Abstract
The high cost of insecticides, the emergence of insecticide resistance in populations of a number of insect species and other problems arising from their continuous use, such as biological imbalance, cotton fiber waste, and environmental pollution, have prompted the development of new technologies aiming the control of Anthonomus grandis Boheman in cotton crops. This study evaluated the level of protection conferred by kaolin clay foliar spraying to cotton plants against boll weevil damage. Treatment-tested spraying kaolin or endosulfan on cotton plants. The highest percentage of oviposition-punctured squares were observed in the control, and the lowest percentages in the treatments sprayed with endosulfan and kaolin in a systematic manner and where the boll weevil reached the economic threshold at all assessments. The greatest numbers of non-attacked bolls by weevils and cotton-seed yield were observed under the endosulfan treatments, followed by the treatments of kaolin spraying. The smallest number of bolls and lowest cotton-seed yield were observed for the control plots. These finding are of practical significance because they may reduce the cotton production cost and environmental impacts of chemical pesticides and make possible the production of organic cotton with the presence of boll weevils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae), is originally from the tropics and subtropics of Mesoamerica (Burke et al. 1986), but its modern-day distribution extends from temperate parts of the U.S. Cotton Belt to Brazil and Argentina (Ramalho and Jesus 1988; Ramalho and Wanderley 1996; Rummel and Summy 1997). This insect has a high capacity for survival, reproduction, and dispersal. These physiologic and behavioral aspects explain why the boll weevil has been responsible for severe economic losses to the Brazilian cotton industry, and why it is considered a major pest of this crop (Ramalho and Silva 1993). The losses caused by the boll weevil in the northern Brazil are both direct and indirect, and extend throughout the entire social, financial, and economic structure of the region (Ramalho and Wanderley 1996). An estimate of the magnitude of yield loss can be estimated from field studies in which boll weevil injury has been eliminated (Ramalho and Santos 1994). In the states of the Paraiba and Pernambuco, Brazil, where the boll weevil originally caused yield losses of 54–87 %, increases in yield of seed-cotton over control (untreated plots) varied from 116 to 657 % (Ramalho and Santos 1994). Estimates indicate that one hectare of cotton (Gossypium hirsutum L.) can produce more than 1.5 million (exclude of) boll weevil adults, and in high pest population levels, the (exclude current) control techniques are not economically viable (Showler 2003, El-Sayed et al. 2006). Therefore, during some periods of cotton cultivation, (excluded commercial) chemical insecticides are (excluded directly) applied to the cotton square to eliminate and protect the crop against losses caused by this pest (Ramalho 1994; Ramalho and Wanderley 1996; Page et al. 1999; Ramalho et al. 2003). However, the high price of these products, increase of resistant insect populations, and other problems arising from their use, such as biological imbalance, cotton fiber waste and environmental pollution, have prompted the development of new technologies to control boll weevils (Ramalho and Wanderley 1996; Ramalho et al. 2003).
The technology of mineral particle films is a potential alternative to some insecticides in the control of different pests (Alavo and Abagli 2011). Kaolin is mineral composed of aluminum silicate (Al4Si4O10(OH)8) of fine grain, white color, flat, porous, and non-expanding, dissolving in water and chemically inert at a wide range of pH (Harben 1995). Preliminary studies have shown that kaolin has a deterrent effect on the feeding and oviposition behaviors of the boll weevil (Showler 2002), although the effectiveness of this product may be specific to some species of insects and must be studied for each pest in their own environment (Glenn and Puterka 2005). Kaolin has been shown to be effective against aphids (Cottrell et al. 2002; Wiss and Daniel 2004, Alavo and Abagli 2011); beetles (Showler 2002); fruit flies (Mazor and Erez 2004; Lo Verde et al. 2011), psyllids, leafhoppers and mites in various cropping systems (Puterka et al. 2000; Peng et al. 2011). The mechanisms of action of kaolin against (excluded targeted) insect pests include repellency, tactile or visual cue interference, impairment or disruption of oviposition and feeding activity, and decreased longevity and survivorship (Glenn and Puterka 2005). The abrasive mineral particles promote insect dissection due the disruption of their cuticle, obstruction of their digestive system, and also change the host plants color, affecting the recognition and attractiveness of the plant (Showler 2002).
