas and P. fullo larvae. Our results show that the EPN species tested, either alone or in combination, are not economically feasible for use against C. elephas or P. fullo, but that further research with other combinations of EPN species or EPNs with other entomopathogens is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chestnut, Castanea sativa (Fagales, Fagaceae), is one of the most important agricultural crops in Turkey with annual production of more than 60,000 tons (Ertan and Seferoglu 2003). One of the most serious pests affecting chestnut production is the chestnut weevil, Curculio elephas (Coleoptera: Curculionidae), which has the potential to proliferate rapidly and is expanding its geographic range (Avtzis and Cognato 2013). The weevil adults emerge from the soil in August and the females lay their eggs on or in the chestnuts where the larvae feed on the kernel for about 2 months. In October, the majority of the last larval stage emerge from the infested kernels and enter the soil to pupate where they remain for at least 9 months (Desouhant 1998; Speranza 1999). Control of the larval stages of chestnut fruit pests is difficult because they occur within the chestnut fruit and then overwinter in the soil.
Polyphylla fullo (Coleoptera: Scarabaeidae) is a major polyphagous agricultural pest because the larvae feed on the roots of many important cultivated plants. It has a 2- to 3-year life cycle with three larval stages that feed on plant roots causing extensive damage, and, in severe infestations, can cause yield loss and plant death (Anonymous 2008). One of the main crops affected by P. fullo is strawberry. Because there is no registered pesticide against this white grub species in Turkey, strawberry producers resort to using chlorpyrifos-ethyl and parathion-methyl which are registered for insect control on fruit trees and vineyards, respectively (Anonymous 2008). These chemical pesticides are used individually or together at high concentrations, but the results have been unsatisfactory.
Research on developing alternative control methods for suppressing C. elephas and P. fullo is needed. One of the possible methods is using entomopathogenic nematodes (EPNs) against the larval stages that occur in the soil. For C. elephas, the approach is to reduce the overwintering population and to prevent or reduce damage to the next generation of chestnuts. For P. fullo, it is to reduce the larval population and protect the strawberry roots from damage.
Entomopathogenic nematodes in the families Steinernematidae and Heterorhabditidae are obligate pathogens in nature with the free-living, third-stage infective juveniles (IJs) searching for and infecting their insect host in the soil environment (Kaya and Gaugler 1993). They are associated with mutualistic bacteria in the genus Xenorhabdus for Steinernema and Photorhabdus for Heterorhabditis, and these bacteria are housed in the IJs in a specialized intestinal lumen in the case of steinernematids and in the intestine of heterorhabditids (Hazir et al. 2003). The IJ infects the insect host by entering through natural openings (mouth, spiracles, or anus) or thin areas of the host’s cuticle (common in heterorhabditids) and penetrates into the host’s hemocoel. The IJ then releases the bacterium which propagates and causes septicemia that kills the host in 48–72 h. The nematode resumes its development, feeding on the bacterial cells and host tissues that have been metabolized by the bacterium and goes through 1–3 generations, depending on host size. As the food resources in the host cadaver are depleted, a new cohort of IJs is produced and emerges from the host cadaver into the soil to search for new hosts (Kaya and Gaugler 1993). Most EPN species can infect a variety of insects, especially if ecological and behavioral barriers are removed. On other hand, some insect species are partially or completely resistant to EPN infection due to behavioral, morphological, or physiological defense mechanisms (Gaugler et al. 1994; Koppenhöfer et al. 2000).
In a laboratory study conducted in Turkey, Karagoz et al. (2009) demonstrated that the last instar larvae of C. elephas and Cydia splendana (Lepidoptera: Tortricidae), another pest of chestnut, responded differently to infection by three Turkish isolates of Steinernema feltiae, S. weiseri, and Heterorhabditis bacteriophora. C. splendana larvae were highly susceptible, whereas C. elephas larvae were relatively resistant to the tested EPN species. In another laboratory study, Kepenekci et al. (2004) showed that two Turkish isolates of H. bacteriophora caused 72.1 and 96.5 % mortality, respectively, of C. elephas larvae at 25 °C using 500 IJs/cup, whereas S. feltiae and S. carpocapsae were less effective. They also demonstrated that the two Turkish H. bacteriophora isolates required 266 and 494 IJs to kill 50 % of C. elephas larvae at 15 °C. Thus, C. elephas larvae are not easily killed by EPNs. Against P. fullo larvae, Karagoz et al. (2007) evaluated the efficiency of 35 new EPN isolates and reported that this white grub species was highly resistant to EPN infection. Similarly, Karimi et al. (2010) tested H. bacteriophora against the white grub Polyphylla adspersa in Iran and obtained only 42 % mortality. We focused our research on increasing the efficacy of EPNs on C. elephas and P. fullo because they are important pests in Turkey, are difficult to control with chemical insecticides, and have immature life cycle stages in the soil, and there is a need for alternate, safer control agents for these pests.
