Abstract
Production and biocontrol efficacy of culture filtrates containing cuticle degrading enzymes from three isolates of Isaria fumosorosea against diamondback moth (Plutella xylostella) was observed during this study. This fungus when cultured in liquid medium having different carbon sources showed maximum biomass production when 1% chitin was added as carbon source. These isolates when grown in liquid culture conditions having 1% chitin as source produced cuticular degrading enzymes (proteases (Pr1 and Pr2), chitinases, chitosanase, and lipase) in a sequential manner and the production of these enzymes differed from control. Biocontrol assays with P. xylostella showed that the culture filtrates of I. fumosorosea were potent antifeedants because reduction in the feeding rate and body weight of the larvae was observed. Similarly, reduction in rates of successful pupation, adult emergence was observed when the culture filtrates were applied topically. At the end of the test period, the lowest ST50 value (1.57 ± 0.20 days) was recorded for insect groups treated with culture filtrates from isolate IF28.2 when compared to the control. In view of the need for safer and environmentally friendly pest management tools, the present study can help in the development of enzyme-based biopesticides against P. xylostella.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae) is a major pest of cabbage, broccoli, and canola. Each year, farmers worldwide spend more than $1 billion to control this pest, primarily by using chemical insecticides (Henrik et al. 2000). As a result, natural antagonists are killed (Xu et al. 2004) and many populations of diamond back moth have become resistant to conventional insecticides (Shelton et al. 1993; Tabashnik 1994). In addition, Bacillus thuringiensis resistant field populations have been detected in several countries, such as, U.S.A., Central America, and Asia (Syed 1992; Ferré and van Rie 2002). Alternative control measures being investigated for diamondback moth include the use of entomopathogenic fungi (Cherry et al. 2004; Wright 2004; Muhammad et al. 2005). Isolates of different fungal species such as, Zoophthora radicans, Paecilomyces fumosorosea, Metarhizium anisopliae, Fusarium sp., and Beauveria bassiana can infect diamondback moth. B. bassiana is virulent against diamondback moth in screen house or field conditions (Pell et al. 1993; Ibrahim and Low 1993; Vandenberg and Ramos 1997; Vandenberg et al. 1998; Altre et al. 1999). Isaria fumosorosea (Paecilomyces fumosorosea designated as Isaria clade, Luangsa-Ard et al. 2005) is one of the most promising fungal species for control of diamondback moth, whiteflies and other insect pests (Altre et al. 1999; Wraight et al. 2000; Ali et al. 2009).
The fungal infection is dependent upon numerous biological events which are initiated by the adhesion of fungal spores to the insect cuticle, spore germination, and hyphal growth. In order to breach the insect cuticle, fungal hyphae exert mechanical pressure and produce cuticle degrading enzymes (Altre et al. 1999). Several cuticle degrading enzymes such as proteases and chitinases have been detected in entomopathogenic fungi such as B. bassiana, Numuraea rileyi, and M. anisopliae (Brey et al. 1986; El-Sayed et al. 1989). In insects, chitin forms a structural barrier that must be overcome by extracellular chitinases so that the fungus can enter and be dispersed throughout the hemocoel. This suggests that chitinases might play an important role in both cuticle degradation and host penetration (St. Leger et al. 1996).
Proteolytic enzymes along with chitinases and lipases are important factors of entomopathogenic fungi (Samuels and Paterson 1995). Pr1, a serine protease, plays a major role in insect penetration and subsequent pathogenicity (St. Leger et al. 1988, 1996). Lipids are also integral to the insect cuticle and improvements in lipase production by an entomopathogen would be highly desirable for use of entomopathogens to be used as bioinsecticides (Clarkson and Charnley 1996). As these enzymes degrade chitin, it might be speculated that these enzymes can cause disruption of cuticle which subsequently causes abnormal molting. Similarly, if these enzymes enter the gut of insect larvae, they can cause significant damage to the peritrophic membrane structure which will result in the larvae being not able to feed and consequently leads to death (Binod et al. 2007). This projects that the cocktail of enzymes is a potential agent to be included in the spraying type of biological control agents, provided that the enzymes remain stable for sufficiently longer duration in the environment with retention of activity. Considering this, we initiated studies for the possible development of an enzyme-based biocontrol program against diamondback moth larvae.
The objective of the present study was to evaluate the efficacy of fungal culture filtrate against diamondback moth. Isaria fumosorosea was cultured under submerged condition and the culture filtrate was analyzed for Pr1, Pr2, chitinases, chitosanase, and lipase activities. Crude culture filtrates were also tested on the larvae for biological activity. Effect of enzyme feeding on larval development was measured by changes in amount of larval feeding and body weight. Effect of topical application of enzymes on larval development was measured by changes in rate of pupation, rate of adult emergence and larval mortality.
