Abstract
The entomopathogenic Beauveria spp. were acquired from insect cadavers and soil rhizosphere of cotton, groundnut, and castor. Among Beauveria, five spp. derived from infected insects, eight Beauveria found from soil, and one strain of Beauveria bassiana collected from MTCC 9544. Beauveria were characterized for morphology and cuticle-degrading enzyme activity associated with virulence against Bemisia tabaci. The colony morphology, conidial arrangement, size, and shape confirmed all isolates as Beauveria. The chitinase (EC 3.2.1.14) and lipase (EC 3.1.1.3) activities were observed the highest in Beauveria JAU2, while higher protease (EC 3.4.21.4) activity found in JAU4 followed by JAU2 at 240 h. The bio-efficacy of Beauveria (1 × 107 conidia.ml−1) illustrated that potent JAU2 was examined with the highest % mortality and corrected mortality of B. tabaci at 144 h followed by JAU1. The LC90 and LC50were determined from potent (JAU1 and JAU2) and weak (JAU6), and it was found the lowest in JAU2. The most potent Beauveria JAU2, isolated from insect cadaver (Harmivora armigera), was illustrated higher virulence than other isolates. The Beauveria JAU2 were recognized as Beauveria bassiana based on the shape of conidia and size (2.00 to 2.09 µm dia) as examined in SEM. Study insight into recognition of potent Beauveria bassiana JAU2 was linked with cuticle-degrading enzyme activity for insecticidal action. The JAU2 isolate established the most positive correlation (P0.01: 0.864) between chitinase activity and corrected mortality of insect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chemical pesticides are used as conventional practices, and it continued to increase over the years to augment higher food productions, which caused adverse action and also deteriorated the environment. Side effects of chemically synthesized pesticides lead to an alternate pest management approach which is eco-friendly as well as sustainable for crop production. Of the various microbial entomopathogens including protozoans, bacteria, viruses, fungi, and nematodes reported, but only some were studied scientifically for their utility. A careful assessment of beneficial pathogen may lead to exploiting effective microbial-based biocontrol programs (Lacey et al. 2015). The major insect can be grouped as soil-inhabiting insects, sucking pest, foliage-feeding insects, insects that damage flowers and emergent parts, and those that transmit the virus (Wightman and Whitford 1982).
The whitefly (Bemisia tabaci) species are major agricultural pests for various vegetables, ornamental, and field crops. These pests damage important crop production by a feed nutrition from plants, reducing the produce quality and transmitting viruses which cause viral diseases. Management practices of sucking pest rely on using chemical pesticides which resulted to develop resistance against insect pathogens and again made a problem in many cropping systems (Sadeh et al. 2017; Ellsworth and Martinez-carillo 2001). It also drew the attention of entomologists to develop economically viable, environmental-friendly, and sustainable management practices. Among such eco-friendly approaches, entomopathogenic fungi form played the most essential components which are being employed to control noxious sucking pests of ecosystem (Feng et al. 2004). Entomopathogenic fungi specifically kill insects and other arthropods. These fungi are not harmful to plants and somewhat non-hazardous to animals and humans (Asi et al. 2010). However, further studies on risks of infections in animals and humans are reported by Luangsa-Ard et al. (2011). Despite the fact that insect infected through fungus can be normally recognized in nature and pest control observed as epizootics. However, pest mortality due to fungal infection through natural way occurred rarely or at earlier stage to stop pest cycle and prevent economic losses. Therefore, application of potent antagonist (entomopathogenic fungi) to control the pest is a requisite for biological control of insect and pest.
Beauveria bassiana, one of the biological control agents, is a common entomopathogenic fungus from family Cordycipitaceae. This family is widely known for the production of lethal secondary metabolites and includes different fungal species which include endophytes as well as insect and plant pathogens (Zimmermann et al. 2013). Applications of these fungi were reported for reduction of the population of whiteflies and thrips in key crops such as cotton, brinjal, cucumbers, chrysanthemums, roses, and carnations. B. bassiana caused epizootic infections and played important roles in the innate control of insect populations (Ebani and Mancianti 2021). The pathogen B. bassiana was used against agricultural insect pests of a large host range (Vey et al. 2001). The B. bassiana is broadly utilized as a mycoinsecticide to control various pests and as a biological alternate approach to chemical insecticides. The key benefits for the microbial controller are their capacity to multiply, stick with the environment, and continued to suppress the insect pest populations. Exploiting this benefit, however, is appropriate with the need to resolve the risks to non-target organisms of mass releasing this fungus. Beauveria bassiana with genetic recombination among strains is not available for usage in agriculture, and this recombination could result in altered virulence and host range (Boopathi et al. 2015).