The standard approach of using EPNs against C. elephas and P. fullo is not feasible because they are resistant to EPN infection. A different application tactic has been used against insect pests that are naturally resistant to EPN infection, and this approach may be feasible against the two pests. For example, Choo et al. (1996) tested the efficiency of combinations of two EPN species against second-stage larvae of Diabrotica undecimpunctata (Coleoptera: Chrysomelidae) in the laboratory. They demonstrated that a combination of two nematode species was more efficacious than one species alone. In another study by Sankar et al. (2009), a combination of H. indica and S. asiaticum killed larvae of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) more quickly than each EPN species applied alone. Combined applications of three different nematode species, H. bacteriophora, S. kushidai, and S. glaseri, showed additive interactions against the third-instar masked chafer Cyclocephala hirta (Coleoptera: Scarabaeidae) and oriental beetle Exomala (=Anomala) orientalis (Coleoptera: Scarabaeidae) (Koppenhöfer et al. 2000). Accordingly, we hypothesized that using a combination of different EPN species against C. elephas and P. fullo larvae may be more efficacious than a single EPN species. Our objective was also to evaluate whether combined application of EPNs results in an antagonistic, additive, or synergistic effect on C. elephas or P. fullo larvae.
Materials and methods
Insects
Last instar larvae of C. elephas emerged from the infested chestnuts were collected from a chestnut processing factory in Aydin and used within 3 days. The 2nd and 3rd instar P. fullo larvae were collected from strawberry fields in the Umurlu district of Aydin, Turkey. The larvae were kept for a minimum of 1 week in 20-l plastic pots (8–10/pot) filled with sterilized soil collected from the strawberry fields (to detect and eliminate diseased and/or injured individuals) and fed sliced carrots before they were used in the experiments.
Late instar larvae of the greater wax moth, Galleria mellonella (Lepidoptera: Pyralidae), reared on an artificial medium as described by Han and Ehlers (2000) were used to produce IJs.
Nematodes
Steinernema glaseri (Belgian isolate) isolated and identified by Ansari et al. (2005), and the Turkish isolates of S. weiseri (09-01) isolated and identified by Unlu et al. (2007) and H. bacteriophora (09-20) identified by Dr. Patricia Stock by morphological/morphometric and molecular techniques (unpublished) were reared in last instar G. mellonella larvae (Kaya and Stock 1997) and stored in distilled water at 10 °C in tetra pak boxes (Gulcu and Hazir 2012) for up to 2 weeks before use in experiments.
EPNs against Curculio elephas larvae
The experiments were conducted in 24-well tissue culture plates. Each well of the tissue culture plates was filled with 0.5 g autoclaved and air-dried sandy soil (87.4 % sand and 12.6 % silt) and 60 μl distilled water was added to each treatment. Soil moisture was 10 % (w/w). After 1 h at room temperature (23–24 °C) to allow for acclimatization of the IJs, one C. elephas larva was added to each well. Treatments were S. glaseri, S. weiseri, or H. bacteriophora alone at 50 and 100 IJs/larva, two-species combinations of each EPN species at 50 IJs per species (100 IJs total), and a water only control. There was one replicate (24 insects) for each treatment and the control, and the experiment was repeated three times on different dates.
The treated tissue culture plates were placed into plastic bags to minimize moisture loss and kept at room temperature. Each larva was checked daily for 10 days using a probe to determine whether it was alive or dead. All dead larvae were transferred individually to White traps to confirm EPN infection by the production of IJs emerging over a 2-week period.