Materials and methods
Fungal strains
Isaria fumosorosea isolates (IF-28-2, IF-32, and IF-49) originally isolated from soil (Liu, 2006), deposited to the collection at Engineering Research Center of Biological Control, South China Agricultural University were used during these studies. To produce the inoculum for each assay I. fumosorosea was cultured on potato dextrose agar (Potato infusion 200 g/l; Dextrose 20 g/l and Agar 20 g/l) and incubated at 26 ± 2°C for 10 days. Conidia were harvested with distilled water containing 0.03% aqueous polysorbate monooleate (Tween™ 80), bought from Whiga chemicals Shanghai and sieved through filter paper into sterile vials. Conidia were counted in Fuchs-Rosenthal hemocytometer using a compound microscope in order to prepare a suspension of 1 × 107 conidia/ml.
Basal medium
Basal medium (pH 7.2) consisted of glucose 0.2% (w/v), peptone 0.5% (w/v), MgSO4 0.01% (w/v), K2HPO4 0.1% (w/v) and SDS 0.25%(w/v).
Growth of entomopathogenic fungi on cuticle components
The effect of chitin (reagent grade from Whiga chemicals, Shanghai), chitosan (reagent grade purchased from Sinopharm chemical reagent, Shanghai, China), amino acids like alanine, isoleucine and glycine (Sinopharm chemical reagent, Shanghai, China) as a carbon source for biomass production by I. fumosorosea was studied by growing the fungal isolates in liquid medium described above containing carbon sources at 1% (w/v) in 50 ml of basal medium in 250 ml Erlenmeyer flasks, heat sterilized at 121°C for 15 min while the basal medium without any carbon source was used as a control. The flasks were inoculated with 1 ml of 1 × 107 spores/ml and incubated at 180 rpm and 30°C for 5 days. For biomass determinations the culture supernatants were separated from the mycelium by filtration through pre-weighed Whatman filter no. 1 and dried at 80°C until a constant weight.
Production cuticle degrading enzyme complex of entomopathogenic fungi on chitin
The cuticle degrading enzyme complex was produced by growing I. fumosorosea in liquid medium described above. Chitin (1% w/v) was added as a carbon source to previously sterilized basal medium (121°C, 15 min) while the basal medium without chitin served as control. The flasks were inoculated with 1 ml of 1 × 107 spores/ml and incubated at 180 rpm and 30°C for 5 days. Samples were removed at 24 h intervals used for further enzymatic analysis. To prepare the culture filtrates for biological control studies the inoculum was harvested by centrifugation at 1,000 g for 10 min at 4°C in a Microfuge®18 with a F241.5P rotor (Beckman Coulter, Inc, USA).
Enzyme assays
Protease assay
Isaria fumosorosea subtilisin and trypsin-like activities are referred to as Pr1 and Pr2, respectively. Subtilisin-like (Pr1) activity was assayed by using succinyl-(alanine)2-proline-phenylalanine-p-nitroanilide (Sigma) as substrate, and trypsin (Pr2) activity using benzoylphenylalanine-valine-arginine-p-nitroanilide (Sigma). Each assay consisted of 0.05 ml substrate (1 mmol l−1), 0.85 ml 15 mmol l−1 Tris–HCl buffer (pH 8.5) and 0.1 ml crude enzyme. The mixture was incubated for 1 h at 28°C and the reaction was terminated by adding 0.25 ml of 30% acetic acid and left to stand for 15 min on ice, after which the samples were centrifuged at 1,250 g for 5 min at 4°C in a Microfuge®18 with a F241.5P rotor (Beckman Coulter, Inc, USA). The supernatants were transferred to the wells of microtitre plate and absorbance was measured using spectrophotometer (Beckman, Coulter) at 410 nm. One unit of activities was expressed as nanomoles nitroanalide (NA) released per mg per hour. Protein was measured using Coomassie Brilliant Blue G-250 (Dingguo chemicals, Beijing) according to Bradford (1976), with bovine serum albumin as standard.
Lipase assay
Lipase activity was determined as described by Pignede et al. (2000). The substrate emulsion was prepared with olive oil, 50 ml (obtained from Nan Hai oil Industry, Shenzhen, China) and gum Arabic (Jinhuadu chemical reagent company, Guangzhou, China.), 50 ml (10% w/v). The reaction mixture contained 1 ml enzyme (sample already obtained from culture flasks), 5 ml substrate emulsion and 2 ml of 50 mM phosphate buffer, pH 6.8, and were incubated for 1 h at 37°C with shaking. The reaction was stopped with 4 ml of acetone–ethanol (1:1) containing 0.09% phenolphthalein (obtained from Jinhuadu chemical reagent company, Guangzhou, China) as an indicator. Enzyme activity was determined by titration of the fatty acid released with 50 mM sodium hydroxide. One international unit was defined as enzyme activity that produced 1 μmol of fatty acid per min.