Therefore, the use of kaolin might reduce the level of damage caused by boll weevils in the semiarid and Cerrado Savannah regions of Brazil where cotton is grown without much negative effects on the environment, because it is non-toxic to humans and relatively safe to natural enemies (Friedrich et al. 2003, Glenn and Puterka 2005, Marko et al. 2008).
This study aimed at assessing the level of protection conferred by kaolin to cotton plants against boll weevil.
Materials and methods
Experimental site and cotton cultivar
The study was conducted between January 31 and June 6, 2009, at an experimental farm of the “Embrapa Algodão”, in Barbalha municipality, State of Ceará, Brazil (latitude 7o18′40′’, longitude 39o18′15′’, elevation 414 m) in an area historically infested with boll weevils. An area of approximately one hectare was used to cultivate cotton (cultivar BRS Safira) under dry land conditions with two plants per hill after thinning. This cotton cultivar has tender leaves with little fiber and was produced by crossing upland cotton introduced from the United States, which exhibits a dark brown colored fiber, with the CNPA 87–33 cultivar, which has good quality white lint. The rows were spaced 0.80 m apart with 0.20 m between plants in rows with two plants per hill. The number of cotton plants per plot was 1,000 plants.
The temperature (°C), relative humidity (%), and weekly rainfall (mm) during the assessment experiment were recorded by the National Institute of Meteorology (INMET) located in the Municipality of Barbalha, in the State of Ceará, Brazil. During this research the mean weekly temperature was 24.9 °C, ranging from 23 to 26.3 °C; the mean weekly relative humidity was 88.7 %, ranging from 76 to 97 %; and the mean weekly rainfall was 11.1 mm, ranging from 0.6 to 19 mm.
Experimental design
The experimental design consisted of randomized blocks with five treatments and four replications. Treatment-tested spraying kaolin or endosulfan on cotton plants, as follows: T1) (excluded a) weekly systematic spraying with kaolin (60 g l−1 of water) after the emergence of the cotton plants at 7-day intervals; T2) spraying with kaolin (60 g l−1 of water) when the number of plants with oviposition-punctured squares reached a level of 5 %; T3) a weekly systematic spraying with endosulfan (Thiodan 35 EC, 1.5 g a.i. ha−1) after the emergence of the cotton plants at 7-day intervals; T4) spraying with endosulfan (Thiodan 35 EC, 1.5 g a.i. ha−1) when the number of plants with oviposition-punctured squares reached a level of 10 % (Ramalho and Silva 1993), and T5) control (no spray). We utilize an economic threshold of 5 % damaged squares for kaolin due to the fact that with this product applications need to occur weekly, and continuous good coverage is important for maintaining its effect (Showler 2002). Because this is not necessary for endosulfan, a 10 % damaged squares threshold was used in this treatment (Ramalho and Silva 1993). Each experimental unit comprised 10 rows of cotton of 10 m long, and 5 m separating each experimental unit. The kaolin-based particle film used in this study was Potiguar wettable powder (Equador, RN, Brazil), here referred to as kaolin. A knapsack sprayer, with a capacity of 20 L of spray and nozzle cone empty, was used. The spray nozzle was positioned laterally to the row and maintained at approximately 20 cm from the plants (Ramalho and Jesus 1988). The flow rate was adjusted depending on the cotton crop growth stage, and varied between 150 and 300 L ha−1.
An outbreak of cotton leafworm, Alabama argillacea (Hübner) (Lepidoptera: Noctuidae) occurred at the seedling stage, and was controlled by sprayings of Bacillus thuringiensis Berliner at the commercial dosage (16 g a.i. ha−1).
Boll weevil damage
Evaluations were performed weekly by the randomly surveying 30 cotton plants per plot. For each plant, one cotton square of median size, taken at random in the upper half of the plant (Ramalho and Jesus 1988), was examined for the presence or absence of oviposition puncture damage. The plant height, number of cotton bolls and cotton-seed yield were determined by observing 5, 6, and 12 plants at random from different rows per experimental unit, respectively.