The production of cuticle-degrading enzymes has been proposed as an important attribute determining the virulence of the entomopathogenic fungi towards their hosts because fungi encounter the insect cuticle by producing a wide range of extracellular enzymes (Pedrini et al., 2007). These enzymes involved in the breakdown of protein, chitin, and lipids, which are the principal components of the insect’s cuticle (Koo et al., 2008; Pelizza et al., 2020). Extracellular enzymes including protease, chitinase, and lipase secretion by entomopathogenic fungi may be involved in the degradation of cuticular polymers during pathogenesis, assisting in the penetration of the insect exoskeleton and providing nutrients for fungal growth (Samuels et al., 2011; Petrisor and Stoian, 2017).
The aim of the study was to isolate entomopathogenic B. bassiana strains from various sources (soil and insect cadaver) and characterize them for morphological and microscopic observations. Parasitic evolution of Beauveria strains gave the idea about potent entomopathogenic activity, which may be applied as IPM strategies. The objectives of the study were to (1) isolate B. bassiana by culturing fungi from soil and infected insects collected from the field; (2) characterize the B. bassiana isolates, viz., micro-morphology; and (3) compare the virulence of representative B. bassiana isolates against B. tabaci. It provided information of potential for indigenous Beauveria fungal strain to utilize for biocontrol of B. tabaci and may exhibit helpful strategies for their exploitation in the field.
Materials and methods
Isolation of Beauveria from infected insects
The cadavers of the insect, viz., H. armigera and crops rhizospheric soil, were collected from the isolation of Beauveria fungi on specific media. The samples of cadavers of the insect were firstly sterilized with 4% sodium hypochlorite followed by rinsing with sterile D/W for few times and then were transferred to Sabouraud dextrose agar (SDA) supplemented with antibiotics and kept up to 5 days followed by sub-culturing to obtain and purify the targeted fungi. The macro- and microscopic observations were taken including morphological parameters of conidia and mycelium (Rakh et al. 2011).
Soil (Oe and Oa horizons) were sampled from five randomly chosen field of Junagadh Agricultural University under crop rhizosphere such as cotton, groundnut, and castor. Each sampling soil was collected in 10-cm depth and placed together as subsamples in one plastic bag. Soil samples were diluted (1:100) in a sterile D/W followed by plated on SDA media to consider the frequency of entomopathogenic fungi found in samples. SDA plates were prepared with a 10 mg.l−1 antibiotic solution. The macro- and micro-characteristics of all fungal growth were examined ( Domsch et al. 2007) to confirm the fungal colony as Beauveria (Tuininga et al. 2009).
Morphological, microscopic, and molecular characterizations of entomopathogenic fungi
All Beauveria isolates from various sources were observed for morphological and microscopic characteristics like colony color, reverse color, mycelial form, mycelial color, and shape of conidia and conidial walls under the light microscope (El Kichaoui et al. 2017). Macro- and microscopic examination of isolated fungi were observed on media. All isolates were incubated for 14 days in darkness at 25 °C, and then pigmentation of the colony was recorded as colony color (El Kichaoui et al. 2017). For mounting of fungi, the fungal mycelia were taken on a glass slide. Then a drop of dye (lactophenol cotton blue) was put on the mycelia, and the slide was covered with a cover slip. The slides were examined in the microscope, using the proper microscopic technique (Campbell et al. 1983). Morphological characteristics were spore shape, and its arrangement are recorded from 14-day-old colonies by using lactophenol cotton blue (0.01% w/v) staining. Fungal spore and spore-bearing structures were mounted on a clean slide followed by lactophenol cotton blue staining and observed under light microscope. The spore’s arrangement and its shape were observed in 100 × and SEM. The isolates JAU1 and JAU2 were identified by using B. bassiana–specific probe pBb22 primer P1 (5′-AAGCTTCGACATGGTCTG-3′) – P3 (5′-GGAGGTGGTGAGGTTCTGTT-3′) pair (Hegedus and Khachatourians 1996; Dhar et al. 2019).