Chitinase and chitosanase assay
Colloidal chitin was prepared by the method of Roberts and Seletrenikoff (1988) with some modification. One hundred grams of chitin flakes of reagent grade chitin (Whiga chemicals, Shanghai) were added slowly to 1.75 l concentrated HCl and agitated gently for 3 h on a magnetic stirrer. This solution was then filtered to 20 l of pre chilled distilled water with constant mixing and allowed to settle. A dense white precipitate formed was then centrifuged at 10,000 rpm for 10 min at 4°C. The precipitate was then washed in cold distilled water repeatedly until the pH of the wash reached near to 5.5. The supernatant was discarded and colloidal chitin was then kept in refrigerator for future use.
Chitinase assay was based on the estimation of reducing sugars released during the hydrolysis of colloidal chitin. The reaction mixture contained 0.5 ml of enzyme, 0.5 ml of 0.5% colloidal chitin, and 1.0 ml of citrate phosphate buffer pH 5.6. The mixture was kept in a water bath at 37°C for 1 h. The amount of reducing sugar liberated was estimated by Miller’s (1959) method. One unit (U) of activity was defined as the amount of enzyme which catalyzed the release of 1 μg of reducing sugar per ml per minute under the reaction conditions. Protein concentration was determined according to Bradford (1976) using bovine serum albumin as a standard.
Chitosanase activity was estimated using acid swollen chitosan (purchased from Sinopharm chemical reagent, Shanghai, China) as the substrate. The acid swollen chitosan were prepared following the protocol used to make acid swollen chitin. The assay mixture will contain 1 ml 0.7% acid swollen chitosan, 1 ml 50 mM acetate buffer, pH 5.0, and 1 ml enzyme that were incubated at 37°C for 1 h. The reducing sugars produced were estimated using Somogyi method (Somogyi 1951) at 520 nm. One international unit of enzyme was defined as the activity that produced 1 μmol glucosamine equivalents per min.
Biocontrol studies
Insect cultures
Larvae of P. xylostella, were obtained from the stock cultures raised on Brassica campestris L. Plants were grown in plastic pots using regular soil. Slow release fertilizer (N: P: K = 13:7:15, Shenzhen Batian ecotypic engineering Co., LTD. Xili Shenzhen China) was added as required to maintain normal plant growth. The pots were incubated in an artificial climate room at 26°C and >95% R.H. Second instar larvae were used for antifeedant studies whereas fourth instar was used for growth inhibition studies.
Studies on antifeedant activity
The efficacy of fungal culture filtrate as an antifeedant was tested by feeding larvae with fresh B. campestris leaves coated with filtrate. Leaf discs (10 cm2) were dipped in enzyme preparation and air dried on paper before being fed to the larvae. Leaf discs similarly treated in culture filtrate produced in the absence of chitin were used as control. The leaf discs were replaced on daily basis. Feeding with treated leaves was continued for 4 days after which normal feeding were resumed. The insects were placed in an air-conditioned room at 26°C and >95% R.H. The rate of feeding was measured by noting the leaf area consumed by the larvae (using AM 300 portable leaf area meter, Dynamax Inc, USA) and was expressed as percent leaf consumption based on the following transformation.
Effect of feeding on larval development was measured by changes in body weight during a 10-day observation period. Each treatment and control was repeated three times with a new batch of insects and new culture fluid, and for each repetition there were four leaves, each leaf having 20 diamondback moth larvae.
Studies on growth inhibition of diamondback moth
In the bioassay experiment for growth inhibition crude fungal filtrates were used. Five microliters of each preparation were applied topically on the thorax back of the 4th instar larvae using a micropipette. Five microliters of culture filtrate produced in absence of colloidal chitin served as control. After this larvae were left to air dry before being transferred to 20-cm diameter clean glass Petri dishes and a piece of filter paper (20 cm in diameter) was placed at the bottom of the dish with a few drops of water for maintenance of moisture. Topical application was continued for 3 days after which the larvae were left undisturbed. Leaf disks were replaced every 2 days except during the pupal stage. The insects were placed at 26°C and >95% R.H. and the effect of filtrate applications on larval development was measured by changes in rate of pupation, adult emergence, and mortality induced by the treatments.