Analysis of data
The effects of kaolin and insecticide sprays on (1) plant height, (2) the mean percentage of oviposited squares (i.e., squares that had oviposition marks), and (3) number of non-attacked cotton bolls were subjected to two-way analysis of variance (ANOVA) [treatment and plant age], using PROC GLM of SAS (SAS Institute 2006). We use the mean of the measurements for each experimental unit in the analyses. The proportions of oviposition-punctured squares made by boll weevil were transformed in arcsine square root before repeated measures analyses; however, untransformed means are presented. The number of non-attacked bolls per cotton plant and cotton-seed yield (kg ha−1) were regressed against percentage of oviposition-punctured squares by boll weevil using PROC REG of SAS (SAS Institute 2006).
Results
The emergence of the cotton plants, the appearances of the first square, and boll opening occurred at 8, 31, and 73 days after planting, respectively.
The two-way ANOVA revealed a significant effect of treatment, plant age, and interaction between treatment and plant age (P < 0.0001 for all; Table 1) for percentages of oviposition-punctured squares by boll weevil.
The percentages of oviposition-punctured squares by boll weevil differed significantly between the treatments at the sixth, seventh, and eighth assessments (Table 2). The highest percentage of oviposition-punctured squares were observed in the control, and the lowest percentages in the treatments sprayed with endosulfan and kaolin in a systematic manner and where the boll weevil reached the economic threshold at all assessments (Table 2). When sprayed consistently, kaolin showed a higher percentage of oviposition-punctured squares (27.5 %) than the obtained after treatment with endosulfan sprayed systematically (5.8 %) at the plants 80 days old, but the percentages did not differ from those obtained at the plants 66 and 73 days old (Table 2).
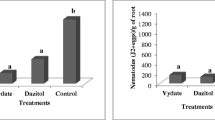
The number of non-attacked bolls per plant 94 days old (F(4, 12) = 83.26, P < 0.0001) (Fig. 1a), plant height at 94 days old (F(4, 12) = 2.26, P < 0.0590) (Fig. 1b), and cotton-seed yield per hectare (F(4, 12) = 18.92; P < 0.0001) (Fig. 2) differed among the tested treatments. The tallest cotton plants were in the control, which differed significantly from those weekly sprayed with endosulfan. Both kaolin treatments and endosulfan at 10 % threshold had intermediate plant heights (Fig. 1b). In contrast, the shortest plants were observed under the systematic spraying with endosulfan (Fig. 1b). The greatest number of non-attacked bolls by weevils (Fig. 1a) and cotton-seed yield (Fig. 2) were observed in the endosulfan treatments, followed by the kaolin treatments. The smallest number of bolls and the lowest cotton-seed yield were observed for the control plots. In the treated plots with kaolin and endosulfan (T1, T2, T3 and T4), the cotton-seed yields were 3.88, 3.03, 2.37, and 2.49 larger than the control cotton plot.
a non-attacked bolls by boll weevil (mean no. per plant (±SE) and b mean (±SE) plant height (cm) of cotton plants and in the following treatments: T1, kaolin weekly after cotton emergence; T2, kaolin weekly from 5 % oviposition-punctured squares; T3, endosulfan weekly after cotton emergence; T4, endosulfan weekly from 10 % oviposition-punctured squares, and T5, control (no spray). Bars capped with different letters are significantly different (P = 0.05) by Student–Newman–Keuls test
Mean (±SE) cotton-seed yield (kg ha−1) in the followings treatments: T1, kaolin weekly after cotton emergence; T2, kaolin weekly from 5 % oviposition-punctured squares; T3, endosulfan weekly after cotton emergence; T4, endosulfan weekly from 10 % oviposition-punctured squares, and T5, control (no spray). Bars capped with different letters are significantly different (P = 0.05) by Student–Newman–Keuls test
Linear models were the best fits to represent the relationship between cotton-seed yield (kg ha−1) (y = 3079.32 − 67.77x, F(1,3) = 50.16, R2 = 0.94, P < 0.0058) (Fig. 3a) or number of non-attacked bolls (n plant−1) (y = 9.99 − 0.22x, F(1, 3) = 45.82, R2 = 0.95, P < 0.0066) (Fig. 3b) as a function of the percentage of oviposition-punctured squares by boll weevil. This mean that 94 and 95 % of the variation in the cotton-seed yield and number of non-attacked bolls, respectively, was due to the percentage of oviposition-punctured squares by boll weevil.