Production of cuticle-degrading enzymes from entomopathogenic fungi
Enzyme extract was prepared by inoculating Beauveria in synthetic medium containing 1.0 g KH2PO4, 0.5 g K2HPO4, 0.5 g MgSO4, 0.2 g FeSO4.7H2O, 1.56 g MnCl2.4H2O, 0.02 g NaMOO4, 3.34 g ZnSO4.7H2O, 0.02 g CuSO4.5H2O, 0.48 g NaCl, dextrose (2%), yeast extract (1%), casein (1%), and chitin (1%) in 1 L D/W as a production media as described by Vyas and Deshpande (1989). The broth flasks were inoculated with 107 spores.ml−1 of Beauveria isolates under study and incubated for 144 h in orbital shaker (120 rpm) at 28 °C.
The protease (EC 3.4.21.4) assay was done using casein as a substrate (Charney and Tomarelli, 1947). The reaction system contained 100 µl of 0.1% (w/v) of casein and 100 µl of culture supernatants. Free amino acids were released by protease which was measured by using Ninhydrin method (Lee and Takahashi, 1966). The activity of protease was expressed as mM.mg−1 protein. The absorbance (OD) was measured at 530 nm (Malik and Singh 1980). Chitinase (EC 3.2.1.14) assay was done by the method given by Boller and Mauch (1988). The reaction mixture comprises 500 µl of 0.5% chitin in 10 mM sodium acetate buffer, pH 5.2, and 500 µl of culture supernatants, which were incubated for 1 h at 50 °C. The formation of sugar N-acetyl-D-glucosamine was determined by the DMAB method, and the activity of chitinase was expressed as μM.mg-1 protein (Reissig et al. 1955). The assay of lipase (EC 3.1.1.3) activity was done according to Nahar et al. (2004), and gum-acacia and olive oil were used as substrate. The substrate mixture was made up of an equal proportion of gum arabic (50 ml, 10% w/v) and olive oil (50 ml). The reaction mixture consisted of 2 ml phosphate buffer (50 mM, pH 6.8), crude enzyme extract (1 ml), and substrate emulsion (5 ml). The reaction mixture was incubated at 37 °C for 1 h with constant agitation, and 4 ml of acetone–ethanol (1:1) containing 0.09% phenolphthalein indicator was used as reaction terminator. The activity of lipase was assayed with fatty acid titration released with sodium hydroxide (50 mM). The activity was expressed as the total of enzyme-released fatty acids (1 mol) per min.
Bio-efficacy of potent Beauveria isolate for their parasitic activity
Preparation of conidial suspension
The fungal isolates, first, were grown in SDA medium for 10–14 days in flasks, and aqueous spore suspensions of various spore concentrations were prepared using sterile D/W. The conidia were harvested by scrapping and were suspended in sterile aqueous 0.1% Tween 80 solution. A suspension was quantified in a Neubauer chamber according to Zafar et al. (2016). A suspension containing 107conidia.ml−1 was prepared by using serial dilutions. Sterile aqueous Tween 80 (0.1%) solution was used as control solution. The conidial suspension was vortex for 5 min to produce uniform conidial suspension. The viability of conidia was evaluated according to studies reported by Javed et al. (2019).
Laboratory bioassay
The field-collected whiteflies (B. tabaci) were used for the assessment of the bio-efficacy of different Beauveria isolates under laboratory condition. The influences of different isolates of B. bassiana on percent mortality of whiteflies were studied under laboratory condition with three replications. The collected whiteflies were released in Petri plates containing cotton leaves. Each treatment was replicated three times. In each treatment, the numbers of whiteflies released were 30. The suspension contains 107 conidia.ml−1 sprayed on the whiteflies. The mortality of each B. tabaci was recorded from the first day to the seventh day after spray. Further, Beauveria has been isolated from the dead whiteflies and characterized for efficacy. The data on % mortality were subjected to statistical analysis to draw the inference. The percent mortality was calculated as described by Javed et al. (2019) and Abbott (1925).