The effect of treatment on larval development was represented as the percentage of successful pupation at the end of the test period. Successful pupation was defined as the proportion of larvae successfully pupating divided by the total number of larvae which can or have already developed into normal adults at the end of the test period (10 days).
Statistical analysis
Biomass production and enzymatic activities after 5 days, percent pupation and adult emergence were analyzed by Analysis of variance (ANOVA) and treatment means were compared by using Tukey’s test for mean comparisons at 5% level of significance. Data regarding sequential production of different enzymes, percent larval feeding, and changes in larval body weight were analyzed by Repeated Measures Anova. For mortality data, Median survival time (ST50) estimates were obtained by using standard Kaplan–Meier survivorship analysis, and were subjected to ANOVA followed by the Dunnet’s test. All statistical analysis was performed using SAS 8.01(SAS 2000).
Results
Growth of entomopathogenic fungi on cuticle components
The biomass produced by IF28.2 in the basal medium having 1% (w/v) solution or suspensions of different carbon sources after 5 days of inoculation was significantly different among different treatments and control (F 5,12 = 17.98, P < 0.0001). The lowest biomass production (1.34 ± 0.34 mg/ml) was observed in control while the highest biomass (10.86 ± 1.02 mg/ml) was obtained from the basal medium having 1% chitin as a carbon source (Table 1).
Biomass production by I. fumosorosea isolate IF32 in the basal medium having different carbon sources differed significantly among different treatments and control (F 5,12 = 27.12, P < 0.0001). The highest biomass production (9.22 ± 0.83 mg/ml) was observed for the basal medium having 1% chitin as a carbon source, whereas the lowest biomass production (1.23 ± 0.13 mg/ml) was observed for control (Table 1).
The production of biomass by I. fumosorosea isolate IF49 in the basal medium having different carbon sources after 5 days of inoculation differed significantly among different treatments and control (F 5,12 = 32.12, P < 0.01). The lowest biomass production (1.09 ± 0.18 mg/ml) was observed in control while the highest biomass (8.11 ± 0.46 mg/ml) was obtained from the basal medium having 1% chitin as a carbon source (Table 1).
Sequential production of cuticle degrading enzymes by entomopathogenic fungi
The pattern of extracellular enzymes production in culture containing 1% chitin was studied with three different I. fumosorosea isolates (Fig. 1a–e). In all cultures enzymes were produced sequentially and showed a distinct phase of rapid production. The analysis of repeated measures ANOVA resulted in significantly different interaction effect between different isolates and time intervals for Subtilisin-like (Pr1) activity (F 15,46 = 26.91, P < 0.0001) and trypsin-like (Pr2) activity (F 15,46 = 36.30, P < 0.01). These enzymes appeared first within 24 h and a rapid increase in production was observed after 48 h (Fig. 1a, b). Similarly a significantly different interaction effect between different isolates and time intervals was also observed for lipase (F 15,46 = 10.83, P < 0.024) and chitosanase activity (F 15,46 = 33.96, P < 0.0001). These enzymes started to appear within first 24 h and then increased rapidly after 48 h (Fig. 1c, d). Interaction effect between different isolates and different time intervals proved to be significantly different for chitinase activity (F 15,46 = 20.59, P < 0.0001). This enzyme started to appear between 24 and 48 h and there was a sudden increase in their production after 48 h which became almost stable between 96 and 120 h (Fig. 1e).
Range of cuticle degrading enzymes produced by entomopathogenic fungi
I. fumosorosea grew in liquid culture medium having 1% chitin as sole carbon source and produced a range of extracellular cuticular degrading enzymes corresponding to the major components of insect cuticle, i.e., chitin, protein, lipids. Enzyme activities in 5 day culture filtrates are shown in Table 2. Marked variations in enzyme levels between different isolates as well as different species were also found which may be accounted for difference in growth rates and sequential phasing of enzyme production.
As shown in Table 2, Subtilisin-like (Pr1) activity was exhibited by different I. fumosorosea isolates under liquid culture conditions; however, the amount of secreted enzyme varied significantly from control (F 3,8 = 10.6, P < 0.021). The highest level of Pr1 activity (29.14 ± 1.34 U/mg) was found in the supernatants from IF28.2 while the lowest Pr1 activity was detected for control (mean of Pr1 production by all three isolates in basal medium without chitin) having a mean value of 7.35 ± 0.49 U/mg.
The trypsin-like (Pr2) activity shown by different I. fumosorosea isolates in the basal medium after 5 days of inoculation was significantly different from control (F 3,8 = 24.21, P < 0.001). The lowest Pr2 activity was observed for control (mean of Pr2 production by all three isolates in basal medium without chitin) having a mean value of 2.91 ± 0.61 U/mg whereas the highest Pr2 activity (14.67 ± 1.05 U/mg) was found in the supernatants inoculated with I. fumosorosea isolate IF28.2 (Table 2).