Discussion
Climatic conditions can affect boll weevil populations and the persistence of kaolin particles on plants (Showler 2007). The survival of insects in cotton agro ecosystems is influenced by high temperature and low humidity (Ramalho and Silva, 1993), and the persistence of kaolin particles is also reduced in environments that experience high levels of precipitation (Lo Verde et al. 2011). In our study, the temperature, relative humidity and weekly rainfall were favorable to the pest population development and not hampered spraying with kaolin.
The tallest plants, lower number of bolls and lowest cotton-seed yield of the control plot may be attributed to the higher percentage of oviposition-punctured squares observed in this treatment. On the other hand, the smaller plant height, larger number of bolls, and higher cotton-seed yields of the cotton plots sprayed systematically with endosulfan, either weekly or from the 10 % economic threshold, can be attributed to the lower percentage of oviposition-punctured squares observed in these treatments. There has been found a negative relationship describing cotton-seed yield and non-attacked bolls as a function of oviposition-punctued squares by boll weevil (Bevers and Slosser 1992, Slosser 1993, Showler et al. 2005).
Irrespective of the tested treatment, the mean percentage of oviposition-punctured squares by A. grandis varied with age of the plants (Table 2). However, the mean percentage of oviposition-punctured squares found for the treatments sprayed with kaolin and endosulfan, both systematically or where the boll weevil reached the economic threshold, differed from the control plot at the last four assessments (Table 2). Plants were smaller, while the mean numbers of bolls and cotton-seed yield were higher with endosulfan treatments. We believe that these results can be attributed to the different modes of action of tested products. Cyclodienes, such as endosulfan, are antagonists of the neurotransmitter gamma-aminobutyric acid (GABA), inducing the absorption of chloride ions by neurons, promoting their blockage and uncontrolled excitement, and as consequence death of the insect (Klaassen and Watkins 1999). On the other hand, the kaolin particle film shows a repellent effect, reducing feeding and oviposition on insects, without causing their death (Showler 2002). Although the real modes of action of kaolin particles on insects are not fully understood, the effects appear to be caused by the color (white), which repels the insects from landing (Liang and Liu 2002), and disruption of feeding and oviposition (Liang and Liu 2002, Liu and Trumble 2004).
The lowest percentage of oviposition-punctured squares, the largest number of bolls and cotton-seed yields in the plots treated with kaolin found in our study indicates that possibly plant color plays an important role in guiding the boll weevils for feeding and egg-laying in cotton. A similar effect was found in experiments with the cotton cultivar “Deltapine-50” sprayed with kaolin at the Kika de la Garza Subtropical Agricultural Center (Showler 2002). As kaolin has a repellent effect as the main mode of action (Glenn and Puterka 2005), the boll weevils appeared to prefer the cotton plants with green leaves (without kaolin) for their feeding and oviposition. Fecundated female weevils first move toward the buds without kaolin, and after contact feeding and oviposition (Showler 2002) stimulated by cotton plant volatiles (Grodowitz et al. 1992) and aggregation pheromone (Hardee et al. 1969). However, if the boll weevil contacts the cotton square treated with kaolin, the feeding and oviposition behaviors will not be arrested (Showler 2002), indicating that the protection conferred by kaolin can be reduced in the absence of choice.
The percentages of oviposition-punctured squares, number of bolls and cotton-seed yield did not differ between the treatments sprayed with kaolin in a systematic manner and after the boll weevil reached the 5 % damaged squares economic threshold.
These results are of practical significance because they may reduce the cotton production cost and environmental impacts of chemical pesticides, making possible the production of organic cotton with the presence of boll weevils. However, there is the possibility that by spraying kaolin to entire field, at high boll weevil populations, the level of effectiveness of this approach may be reduced given the need of the female weevils to oviposit. The mechanism of action of this material is based on creating a kaolin-based particle barrier on the plant surface, so understanding how it affects the behavior and biology of the boll weevil is important in developing an effective deployment strategy for kaolin particle film against this pest.
The fact that yield was not only affected but also indicates that this product probably does not affect the photosynthetic capacity of the cotton plants. The kaolin particle film may contribute to the physiologic balance of the plant due to the reflective properties, which can reduce heat stress, protecting against sunburn (Glenn et al. 2003), and increase photosynthesis by reducing the plant temperature, resulting in increases in size and yield (Glenn et al. 1999; Thomas et al. 2004; Glenn and Puterka 2005; Lapointe et al. 2006).