EPNs against Polyphylla fullo larvae
The first experiment was conducted at room temperature in 350-ml plastic containers (30 cm2 surface area) filled with 100 g autoclaved and air-dried loamy soil (48 % sand, 10 % clay, 42 % loam) prepared at 10 % (w/w) soil moisture. The soil had been collected from an infested strawberry field. One P. fullo larva was added to each container, and a slice of carrot was placed into the soil for food. After 1 h, treatments in 3 ml of water were applied to soil surface with a pipette. Treatments were S. glaseri alone and H. bacteriophora alone [both at 1,500 and 3,000 IJ per container (50 and 100 IJs/cm2)], their combination (1,500 IJs of each species per container), and a water only control. There were 10 replications per treatment, and the experiment was conducted three times on different dates. The mortality of P. fullo larvae was recorded daily for 10 days. Dead larvae were transferred individually to White traps, incubated at room temperature, and checked for IJ emergence over a 3-week period.
A second experiment was conducted in plastic pots (1.3 l volume; 13 cm depth × 12 cm diameter; surface area = 113 cm2) filled with 1 kg of the same sandy soil as used above. One larva along with a piece of carrot was placed into each pot. The treatments were (1) S. glaseri alone, (2) H. bacteriophora alone [both at 11,300 IJs per container (100 IJs/cm2)], (3) their combination (11,300 IJs of each species per container), and (4) water only control.
There were 10 replications for each treatment and the experiment was repeated three times on different dates. The mortality of P. fullo larvae was recorded after 10 days, and dead larvae were transferred individually to White traps for monitoring nematode emergence for 3 weeks.
Further verification of nematode-caused mortality
The color of the cadaver and the length of the new generation of IJs were used to determine which nematode species caused the larval mortality and reproduced in the cadaver. This verification was done for the nematode combination treatments for both C. elephas and P. fullo cadavers. Red cadavers indicated H. bacteriophora infection, whereas light-brown cadavers indicated S. glaseri or S. weiseri infections. A subsample was taken from each cadaver and the IJs were heat-killed by hot water (60 °C for 2 min). Twenty IJs were randomly selected and measured with a Leica IM50 microscope equipped with automatic measurement system.
Statistics
The treatment mortality was adjusted for control mortality (<10 %) using Abbott’s formula (1925). Percentage data obtained from 3 replications were combined in the same analyses and arcsine-transformed before statistical analysis. One-way ANOVA was used to compare larval mortality among treatments and the means separated using Tukey’s test (P = 0.05) (SPSS 2004).
The nature of interaction in all experiments between nematode species on larval mortality was determined through a comparison of expected and observed percentage larval mortality (Shapiro-Ilan et al. 2004). Expected mortality was calculated with the formula P E = P 0 + (1−P 0)(P 1) + (1−P 0)(1−P 1)(P 2), (P E: expected mortality; P 0: control mortality; P 1: mortality from one nematode applied alone; P 2: mortality from the other nematode applied alone). A Chi square (χ 2) test was applied to the observed and expected results. χ 2 = (L 0−L E)2/L E + (D 0−D E)2/D E (Lo: the number of living larvae observed; L E: the number of living larvae expected; Do: the number of dead larvae observed; D E: the number of dead larvae expected). If the value of χ 2 < 3.84 = additive, χ 2 > 3.84 and Pc–PE positive = synergistic, χ 2 > 3.84 and PC–PE negative = antagonistic (PC: observed mortality; PE: expected mortality).
Results
EPNs against Curculio elephas larvae
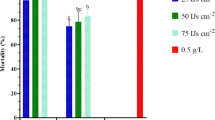
Statistically, the highest larval mortalities were obtained with S. weiseri+H. bacteriophora combination application followed by H. bacteriophora and S. weiseri at 100 IJs alone, and H. bacteriophora at 50 IJs alone. These mortalities were significantly different from S. glaseri alone at 50 and 100 IJ concentrations, respectively (F = 15, 414; df = 8, 17; P < 0.0001) which had the lowest larval mortality (Fig. 1). However, there were no significant differences among H. bacteriophora or S. weiseri at 100 IJs alone or H. bacteriophora at 50 IJs alone or S. weiseri at 50 IJs alone, or S. weiseri+S. glaseri combination, or S. glaseri+H. bacteriophora combination. In addition, there were no significant differences among S. weiseri at 50 IJs alone, or S. weiseri+S. glaseri or S. glaseri+H. bacteriophora combination when compared with S. glaseri at 100 IJs alone (P > 0.05) (Fig. 1). S. weiseri+S. glaseri (57 % ± 9.3) and S. weiseri+H. bacteriophora (81 % ± 3.1) resulted in additive effects, whereas the combination of S. glaseri+H. bacteriophora (50 % ± 7.1) showed an antagonism interaction (Fig. 1; Table 1).