The lipase production by different I. fumosorosea isolates (except IF49) differed significantly from control (F 3,8 = 15.68, P < 0.001). Maximum lipase activity (1.51 ± 0.36 U/mg) was shown by I. fumosorosea isolate IF28.2 whereas the lowest lipase activity was observed for control (mean of lipase production by all three isolates in basal medium without chitin) a mean value of 0.69 ± 0.13 U/mg.
Chitinases were produced in varying amounts by different I. fumosorosea isolates when compared to the control (F 3,8 = 28.01, P < 0.001). I. fumosorosea isolate IF28.2 proved to be the most active producer of chitinases showing average activity of 16.86 ± 0.54 mU/mg while the lowest chitinases activity (4.94 ± 0.72 mU/mg) was observed for IF49 (Table 2).
Significant differences were also observed among different I. fumosorosea isolates for chitosanase activity when compared to the control (F 3,8 = 14.88, P < 0.0001). Maximum chitosanase activity was shown by I. fumosorosea isolate IF28.2 having a mean value of 6.38 ± 0.70 mU/mg whereas the lowest chitosanase activity (0.91 ± 0.37 mU/mg) was observed for control (mean of chitosanase production by all three isolates in basal medium without chitin) .
Studies on antifeedant activity
Effect on larval feeding rate
A significantly different interaction effect between the culture filtrates of different isolates used for coating the leaves and different time intervals was observed for cumulative percent leaf consumption (F 12, 38 = 13.03, P < 0.0001). Lowest rates of % leaf consumption at different time periods were observed for I. fumosorosea isolate IF28.2. After 5 days feeding rates for IF49 were comparatively higher but these were still lower than the control (mean of all the three isolates in the basal medium without chitin) on that day (Fig. 2).
Effect on larval body weight
Interaction effect between the culture filtrates of different isolates used for coating the leaves and different time intervals for increase in body weight by the larvae was significant (F 27,78 = 15.57, P < 0.001).The control group performed better in terms of growth as indicated by increased body weight. Almost similar rates of increase in body weight were observed for I. fumosorosea isolates IF32 and 49 while for IF28.2 the rates of larval growth were lowest when compared to the other treatments (Fig. 3).
Growth inhibition studies
Effect on larval development
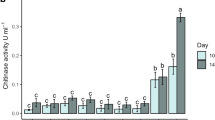
The percentage pupation after 10 days culture filtrates application was significantly different among different I. fumosorosea isolates and control (F 3,8 = 40.42, P < 0.001). The lowest rates of successful pupation were observed for IF28.2 having mean value of 11.50 ± 0.64% while the highest rates of % pupation (74.30 ± 1.08%) was observed for the control (mean of all the three isolates in basal medium without chitin) (Fig. 4).
Effect of topical application of culture filtrates of hydrolytic enzymes from I. fumosorosea on % pupation. Legends with different letters are significantly different from each other (Tukey’s, P < 0.05); Bars represent standard error of means (Based on three independent replicates). * Control stands average activity of all the three isolates in the basal medium without chitin
Effect on adult emergence
Significantly different rates of % adult emergence were observed among culture filtrates of different I. fumosorosea isolates and the control (F 3,8 = 23.61, P < 0.001). It can be observed that the percentage of adult emergence was lowest (4.67 ± 0.57%) for I. fumosorosea isolate IF28.2 where the highest rate of adult emergence was observed for control (Fig. 5).
Effect of topical application of culture filtrates of hydrolytic enzymes from I. fumosorosea on % adult emergence. Legends with different letters are significantly different from each other (Tukey’s, P < 0.05); Bars represent standard error of means (Based on three independent replicates). * Control stands average activity of all the three isolates in the basal medium without chitin
Effect on larval mortality
Mortality of larvae/pupae due to the treatment was measured until the 10th day. At the end of the test period, the analysis of median survival time (ST50) showed significant differences between different isolates and control (F 3,8 = 17.78, P < 0.001). Culture filtrates from IF28.2 proved to the most aggressive showing lowest ST50 value (1.57 ± 0.20 days), and those from control were the least virulent (Fig. 6).