Other advantages of kaolin particle film are the difficult of pests develop resistance to it (Liu and Trumble 2004); does not show phytotoxic effects, and lasts longer than most insecticides on the plants when it does not rain or there is no excessive dew formation (Sugar et al. 2005), being non-toxic to humans, and relatively safe to natural enemies (Delate and Friedrich 2004). Besides, it is washable and forms a suspension in water, being easily applied using conventional spray equipment, and it may eventually reduce the number of chemical insecticides applications (Peng et al. 2011).
References
Alavo TBC, Abagli AZ (2011) Effect of kaolin particle film formulation against populations of the aphid Lipaphis erysimi Kalt. (Homoptera: Aphididae) in Cabbage. Open Entomol J 5:49–53
Bevers SJ, Slosser JE (1992) Assessing cost effectiveness of planting dates and insecticide chemicals in Texas dryland cotton production. J Crop Prod Agric 5:374–377
Burke HR, Clark WE, Cate JR, Fryxell PA (1986) Origin and dispersal of the boll weevil. Bull Entomol Soc Am 32:228–238
Cottrell TE, Wood BW, Reilly CC Reilly (2002) Particle film affects black pecan aphid (Homoptera: Aphididae) on pecan. J Econ Entomol 95:782–788
Delate K, Friedrich H (2004) Organic apple and grape performance in the Midwestern U.S. Acta Hortic 638:309–320
El-Sayed AM, Suckling DM, Wearing CH (2006) Potential of mass trapping for long-term pest management and eradication of invasive species. J Econ Entomol 99:1550–1564
Friedrich H, Delate K, Domoto P, Nonnecke G, Wilson L (2003) Effect of organic pest management practices on apple productivity and apple food safety. Biol Agric Hortic 21:1–14
Glenn DM, Puterka GJ (2005) Particle films: a new technology for agriculture. Hortic Rev 31:1–44
Glenn DM, Puterka GJ, Vanderzwet T, Byers RE, Feldman C (1999) Hydrophobic particle films: a new paradigm for of arthropod pests and plant diseases. J Econ Entomol 92:759–771
Glenn DM, Erez A, Puterka GJ, Gundrum P (2003) Particle film affect carbon assimilation and yield in ‘Empire’ apple. J Am Soc Hortic Sci 128:356–362
Grodowitz MJ, Lloyd EP, McKibben GH (1992) Comparison of feeding and olfactory behaviors between laboratory-reared and overwintered native boll weevils (Coleoptera: Curculionidae). J Econ Entomol 85:2201–2210
Harben PW (1995) The industrial minerals handbook II: a guide to markers, specifications, and prices. Arby Industrial Mineral Division Metal Bulletin, PLC, London
Hardee DD, Cross WH, Mitchell EB (1969) Male boll weevils are more attractive than cotton plants to boll weevils. J Econ Entomol 62:165–169
Klaassen CD, Watkins JB (1999) Casarett & Doull’s toxicology: the basic science of poisons. MacGraw-Hill, New York
Lapointe SL, Mckenzie CL, Hall DG (2006) Reduced oviposition by Diaprepes abbreviatus (Coleoptera: Curculionidae) and growth enhancement of citrus by Surround particle film. J Econ Entomol 99:109–116
Liang GM, Liu TX (2002) Repellency of a kaolin particle film, Surround, and a mineral oil, sunspray oil, to silverleaf whitefly (Homoptera: Aleyrodidae) on melon in the laboratory. J Econ Entomol 95:317–324
Liu DG, Trumble JT (2004) Tomato psyllid behavioral responses to tomato plant lines and interactions of plant lines with insecticides. J Econ Entomol 97:1078–1085
Lo Verde G, Caleca V, Lo Verde V (2011) The use of kaolin to control Ceratitis capitata in organic citrus groves. Bull Insectol 64:127–134
Marko V, Blommers LHM, Bogya S, Helsen H (2008) Kaolin particle films suppress many apple pests, disrupt natural enemies and promote woolly apple aphid. J Appl Entomol 132:26–35
Mazor M, Erez A (2004) Processed kaolin protectant fruits from Mediterranean fruit fly infestations. Crop Prot 23:47–51