Mean mortality (%) of Curculio elephas larvae by entomopathogenic nematode species alone or in combination. Different lower case letters above the bars indicate significant differences at P < 0.05. S. w Steinernema weiseri, S. g S. glaseri, H. b Heterorhabditis bacteriophora. White bar 50 IJ concentration of one nematode species; black bar 100 IJ concentration of one nematode species; hatched bar 100 IJ concentration with 50 IJs of one species and 50 IJs of another species. Symbol above shaded bars indicates (hyphen) antagonistic or (filled star) additive interaction
Verification of nematode-caused mortality in Curculio elephas cadavers
Combination of S. glaseri+H. bacteriophora
When S. glaseri and H. bacteriophora species were applied together, H. bacteriophora reproduced in 56 % (±11.0) and 53 % (±15.6) of the cadavers, whereas S. glaseri species reproduced in 44 % (±13.5) and 47 % (±15.7) of the nematode-killed insects at 50 and 100 IJs/larva, respectively. S. glaseri and H. bacteriophora did not produced progeny from the same cadavers.
Combination of S. weiseri+H. bacteriophora
At both concentrations, S. weiseri had more cadavers with progeny than H. bacteriophora. At 50 IJs/larva, S. weiseri and H. bacteriophora had 62 % (±22.0) and 38 % (±22.5) of the cadavers with progeny, respectively. At 100 IJs/larva, 60 % (±24.9) and 40 % (±24.9) reproduction were observed for S. weiseri and H. bacteriophora, respectively. No progeny of S. weiseri and H. bacteriophora together was observed in the same cadaver.
Combination of S. weiseri+S. glaseri
At 50 IJs/larva, S. weiseri had 58 % (±11.5) of the cadavers with progeny followed by S. glaseri at 36 % (±8.0) and S. weiseri and S. glaseri together in the same cadaver at 6 % (±6.9). Only 2 from 34 cadavers produced co-progeny. At 100 IJs/larva, S. glaseri reproduced in 69 % (±14.4) of the cadavers, whereas S. weiseri (±14.4) reproduced in 31 %. At this concentration, no progeny of S. weiseri and S. glaseri occurred together in the same cadaver.
EPNs against Polyphylla fullo larvae
In the plastic container experiments conducted at 50 IJs/cm2, the larval mortality was 2.9, 5.7, and 6.3 % for S. glaseri, H. bacteriophora, and the S. glaseri+H. bacteriophora combination, respectively. No statistical difference was observed among the treatments (F = 0.601; df = 2, 6; P > 0.05).
The combination of S. glaseri+H. bacteriophora resulted in an additive interactions of P. fullo mortality (Table 1). At 100 IJs/cm2, S. glaseri resulted in 8.8 % larval mortality followed by H. bacteriophora at 12 % and S. glaseri+H. bacteriophora at 9.9 %. There was no significant difference among the groups (F = 0.275; df = 2, 6; P > 0.05).
In the pot experiments, although S. glaseri+H. bacteriophora combination showed numerically more mortality (24 % ± 20.4) than S. glaseri (7 % ± 4.9) and H. bacteriophora (11 % ± 6.0) alone applications, there was no statistical difference among the treatments (F = 0.46; df = 2, 6; P > 0.05). However, the combination treatment of S. glaseri+H. bacteriophora did cause an additive interaction of P. fullo mortality (Table 1).
Verification of nematode-caused mortality in Polyphylla fullo cadavers
In the plastic container experiments, when S. glaseri and H. bacteriophora were applied together, only S. glaseri reproduced into the cadavers. In the pot experiments, 71 % of the cadavers produced S. glaseri IJs, whereas 29 % of the cadavers produced H. bacteriophora. No mixed progeny were observed in the same cadaver.