Effect of topical application of culture filtrates of hydrolytic enzymes from I. fumosorosea on median survival time (ST50). Legends with different letters are significantly different from each other (Tukey’s, P < 0.05); Bars represent standard error of means (based on three independent replicates). * Control stands average activity of all the three isolates in the basal medium without chitin
Discussion
Effect of chitin, chitosan, and amino acids components (alanine, glycine, and isoleucine) of insect cuticle as a sole carbon source on biomass production by I. fumosorosea was determined. This approach was used to ensure that any possible delay or absence in enzyme production was not due merely to non utilization of possible inducing substrates. Higher biomass values were obtained for chitin and amino acid alanine which is similar to the findings of St. Leger et al. (1986b) who observed rapid and extensive growth of M. anisopliae isolate ME1 on NAG and alanine. They also observed good growth on olive oil but only in the presence of 0.2% NaNO3 as nitrogen source.
To gain an indication how host influences the growth of cuticle degrading enzymes; isolates of I. fumosorosea were grown on 1% chitin as a sole carbon source. During these studies higher levels of chitinase and protease were observed which are similar to the findings of St. Leger et al. (1986a) who also suggested that protease play an important role in cuticle as chitinolytic enzymes appear after proteolytic enzymes. Therefore, it was important to investigate the other chitin metabolizing enzymes such as chitosanase, NAGase, and lipase that can help in infection process. Detection of higher levels of these enzymes in the culture filtrates suggested that these enzymes are also involved in the infection process which is similar to the findings of St. Leger et al. (1986b) who also observed these enzymes in the fungal culture filtrates having insect cuticle or chitin as sole carbon source. Qazi and Khachatourians (2007) showed that B. bassiana can produce higher amount of proteases and other cuticle degrading enzymes when aphid exuviae were added to the basal medium. Similarly, St. Leger et al. (1991) showed that conidia of M. anisopliae growing on insect cuticle have higher level of protease, chitinase, esterase in comparison to Sabouraud dextrose agar. A possible reason for higher levels of enzyme can be the composition of insect cuticle that comprises of about 70% protein and these enzymes may have an important role in host penetration (Gillespie et al. 1998).
Cuticle degrading enzymes were produced rapidly and sequentially in the culture medium. The first to appear were proteases and lipases and their rapidity increased after 24 h which can be an important factor in pathogenesis providing nutrients and enabling onset of infection process before host defenses become effective. Chitinase and other related enzymes, which are induced by chitin breakdown appears substantially later than proteases. These findings are similar to St. Leger et al. (1986a) who observed that appearance of extracellular enzymes was paralleled by the sequential solubilization of cuticle constituents. This sequence of enzyme production can be explained by the fact that naturally, fungal conidia are endowed for a robust mode of life, and process of pathogenesis only starts if the release of degradation products from cuticle exceeds the needs for fungal growth which can only occur in nutrient rich hemolymph and tissues but not during the pre-penetration phase (Qazi and Khachatourians 2008). Therefore, the appearance of Pr1 and Pr2 shows a clear advantage for different fungal isolates used during these studies in acquiring nutrients to enable the onset of cuticle degradation and fungal penetration.
Reports are available for the degradation of insect gut peritrophic membrane by chitinase. Brandt et al. (1978) proposed that chitinases cause perforations in the membranes, thus facilitating entry of the pathogens into the tissues of susceptible insects. The addition of exogenous chitinase from Streptomyces griseus to the blood meal of the mosquito, Anopheles freeborni, prevented the peritrophic membrane from forming (Shahabuddin et al. 1993). Perforation of peritrophic membranes occurred in vivo after Spodoptera fifth instar larvae were fed on a diet containing recombinant ChiAII, a recombinant endochitinase encoded by Serratia marcescens (Regev et al. 1996). Our results about the effects of culture filtrates having cuticle degrading enzymes showed that the control group performed better in terms of growth as indicated by increased body weight which are similar to the findings of Binod et al. (2007) who showed that hydrolytic enzyme culture filtrates from entomopathogenic fungi, T. harzianum, were capable of negatively effecting the growth and metamorphosis of Heliothis larvae.
Microbial cuticle degrading enzymes have been used in mixing experiments to increase the potency of entomopathogenic micro-organisms. Synergistic effects among chitinolytic enzymes and microbial insecticides have been known to occur since the early 1970s. Larvae of the spruce bud worm, Choristoneura fumiferana, died more rapidly when exposed to chitinase-Bt mixtures than when exposed to the enzyme or bacterium alone (Smirnoff 1974). The mortality of gypsy moth (Lymantria dispar) larvae was enhanced when chitinase was mixed with Bt relative to a treatment with Bt alone, in laboratory experiments. The toxic effect was correlated positively with enzyme levels. Crude chitinase preparation from Bacillus circulans enhanced the toxicity of Bt kurstaki toward diamond back moth larvae (Kramer and Muthukrishnan 1997). The studies conducted for testing the efficacy of fungal culture filtrates of cuticle degrading enzymes against the insect pest P. xylostella have shown that the cuticle degrading enzymes are capable of negatively affecting the growth and metamorphosis of the larvae. This is true when these enzymes are used in feed or when topically applied. The conditions used for the experiment tried to simulate the field conditions of spraying the agent in a water base when the leaves are coated with enzymes or the larvae are directly exposed. It is evident that at the different isolates tested can induce very low ST50 values when compared to the control. It may still be speculated that the adults emerging from treated larvae may be abnormal and incapable of normal life, although this needs further experimental evidence. In view of the need for new pest management tools, a possible alternative is insect pathogens that can secrete high levels of cuticle metabolizing enzymes. This might enable the use of cuticle metabolizing enzyme sprays combined with other pesticide formulations to facilitate with other pesticide formulations to facilitate faster kill.