Determination of LC50 and LC90 values of Beauveria
The cotton leaves were taken and surface sterilized by rinsing them in sodium hypochlorite (0.2%) for 3 min and then rinsed three times with distilled water. Then, the leaves were dried under a fan. The amount of Beauveria ranging from 105 to 109 conidia.ml−1 was prepared in laminar airflow. Then the fan dried leaves of cotton were dipped (leaf dip method) in respective concentrations, as such 5 sets of treated leaves were pre-arranged and put on Petri plates to use it for subsequent investigation against the whiteflies. An average of 30 whiteflies were released using a sterile hairbrush on the respective treated leaf. The respective concentrations were also sprayed over the whiteflies. The number of dead whiteflies was recorded daily for 7 days. The fungal infection was confirmed by observing the development of mycosis with the help of a microscope, and the mode of action of fungus was studied. The same experiment was performed with all five Beauveria isolates which were screened by their bio-efficacy, and the number of dead whiteflies was recorded daily in the respective experiment up to 7 days after treatment. During each experiment, a control set with untreated leaves of cotton was also maintained, and daily observations on whiteflies mortality were taken. Data generated on whiteflies mortality by bioassay of whiteflies were used to determine LC50 and LC90 values (Bugti et al. 2018). The potent Beauveria isolate was recognized at the species level based on colony characteristics and conidial shape and size (SEM morphology) (Afandhi et al. 2012; Imoulan et al. 2017).
Data analysis
The experiment on insect mortality and enzyme assay was conducted in three replications, and statistical analysis was carried out using a completely randomized design (Fisher and Yates, 1948). The critical differences were determined to distinguish the level of significance at 0.05% among treatments (isolates). The correlation between corrected mortality and cuticle-degrading enzymes were examined to identify precise enzyme for insect mortality (SPSS software). Prior to correlation analysis, the Tukey’s HSD (honestly significant difference) test was carried out for pairwise significance comparisons between mortality and enzyme activity. The F-statistic analysis indicated the significance (p value) of the observed Q-statistic. The Tukey’s HSD results suggested significant differences (p < 0.01) between mortality percent and enzymes—protease (Q = 35.82), chitinase (Q = 36.07), and lipase (Q = 35.58). The LC50 and LC90 of potent, moderate, and weak isolates were determined using Probit analysis, and values were calculated based on Finney’s method (Finney and Stevens, 1948).
Results and discussion
Isolation of Beauveria from infected insects
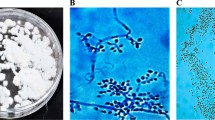
The cadavers of insects and soil samples were collected from different fields of Junagadh region to the laboratory, and the fungi were isolated on SDA medium fortified with 1% yeast extract. The result showed that a total of five Beauveria isolates were isolated from infected insects and designated as JAU1, JAU2, JAU3, JAU4, and JAU5. However, a total of eight Beauveria isolates were isolated from different soil which was collected from the soil of cotton, groundnut, and castor field and designated as JAU6, JAU7, JAU8, JAU9, JAU10, JAU11, JAU12, and JAU13. The standard strain of Beauveria collected from MTCC (JAU14) was also included in study (Table 1). Total fourteen isolates derived from different sources were recognized as Beauveria based on their microscopic examination and colony characteristics (Table S1 and Fig. 1).
Morphological, microscopic, and molecular characterizations of entomopathogenic fungi
The conidiophores of Beauveria appeared in compact to nearly stomatic patches, mononematous, conidiogenous cells with phialides in whorls, often arranged in a candle like fashion, clavate to cylindrical and conidia were single celled, hyaline, smooth walled and produced in slimy heads. Morphologically, all the isolates exhibited typical Beauveria characteristics. All isolates showed white colony color except JAU1 and JAU14 isolate which was cream and off white, respectively (Fig. 1). Reverse colony color of JAU1, JAU2, JAU4, JAU5, JAU7, JAU9, JAU10, and JAU12 showed pale yellow. JAU3, JAU6, JAU11, and JAU14 isolates showed light yellow reverse colony color, and two isolates JAU8 and JAU13 showed white color on SDA medium (Table S1).