Discussion
The combined application of entomopathogens can result in antagonistic, additive, or synergistic effects on the target pest. In our study, additive and antagonistic interactions were encountered in certain nematode combinations against C. elephas and P. fullo larvae. However, no synergistic effect was observed from any of the combinations.
Entomopathogenic nematodes are often applied to systems that are regularly treated with many other agents, including chemical or biorational pesticides, other biological control agents, soil amendments, and fertilizers (see review by Koppenhöfer and Choo 2005). With entomopathogens, synergism was observed with the combine application such as the nematode–fungal interactions (Barbercheck and Kaya 1990; Choo et al. 2002). In a field study conducted in Korea, Beauveria brogniartii, H. bacteriophora, and S. carpocapsae species alone and their combinations were tested against Exomala orientalis larvae. The nematode combination did not increase the efficacy compared to treatments with one nematode species alone. However, the EPN and fungal combination (S. carpocapsae+B. brongniartii) significantly enhanced grub mortality over the application of the fungus alone (Choo et al. 2002). In another study, the combinations of Beauveria bassiana with S. carpocapsae or H. indica showed an antagonistic interaction for suppression of Curculio caryae (Coleoptera: Curculionidae) larvae (Shapiro-Ilan et al. 2004). Antagonism was also observed between the fungus, Paecilomyces fumosoroseus, combined with H. indica or S. carpocapsae.
The results obtained with EPN and entomopathogenic bacterium combinations varied from synergism to antagonism. Some studies reported synergistic interaction (Thurston et al. 1993, 1994; Koppenhöfer and Kaya 1997; Koppenhöfer et al. 1999), whereas antagonism was also observed from EPN and entomopathogenic bacterium combinations (Shapiro-Ilan et al. 2004). Laboratory and field studies showed that synergistic or additive effect occurred when Bacillus thuringiensis subsp. japonensis was combined with H. bacteriophora or S. glaseri against second or early third instar Cyclocephala hirta and C. pasadenae and third instar E. orientalis (Koppenhöfer and Kaya 1997; Koppenhöfer et al. 1999).
In the combined application of two different EPN species against different insect hosts, synergism or additive interactions have been reported. Choo et al. (1996) tested the efficacy of four different nematode species or strains applying them alone or in combination with one of the other species against D. undecimpunctata larvae. In the laboratory study, there was no significant difference between expected and observed mortality and the interaction of the nematodes was additive (χ 2 = 0.076), but in the greenhouse study, no advantage was obtained from the nematode combination. Sankar et al. (2009) applied H. indica+S. asiaticum against the rice leaf folder, C. medinalis, and observed faster and higher percent mortality in the combination treatment than with one EPN species alone. As the authors did not calculate the expected and observed mortality and χ 2 values, it is not known whether the interactions were synergistic or additive. In our study, combinations of S. weiseri showed additive interactions, whereas combinations of S. glaseri and H. bacteriophora resulted in antagonistic interaction against C. elephas larvae. S. glaseri alone was not as effective as other EPN species when it was used alone. On the other hand, when host species changed, S. glaseri and H. bacteriophora combination showed additive effects on P. fullo larvae. Several hypotheses have been proposed to explain these different results varying from synergism to antagonism. It was speculated that the differences might be related to the isolates or species of nematodes and combined pathogens (Koppenhöfer and Kaya 1997; Choo et al. 2002; Shapiro-Ilan et al. 2004, 2011), host species and stages (Koppenhöfer et al. 1999), timing of application (Koppenhöfer and Kaya 1997), and interaction between their mutualistic bacteria and the environmental conditions such as temperature and humidity (Sankar et al. 2009). The control potential of H. bacteriophora+S. kushidai, H. bacteriophora+S. glaseri, and S. kushidai+S. glaseri combinations were evaluated against the third instar C. hirta, and E. orientalis in the greenhouse. Mortality in the combinations was not significantly higher than single nematode treatments and the interactions were additive for all combinations (Koppenhöfer et al. 2000).