References
Ali S, Huang Z, Ren SX (2009) Media composition influences on growth, enzyme activity and virulence of the entomopathogen hyphomycetes Isaria fumosorosea. Entomol Exp Appl 131:30–38
Altre JA, Vandenberg JD, Cantone FA (1999) Pathogenicity of Paecilomyces fumosorosea isolates to diamondback moth, Plutella xylostella: correlation with spore size, germination speed, and attachment to cuticle. J Invert Pathol 73:332–338
Binod P, Sukumaran RK, Shirke SV, Rajpur JC, Pandey A (2007) Evaluation of fungal culture filtrate containing chitinase as a biocontrol agent against Helicoverpa armigera. J Appl Microbiol 103:1845–1852
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brandt CR, Adang MJ, Spence KD (1978) The peritrophic membrane: ultrastructural analysis and function as a mechanical barrier to microbial infection in Orgyia pseudotsugata. J Invert Pathol 32:12–24
Brey PT, Latge JP, Prevost MC (1986) Intergumental penetration of the pea aphid, Acyrthosiphon pisium, by Conidiobolus obscurus (Entomophthoraceae). J Invert Pathol 48:34–41
Cherry AJ, Mercadier G, Meikle W, Castelo-Branco M, Schroer S (2004) The role of entomopathogens in DBM biological control. Improving biocontrol of Plutella xylostella. In: Kirk AA, Bordat D (eds) Procceedings of international symposium. Montpellier, France, pp 51–70
Clarkson JM, Charnley AK (1996) New insights into mechanisms of fungal pathogenesis in insects. Trends Microbiol 4:197–204
El-Sayed GN, Coudron TA, Ignoffo CM, Riba G (1989) Chitinolytic activity and virulence associated with native and mutant isolates of an entomopathogenic fungus Nomuraea rileyi. J Invert Pathol 54:394–403
Ferré J, van Rie J (2002) Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Ann Rev Entomol 47:501–533
Gillespie JP, Bateman R, Charnley K (1998) Role of cuticle degrading proteases in the virulence of Metarhizium spp. for the desert locust Schistocerca gregaria. J Invert Pathol 7:1128–1137
Henrik US, Pittendrigh BR, Kroymann J, Weniger K, Fritsche J, Antje BA, Mitchell-Olds T (2000) Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiol 124:1007–1018
Ibrahim YB, Low W (1993) Potential of mass-production and field efficacy of isolates of the entomopathogenic fungi Beauveria bassiana and Paecilomyes fumosorosea against Plutella xylostella. Int J Pest Manag 39:288–292
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 27:887–900
Liu SY (2006) Investigating and screening the intensive pathogenicity of the fungi in genera Paecilomyces and Metarhizium, Masters thesis. Department of Entomology, South China Agricultural University, Guangzhou, P.R. China
Luangsa-Ard JJ, Hywel-Jones NL, Manoch L, Samson RA (2005) On the relationships of Paecilomyces sect. Isarioidea species. Mycol Res 109:581–589
Miller GL (1959) Use of dinitrosalicylic acid for estimation of reducing sugar. Anal Chem 31:426–428
Muhammad S, Andrew BK, Lloyd MD (2005) Biological control of the diamondback moth, Plutella xylostella: a review. Biocontrol Sci Technol 15:763–789
Pell JK, Wilding N, Player AL, Clark SJ (1993) Selection of an isolate of Zoophthora radicans (Zygomycetes: Entomophthorales) for biocontrol of the diamondback moth. J Invert Pathol 61:75–80
Pignede G, Wang H, Fudalei F (2000) Characterization of an extracellular lipase encoded by LIP2 in Yarrowia lipolytica. J Bacteriol 182:2802–2810
Qazi SS, Khachatourians GG (2007) Hydrated conidia of Metarhizium anisopliae release family of metalloproteases. J Invert Pathol 95:48–59
Qazi SS, Khachatourians GG (2008) Addition of exogenous carbon and nitrogen sources to aphid exuviae modulates synthesis of proteases and chitinase by germinating conidia of Beauveria bassiana. Arch Microbiol 189:589–596
Regev A, Keller M, Strizhov N, Sneh B, Prudovsky E, Chet I, Ginzberg I, Koncz-Kalman Z (1996) Synergistic activity of a Bacillus thuringiensis delta-endotoxin and a bacterial endochitinase against Spodoptera littoralis larvae. Appl Environ Microbiol 62:3581–3586