Morphological characters of all isolates revealed differences with respect to hyphal and conidial characters except in size of conidia under observation in light microscope and SEM. All Beauveria isolates were identified according to the conidium morphology. There was variation in conidial shape and pigmentation between the isolates. The results showed that all Beauveria isolates show white mycelial and conidial color with different shape of conidia such as globose, ellipsoidal, globose to flask-shaped, and subglobose. Mycelial form of all isolates shows different form such as cushion-like, funiculose, granular-pulverulent, and aerial mycelium. However, all isolates exhibited significant variation in the number of days for sporulation. These macro- and micro-morphological characteristics of all isolates were in line with the result of the previous research (Afandhi et al. 2012; Imoulan et al. 2017).
Castillo et al. (2012) isolate B. bassiana in the soil of plantation area and infected insect. B. bassiana was identified according to the conidium morphology. These phenotypic characters in colony morphology were in line with the result of the previous research by Afandhi et al. (2012). The colony of B. bassiana showed yellowish white or opaquely white color. The hypha is shaped like floss in cotton where it characterized B. bassiana which is categorized in class of Hyphomycetes. The shape of colony B. bassiana was round which was correlated with the apical growth of the colony where the colony grew in all directions. The morphological analysis of our study was supported by the result of Humber (2005) which describes that Beauveria isolates showed colonies with white and flat at first, then expanded to some stage, and appeared ochroleucous and powdery at last when they generated conidia, when the bottom of the culture media was colorless (Kulu et al. 2015; Gurlek et al. 2018).
Production of cuticle-degrading enzymes from entomopathogenic fungi
Data on activity of protease (EC 3.4.21.4) revealed that the highest activity were found in JAU4 (0.484 µM.ml−1) followed by JAU2 (0.483 µM.ml−1), while the lowest specific activity was recorded by JAU6 (0.328 µM.ml−1) in the SDA culture medium (Fig. 2). The result of the specific activity of chitinase (EC 3.2.1.14) enzyme is shown in Fig. 2. In vitro chitinase activity was found the highest (0.107 µM.ml−1) in JAU2 followed by JAU1 (0.102 µM.ml−1) and JAU4 (0.100 µM.ml−1). Chitinase activity was significantly reduced to 0.052 µM.ml−1 in JAU6. Data on lipase (EC 3.1.1.3) activity revealed that the highest activity in JAU2 (0.80 µM.min−1) was followed by JAU1 (0.77 µM.min−1), while the lowest activity was recorded by JAU6 (0.60 µM.min−1) in the culture medium (Fig. 2). The correlation between % corrected mortality of insect B. tabaci and cuticle-degrading enzymes (chitinase, protease, and lipase) demonstrated a significant positive relationship. Results indicated that % corrected mortality was significantly (p = 0.01) positively correlated with chitinase (0.864) followed by lipase (0.7662) and protease (0.765) enzymes. The B. bassiana JAU2 isolate illustrated the highest activity of chitinase followed by lipase and hence JAU2 might be a potent isolate for insecticidal activity.
Insect contain many protein compounds (Hunsley and Burnett 1970). It, therefore, was expected that Beauveria synthesized proteases which may act on insect. High protease production during 8th to 9th days of culture is comparable with the observations by Dhar and Kaur (2010), demonstrating high protease activity on 9th day of culture in B. bassiana. The time for maximum protease production does not necessarily depend upon the media constituents. Pelizza et al. (2012) reported chitinase activity in nine isolates of M. anisopliae and 28 isolates of B. bassiana, and activity was elevated in potent fungal strains correlating with mortality of Tropidacris collaris Stoll pest. Our data are supported by Dhawan and Joshi (2017) who reported the mean lipase activities of various isolates of B. bassiana. The maximum activity of lipase was recorded in B. bassiana MTCC 4495 (1.36 U/ml) followed by B. bassiana MTCC 2028 (1.11 U/ml). The maximum lipase activity was recorded on the sixth day (1.64 U/ml), and the minimum lipase activity was recorded on the second day (0.61 U/ml) of incubation.