As discussed above, the studies with combining an EPN species with another EPN species or other entomopathogens have provided mixed laboratory, greenhouse, and field results. The results depended on which EPN species is combined with the other entomopathogens including other EPN species, the target pest, and environmental conditions. As far as we are aware, the only EPN combination field studies have been conducted by Choo et al. (2002) combining EPNs with entomopathogenic fungi and by Koppenhöfer et al. (1999) combining EPNs with an entomopathogenic bacterium. In our laboratory study, although we showed an additive effect with S. weiseri combined with S. glaseri or H. bacteriophora against C. elephas larvae, the increased mortality was not sufficient to justify field applications. Koppenhöfer and Grewal (2005) stated that “Combined aaplication of two agents is only useful if target mortality is synergistically increased.” With EPNs and other entomopathogens, they further stated that only the B. thuringiensis ssp. japonensis+H. bacteriophora or S. glaseri combination has provided synergistic interactions and can be recommended as a control measure against certain white grub species. However, B. thuringiensis subsp. japonensis is currently not commercially available, and, even if it is, the economic feasibility of the combination with EPNs would depend on the cost of each agent alone versus combining the two agents.
What is the future of combining EPN species or EPNs with other entomopathogens? There are so many EPN species and other entomopathogens that are being discovered that certain combinations may provide synergistic interactions and prove useful against a given notably resistant soil pest (Koppenhöfer and Kaya 1997; Koppenhöfer et al. 1999).
Interspecific competition of the nematodes within the same cadaver showed that nematode species, host species, inoculum size, and physical factors such as experimental arena were crucial factors that determined which nematode species reproduced (Akhurst 1983; Dunphy et al. 1985; Alatorre-Rosas and Kaya 1990; Koppenhöfer et al., 1995). With the H. bacteriophora+Steinernema spp. combinations in C. elephas larvae, H. bacteriophora species produced progeny in some cadavers in the combined application with S. weiseri or S. glaseri, but only one nematode species successfully reproduced in the cadaver. In the H. bacteriophora+S. glaseri combination, more cadavers produced H. bacteriophora progeny than S. glaseri. However, when the host was P. fullo larva, H. bacteriophora did not produce any progeny in the pathogenicity tests, whereas S. glaseri did. Koppenhöfer et al. (1995) indicated that some host species are more susceptible and better hosts for some nematode species. On the other hand, H. bacteriophora or S. glaseri produced progeny from P. fullo cadavers in the pot experiments. This result may be related to the physical conditions of the experimental arena. Pathogenicity tests were conducted in a small arena (350 ml volume and 100 g soil), whereas the pot experiments were conducted in larger arena (1,300 ml volume and 1 kg soil). H. bacteriophora is a cruiser characterized by high motility and is distributed throughout the soil profile (Campbell et al. 2003). Therefore, this species could have used its foraging strategy more effectively in the larger arena and compete with the cruiser, S. glaseri, for a host.
In our study, coexistence and progeny production were observed between Steinernema spp. but not between steinernematids and heterorhabditids. It is known that the association between nematode and bacterial symbiont is not completely specific. That is, several Steinernema spp. have been cultured with the Xenorhabdus symbionts isolated from other Steinernema spp. (Akhurst 1983). Accordingly, the occurrence of more than one Steinernema spp. in an insect host has been reported in several studies (Kaya 1984; Dunphy et al. 1985; Choo et al. 1987; Kondo 1989; Koppenhöfer et al. 1995). We confirmed that S. weiseri and S. glaseri can reproduce in the same C. elephas cadaver. In contrast, Akhurst (1983) found that Steinernema spp. could not be cultured on the Photorhabdus symbionts of Heterorhabditis spp. and Alatorre-Rosas and Kaya (1990) reported that both Steinernema and Heterorhabditis could infect the same insect, yet neither nematode species survived to produce progeny. In our study, we did not check for nematode development within the cadavers, but we did observe the emergence of new generation IJs. As only one nematode species emerged when challenged with both a Steinernema spp. and H. bacteriophora, we conclude that only one species successfully infected a C. elephas and P. fullo host.
In conclusion, although the combination of S. weiseri+H. bacteriophora did produce higher larval mortality of C. elephas than the other treatments, it was not significant compared with some of the one nematode species alone treatment. Therefore, the use of combining nematode species to control C. elephas cannot be justified until a more effective nematode or other pathogen combination is found. We did observe an additive effect on the combined application of S. glaseri+H. bacteriophora against P. fullo larvae, but the result was far from satisfactory. If a combination of biological control agents is to be used for P. fullo larvae or even C. elephas, different control tactics with different combinations of pathogens such as entomopathogenic fungus, virus, or bacterium may be needed. Possibly, a more effective pathogen combination that controls these pests can be found.