Page LM, Johnson DR, Moret MP, Amaden SR (1999) Summary of insecticide performance for boll weevil (Anthonomus grandis) control in Arkansas cotton. In: Proceedings of the Beltwide Cotton Conference, National Cotton Council, Memphis, p 1168–1169
Peng L, Trumble JT, Munyanezae J, Liua T-X (2011) Repellency of a kaolin particle film to potato psyllid, Bactericera cockerelli (Hemiptera: Psyllidae), on tomato under laboratory and field conditions. Pest Manag Sci 67:815–824
Puterka GJ, Glenn DM, Sekutowski DG, Unruh IR, Jones SK (2000) Progress toward liquid formulations of particle films for insect and disease control in pear. Environ Entomol 29:329–339
Ramalho FS (1994) Cotton pest management: part 4. A brazilian perspectives. Annu Rev Entomol 39:563–578
Ramalho FS, Jesus FMM (1988) Distribution of boll weevil (Anthonomus grandis Boheman) within cotton plants. Trop Agric 65:245–248
Ramalho FS, Santos RF (1994) Impact of the introduction of the cotton boll weevil in Brazil. In: Constable GA, Forrester NW (eds) Challenging the future: proceedings of the world cotton research conference-1. CSIRO, Melbourne, pp 466–474
Ramalho FS, Silva JRB (1993) Período de emergência e mortalidade natural do bicudo-do-algodoeiro. Pesqui Agropecu Bras 28:1221–1231
Ramalho FS, Wanderley PA (1996) Ecology and management of boll weevil in south American cotton. Am Entomol 42:41–47
Ramalho FS, Medeiros RS, Lemos WP, Wanderley PA, Dias JM, Zanuncio JC (2003) Evaluation of Catolaccus grandis (Burks) (Hym., Pteromalidae) as a biological control agent against cotton boll weevil. J Appl Entomol 124:359–364
Rummel DR, Summy KR (1997) Ecology of the boll weevil (Anthonomus grandis) in the United States cottonbelt. Southwest Entomol 22:356–376
SAS Institute (2006) SAS/STAT user’s guide. SAS Institute, Cary
Showler AT (2002) Effects of kaolin-based particle film application on boll weevil (Coleoptera: Curculionidae) injury to cotton. J Econ Entomol 95:754–762
Showler AT (2003) Effects of routine late-season field operations on numbers of boll weevils (Coleoptera: Curculionidae) captured in large-capacity pheromone traps. J Econ Entomol 96:680–689
Showler AT (2007) Subtropical boll weevil ecology. Am Entomol 53:240–249
Showler AT, Greenberg SM, Scott AW, Robinson JRC (2005) Effects of planting dates on boll weevils (Coleoptera: Curculionidae) and cotton fruit in the subtropics. J Econ Entomol 98:796–804
Slosser JE (1993) Influence of planting date and insecticide treatment on insect pest abundance and damage in dryland cotton. J Econ Entomol 86:1213–1222
Sugar D, Hilton RJ, VanBuskirk PD (2005) Effects of kaolin particle film and rootstock on tree performance and fruit quality in ‘Doyenne du Comice’ pear. Hortic Sci 40:1726–1728
Thomas AL, Muller ME, Dodson BR, Ellersieck MR, Kaps M (2004) A kaolin-based particle film suppresses certain insect and fungal pests while reducing heat stress in apples. J Am Pomol Soc 58:42–51
Wiss E, Daniel C (2004) Effects of autumn kaolin and pyrethrin treatments on the spring population of Dysaphis plantaginea in apple orchards. J Appl Entomol 128:147–149
Acknowledgments
This research was supported by the following Brazilian Agencies: “Financiadora de Estudos e Projetos” (FINEP), and “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Zaviezo.
Rights and permissions
About this article
Cite this article
Silva, C.A.D., Ramalho, F.S. Kaolin spraying protects cotton plants against damages by boll weevil Anthonomus grandis Boheman (Coleoptera: Curculionidae). J Pest Sci 86, 563–569 (2013). https://doi.org/10.1007/s10340-013-0483-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-013-0483-0