Bio-efficacy of potent Beauveria isolate for their parasitic activity
Parasitic activity of Beauveria isolates against test pest B. tabaci
The formulation of Beauveria was evaluated at 1 × 107conidia.ml−1 against B. tabaci in laboratory. Mortality in the B. tabaci occurred in the 48 h of treatment (Table 2) and gradually increased until it reached above 50% in the 168 h after treatment. The highest percent of mortality was observed at 144 h which was shown in JAU2 (72.27%) followed by JAU1 (63.98%). The percent mortality was above 25% in all Beauveria isolate in 96 h from treatment except two isolates which was JAU6 and JAU11. That means JAU2 was highly affected B. tabaci population followed by JAU1 and then other Beauveria isolates. All isolates show clear infection of different Beauveria isolates, but their infection shows different rates such as infection which starts on the second day of treatment, but some isolates show fast growth of Beauveria on B. tabaci, and some isolates show slow growth of fungi on B. tabaci (Fig. 3).
Above 70% mortality was recorded from 144 h after treatment in only one isolate including JAU2 which was 72.27. The maximum mortality was 72.27% recorded in JAU2 followed by JAU1 which was 63.98%, and the lowest % mortality was recorded in JAU6 which was 36.42% from 144 h after treatment. Similarly corrected % mortality was the highest in JAU2 (70.29%) followed by JAU1 (61.41%) and least in JAU6 which was 31.88% (Table 2). Thus, on the basis of % mortality, JAU2 was recorded as a potent isolate followed by JAU1 and JAU14 observed as moderate parasitic activity, whereas JAU6 was recorded as the least effective isolate. SEM image of B. tabaci indicated clear infection of Beauveria JAU 2 as compared to control (Fig. 4).
Determination of LC50 and LC90 values of Beauveria
Two best isolates JAU1 and JAU2 and two moderate isolates JAU4 and JAU14 as well as one least parasitic isolate JAU6 were used for determination of LC50 and LC90 value to determine the concentration of formulation required to control 50 and 90% whiteflies in the test. According to Probit analysis, results showed that the lowest LC50 value was recorded in JAU2 isolate which was 0.043 × 105 conidia.ml−1 as compared to JAU1 isolate which was 0.19 × 105 conidia.ml−1 as well as JAU14 and JAU4 isolates showing 423.6 × 105 and 1817.1 × 105 conidia.ml−1, respectively, but the highest LC50 was 4522.8 × 105 conidia.ml−1 which was recorded in JAU6 isolate. Similarly LC90 was lowest in JAU2 (0.05 × 1014 conidia.ml−1) followed by JAU1 (0.12 × 1014 conidia.ml−1), JAU14 (8.3 × 1014 conidia.ml−1), and JAU4 (31.8 × 1014 conidia.ml−1), whereas the highest is JAU6 (144.7 × 1014 conidia.ml−1). Results interpreted that the lowest LC50 and LC90 value of JAU2 showed highly virulent isolate as compared to other isolates, and the highest LC50 and LC90 was showed least virulent isolates as compared to others (Table 3). The potent isolates JAU1 and JAU2 were found yellowish white and white in colony, respectively. JAU1 and JAU2 have the surface raised curved and bowl shape with conidial shape globose and round, respectively. The SEM images showed that the conidial size of JAU1 was found in the range of 1.90 to 2.01 µm dia. and that of JAU2 was found with 2.0–2.09 µm dia. The potent Beauveria strains JAU1 and JAU2 were recognized as B. bassiana based on colony characteristics and SEM morphology (Table 4) (Afandhi et al. 2012; Imoulan et al. 2017). In addition, the two potent isolates JAU1 and JAU 2 were characterized using probe-specific primer amplification, and DNA fragment was amplified with the size of 522 bp. The PCR product was observed near the 524 bp size, and it confirmed B. bassiana (Table 4) (Hegedus and Khachatourians 1996; Dhar et al. 2019).