References
Abbott WS (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Akhurst RJ (1983) Neoaplectana species: specificity of association with bacteria of the genus Xenorhabdus. Int J Syst Bacteriol 33:38–45
Alatorre-Rosas R, Kaya HK (1990) Interspecific competition between entomopathogenic nematodes in the genera Heterorhabditis and Steinernema for an insect host in sand. J Invertebr Pathol 55:179–188
Anonymous. 2008. T.C. Tarim ve Koyisleri Bakanligi Tarimsal Arastirmalar Genel Mudurlugu, Zirai Mucadele Teknik Talimatlari. Cilt 4, Ankara, 316–318
Ansari MA, Waeyenberge L, Moens M (2005) First record of Steinernema glaseri Steiner, 1929 (Rhabditida: Steinernematidae) from Belgium: a natural pathogen of Hoplia philantus (Coleoptera: Scarabeidae). Nematology 7:953–956
Avtzis DN, Cognato AI (2013) Genetic structure of Curculio elephas Gyll. (Coleoptera, Curculionidae) in Greece: an important pest of sweet chestnut. J Pest Sci 86:491–497
Barbercheck ME, Kaya HK (1990) Interactions between Beauveria bassiana and the entomogenous nematodes Steinernema feltiae and Heterorhabditis heliothidis. J Invertebr Pathol 55:225–234
Campbell JF, Lewis EE, Stock SP, Nadler S, Kaya HK (2003) Evolution of host search strategies in entomopathogenic nematodes. J Nematol 35:142–145
Choo HY, Kaya HK, Shea P, Noffsinger EM (1987) Ecological study of nematode parasitism in Ips beetles from California and Idaho. J Nematol 19:495–502
Choo HY, Koppenhöfer AM, Kaya HK (1996) Combination of two entomopathogenic nematode species for suppression of an insect pest. J Econ Entomol 89:97–103
Choo HY, Kaya HK, Huh J, Lee DW, Kim HH, Lee SM, Choo YM (2002) Entomopathogenic nematodes (Steinernema spp. and Heterorhabditis bacteriophora) and a fungus Beauveria brongniartii for biological control of the white grubs, Ectinohoplia rufipes and Exomala orientalis, in Korean golf courses. Biocontrol 47:177–192
Desouhant E (1998) Selection of fruits for oviposition by the chestnut weevil, Curculio elephas. Entomol Exp Appl 86:71–78
Dunphy GB, Rutherford TA, Webster JM (1985) Growth and virulence of Steinernema glaseri influenced by different subspecies of Xenorhabdus nematophilus. J Nematol 17:476–482
Ertan E, Seferoglu G (2003) The comparison of the biochemical characteristics of chestnut at fruit ripening and after traditional storage periods. Biosci Res Bull 19:139–149
Gaugler R, Wang Y, Campbell JF (1994) Aggressive and evasive behaviors in Popillia japonica (Coleoptera: Scarabaeidae) larvae: defenses against entomopathogenic nematode attack. J Invertebr Pathol 64:193–199
Gulcu B, Hazir S (2012) An alternative storage method for entomopathogenic nematodes. Turk J Zool 36:562–565
Han RC, Ehlers RU (2000) Pathogenicity, development, and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. J Invertebr Pathol 75:55–58
Hazir S, Kaya HK, Stock SP, Keskin N (2003) Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turk J Biol 27:181–202
Karagoz, M., Gulcu, B. and Hazir, S. 2007. Manas larvalarının (Coleoptera: Scarabeidae) biyolojik mücadelesinde entomopatojenik nematodların test edilmesi. I. Entomopatojenler ve Mikrobiyal Mücadele Sempozyumu, 21–24 Haziran, Trabzon
Karagoz M, Gulcu B, Hazir S, Kaya HK (2009) Laboratory evaluation of Turkish entomopathogenic nematodes for suppression of the chestnut pests, Curculio elephas (Coleoptera: Curculionidae) and Cydia splendana (Lepidoptera: Tortricidae). Biocontrol Sci Techn 19:755–768
Karimi, J., Rezapanah, M., Monfared, F. and Mirsaeidi, H. 2010. Biological control potential of an entomopathogenic preparation of Heterorhabditis bacteriophora on the white grub Polyphylla adspersa. Society for Invertebrate Pathology meeting, 11–15 July, Trabzon Turkey