Roberts WA, Seletrenikoff CP (1988) Plant and bacterial chitinase differ in antifungal activity. J Gen Microbiol 134:169–176
Samuels RI, Paterson IC (1995) Cuticle degrading proteases from insect moulting fluid and culture filtrates of entomopathogenic fungi. Comp Biochem Physiol 110:661–669
SAS Institute (2000) SAS user’s guide. Statistics. SAS Institute, Cary, North Carolina
Shahabuddin M, Toyoshima T, Aikawa M, Kaslow DC (1993) Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Nat Acad Sci USA 90:4266–4270
Shelton AM, Wyman JA, Cushing NL, Apfelbeck K, Dennehy TJ, Mahr S, Eigenbrode SD (1993) Insecticide resistance of diamondback moth in North America. J Econ Entomol 86:11–19
Smirnoff WA (1974) Three years of aerial field experiments with Bacillus thuringiensis plus chitinase formulation against the spruce bud worm. J Invert Pathol 24:344–348
St. Leger RJ, Charnley AK, Cooper RM (1986a) Cuticle-degrading enzymes of entomopathogenic fungi mechanisms of interaction between pathogen enzymes and insect cuticle. J Invert Pathol 47:295–302
St. Leger RJ, Cooper RM, Charnley AK (1986b) Cuticle-degrading enzymes of entomopathogenic fungi—regulation of production of chitinolytic enzymes. J Gen Microbiol 132:1509–1517
St. Leger RJ, Durrands PK, Charnley AK, Cooper RM (1988) Role of extracellular chymoelastase in the virulence of Metarhizium anisopliae for Manduca sexta. J Invert Pathol 52:285–293
St. Leger RJ, Staples RC, Roberts DW (1991) Changes in translatable mRNA species associated with nutrient deprivation and protease synthesis in Metarhixiurn anisopliae. J Gen Microbiol 137:807–815
St. Leger RJ, Joshi L, Bidochka M, Rizzo MJ, Roberts DW (1996) Biochemical characterization and ultrastructural localization of two extracellular trypsins produced by Metarhizium anisopliae in infected insect cuticles. Appl Environ Microbiol 62:1257–1264
Syed AR (1992) Insecticide resistance on diamondback moth in Malaysia, Diamondback Moth and Other Crucifer Pests. In: Talekar NS (eds) Proceedings of 2nd international workshop, AVRDC, Taiwan, pp 437–442
Tabashnik BE (1994) Evolution of resistance to Bacillus thuringiensis. Ann Rev Entomol 39:7–79
Vandenberg JD, Ramos MR (1997) Screening of fungal isolates against diamondback moth larvae. Arthropod Manag Tests 22:420–421
Vandenberg JD, Ramos MR, Altre J (1998) Dose-response and age and temperature-related susceptibility of the diamondback month (Lepidoptera: Plutellidae) to two isolates of Beauveria bassiana (Hyphomycetes: Moniliaceae). Environ Entomol 64:1402–1412
Wraight SP, Carruthers RJ, Bradley CA, Garza CJ, Galani-Wraight S (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosorosea for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Control 17:203–217
Wright DJ (2004) Biological control of Plutella xylostella—a global perspective. Improving biocontrol of Plutella xylostella. In: Kirk AA, Bodart D (eds) Proceedings of international symposium. Montpellier, France, pp 9–16
Xu YY, Liu TX, Leibee GL, Jones WA (2004) Effects of selected insecticides on Diadegma insulare (Hymenoptera: Ichneumonidae), a parasitoid of Plutella xylostella (Lepidoptera: Plutellidae). Biocontrol Sci Technol 14:713–723
Acknowledgments
This research was funded by grants from the Chinese National Basic Research Program (called 973 Program) (No. 2006CB102005), Public sector specific research projects (No. 200803005) and the eleventh five year forest support program of China (No. 2006BAD08A1903).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.T. Jaronski.
Zhen Huang is joint first author.
Rights and permissions
About this article
Cite this article
Ali, S., Huang, Z. & Ren, S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J Pest Sci 83, 361–370 (2010). https://doi.org/10.1007/s10340-010-0305-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-010-0305-6