Bio-efficacy of Beauveria was studied under lab condition which shows above 50% mortality and LC50 values of B. tabaci. Similar results were observed by Shams et al. (2011) which LC50 values on day 9 post-treatment of Beauveria were 3.17 × 106 and 6.08 × 107 conidia.ml−1 for C. maculatus and S. granarius, respectively. These results were also supported by different scientists who reported that Beauveria showed good pathogenicity to B. tabaci and mortalities observed above 50% when exposed to Beauveria (Zafar et al. 2016; Bugti et al. 2018).
The current study showed the effectiveness of Beauveria strains against B. tabaci. The study showed that Beauveria is effective for the control of B. tabaci on cotton crops at different concentrations. The fungi causing pathogenicity in insects were observed for mortality of insect pests and allowed them as biocontrol means (Sain et al. 2019; Akmal et al. 2013) or effectively developed for the biological control of several insect pests, which include B. tabaci also (Shah and Pell 2003; Faria and Wraight 2007). The B. bassiana virulence potential against target B. tabaci populations is different for different isolates and also varies from isolate to isolate. The B. tabaci can have different susceptibility ratio to different isolate (Blanford et al. 2005; Sevim et al. 2012). Similarly, the isolates of B. bassiana were reported to be virulent against B. tabaci (71–86% mortality; eight days), with LC50 values ranging from three to four DAI with 107conidia.ml−1 (Mascarin 2013). Obtained results showed that there was a variable response of the Beauveria isolates to insect mortality. The mortality of B. tabaci with B. bassiana increased with spore concentration of conidial suspensions and time exposure (Wraight et al. 2007). Ansari et al. (2011) illustrated that insect mortality was depend on concentration of fungal conidia and time exposure.
The insect gut and crop soil rhizosphere were targeted to isolate entomopathogenic fungi—Beauveria. A total of 14 isolates were derived from different rhizosphere including one standard MTCC strain. The Beauveria isolates were characterized with macro- and micro-morphological observations. The colony of Beauveria was found mostly intact and white in color. The mycelia form and shape of conidia were found diverse for each of isolated Beauveria. The cuticle-degrading enzyme (protease, chitinase, and lipase) activities of Beauveria isolates indicated that the highest activity of all three enzymes was found in JAU2 followed by JAU1 for chitinase and lipase and JAU4 for protease activities. The parasitic activity of 14 strains illustrated that Beauveria JAU2 strain was found with the highest percent of mortality and corrected mortality against B. tabaci followed by JAU1. The mortality incidence was correlated to cuticle-degrading enzyme activity mainly lipase and chitinase. The lowest LC50 and LC90 values of potent Beauveria JAU2 found were 0.043 × 105 and 0.05 × 1014, respectively. The potent JAU2 were identified as B. bassiana based on colony characteristics and SEM morphology. The association between enzyme activity and parasitism of Beauveria on sucking pest was established which may be useful for the recognition of enzyme-based potent Beauveria isolates for insecticidal activity.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:256–267
Afandhi A, Syamsidi SRC, Mimbar SM, Wiroatmodjo B (2012) Isolation and phenotypic characterization of morphology in fungus Beauveria bassiana (Balsamo) Vuillemin colony naturally from leaf surface soil and insect as host in tomato plantation. Agrivita 34(3):303–309
Akmal M, Freed SH, Malik MN, Gul HT (2013) Efficacy of Beauveria bassiana (Deuteromycotina: Hypomycetes) against different aphid species under laboratory conditions. J Zool 45(1):71–78
Ansari MA, Pope EC, Carpenter S, Scholte EJ, Butt TM (2011) Entomopathogenic fungus as a biological control for an important vector of livestock disease: the Culicoides biting midge. PLoS ONE 6:e16108