Kaya HK (1984) Nematode parasites of bark beetle. In: Nickle WR (ed) Plant and insect nematodes. Marcel Dekker, New York, pp 727–754
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Annu Rev Entomol 38:181–206
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey LA (ed) Manual of techniques in ınsect pathology. Academic, London, pp 281–324
Kepenekci I, Gokce A, Gaugler R (2004) Virulence of three species of entomopathogenic nematodes to the chestnut weevil, Curculio elephas (Coleoptera: Curculionidae). Nematropica 34:199–204
Kondo E (1989) Studies on the infectivity and propagation of entomogenous nematodes, Steinernema spp. (Rhabditida: Steinernematidae) in the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Bull Fac Agric 67:1–87
Koppenhöfer AM, Grewal PS (2005) Compatibility and interactions with agrochemicals and other biological control agents. In: Grewal PS, Ehlers RU, Shapiro-Ilan DI (eds) Nematodes as biocontrol agents. CABI, Wallingford, pp 363–381
Koppenhöfer AM, Kaya HK (1997) Additive and synergistic interactions between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biol Control 8:131–137
Koppenhöfer AM, Kaya HK, Shanmugan S, Wood GL (1995) Interspecific competition between steinernematid nematodes within an insect host. J Invertebr Pathol 66:99–103
Koppenhöfer AM, Choo HY, Kaya HK, Lee DW, Gelernter WD (1999) Increased field and greenhouse efficacy against scarab grubs with a combination of an entomopathogenic nematode and Bacillus thuringiensis. Biol Control 14:37–44
Koppenhöfer AM, Grewal PS, Kaya HK (2000) Synergism of imidacloprid and entomopathogenic nematodes against white grubs: the mechanism. Entomol Exp Appl 94:283–293
Sankar M, Prasad JS, Padmakumari AP, Katti G, Divya K (2009) Combined application of two entomopathogenic nematodes, Heterorhabditis indica and Steinernema asiaticum to control the rice leaf folder, Cnaphalocrosis medinalis (Goen.). J Biopestic 2:135–140
Shapiro-Ilan DI, Jackson M, Reilly CC, Hotchkiss MW (2004) Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio caryae (Coleoptera: Curculionidae). Biol Control 30:119–126
Shapiro-Ilan DI, Cottrell TE, Wood BW (2011) Effects of combining microbial and chemical insecticides on mortality of the pecan weevil (Coleoptera: Curculionidae). J Econ Entomol 104:14–20
Speranza S (1999) Chestnut pests in central Italy. Acta Hort 494:417–423
SPSS. 2004. SPSS v.13.0 for windows. SPSS, Chicago
Thurston GS, Kaya HK, Burlando TM, Harrison RE (1993) Milky disease bacterium as a stressor to increase susceptibility of scarabaeid larvae to an entomopathogenic nematode. J Invertebr Pathol 61:167–172
Thurston GS, Kaya HK, Gaugler R (1994) Characterizing the enhanced susceptibility of milky disease-infected scarabaeid grubs to entomopathogenic nematodes. Biol Control 4:67–73
Unlu I, Ehlers R-U, Susurluk A (2007) Additional data and first record of the entomopathogenic nematode Steinernema weiseri from Turkey. Nematology 9:739–741
Acknowledgments
We thank Dr. Albrecht M. Koppenhöfer, Rutgers University, New Brunswick, NJ, for comments on an earlier version of the manuscript and Adnan Menderes University for support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.-U. Ehlers.
Rights and permissions
About this article
Cite this article
Demir, S., Karagoz, M., Hazir, S. et al. Evaluation of entomopathogenic nematodes and their combined application against Curculio elephas and Polyphylla fullo larvae. J Pest Sci 88, 163–170 (2015). https://doi.org/10.1007/s10340-014-0571-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0571-9