Asi MR, Bashir MH, Afzal M, Ashfaq M, Talib-Sahi S (2010) Compatibility of entomopathogenic fungi Metarhizium anisopliae and Paecilomyces fumosoroseus with selective insecticides. Pak J Bot 42:4207–4214
Blanford S, Chan BH, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB (2005) Fungal pathogen reduces potential for malaria transmission. Science 308:1638–1641
Boller T, Mauch F (1988) Colorimetric Assay for Chitinase. Method Enzymol 161:430–435
Boopathi T, Karuppuchamy P, Kalyanasundaram M (2015) Microbial control of the exotic spiralling whitefly Aleurodicus disperses (Hemiptera:Aleyrodidae) on eggplant using entomopathogenic fungi. Afr J Microbiol Res 9:39–46
Bugti GA, Bin W, Na C, Feng LH (2018) Pathogenicity of Beauveria bassiana strain 202 against sap-sucking insect pests. Plant Protect Sci 54:111–117
Campbell RK, Barnes GL, Cartwright BA, Eikenbary RD (1983) Growth and sporulation of Beauveria bassiana and Metarhizium anisopliae in a basal medium containing various carbohydrates sources. J Invertebr Pathol 41:117–121
Castillo MG, Rivera IA, Padilla AB, Victoriano FL, Aguilar CN, Herrera RR (2012) Isolation and identification of entomopathogenic fungal strains of the Beauveria and Metarhizium generous. Indian J Biotechnol 6(12):386–395
Charney MS, Tomarelli RM (1947) A colorimetric method for the determination of the proteolytic activity of duodenal juice. J Biol Chem 171:501–505
Dhar P, Kaur G (2010) Production of cuticle-degrading proteases by B. bassiana and their induction in different media. Afr J Biochem Res 4(3):65–72
Dhar S, Jindal V, Jariyal M et al (2019) Molecular characterization of new isolates of the entomopathogenic fungus Beauveria bassiana and their efficacy against the tobacco caterpillar, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Egypt J Biol Pest Control 29(1):1–9
Dhawan M, Joshi N (2017) Enzymatic comparison and mortality of Beauveria bassiana against cabbage caterpillar Pieris brassicae LINN. Vet Microbiol 48:522–529
Domsch KH, Gams W, Anderson TH (2007) Compendium of Soil Fungi, 2nd edn, taxonomically revised by W. Gams. IHW-Verlag, Eching, p.672
Ebani VV, Mancianti F (2021) Entomopathogenic fungi and bacteria in a veterinary perspective. Biology 10(6):479
El Kichaoui AY, Asaker BA, El Hindi MW (2017) Isolation molecular identification and under lab evaluation of the entomopathogenic fungi M anisopliae and B bassianaagainst the red palm weevil R ferrugineusin Gaza Strip. Adv Microbiol 7:109–124
Ellsworth PC, Martinez-carillo JL (2001) IPM for Bemisia tabaci a case study from North America. Crop Protec 20:853–869
Faria MR, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43:237–256
Feng MG, Pu XY, Ying SH, Wang YG (2004) Field trials of an oil based emulsifiable formulation of Beauveria bassiana conidia and low application rates of imidacloprid for control of false-eye leafhopper Empoasca vitisin Southern China. J Crop Prot 23(6):489–496
Finney DJ, Stevens WL (1948) A table for the calculation of working probits and weights in probit analysis. Biometrika 35(1–2):191–201
Fisher RA, Yates ND (1948) “Statistical methods for research workers”. Oliver and Boyd, Edinburgh, London. 12th edition-Biological Monographs and Manuals, 5:130–131
Gurlek S, Sevim A, Sezgin FM, Sevim E (2018) Isolation and characterization of Beauveria and Metarhizium spp from walnut fields and their pathogenicity against the codling moth Cydiapomonella (L) (Lepidoptera: Tortricidae). Egypt J Biol Pest Co 28:50–56
Hegedus DD, Khachatourians GG (1996) Identification and differentiation of the entomopathogenic fungus Beauveria bassiana using polymerase chain reaction and single-strand conformation polymorphism analysis. J Invertebr Pathol 67(3):289–299
Humber RA (2005) Entomopathogenic fungal identification, key to major genera. Available on: https://www. ars. usda. gov/SP2UserFiles/Place/1907051 0. APSwkshoprev. pdf
Hunsley D, Burnett JH (1970) The ultrastructural architecture of the walls of some hyphal fungi. J Gen Microbiol 62:203–218
Imoulan A, Hussain M, Kirk PM, Meziane YY (2017) Entomopathogenic fungus Beauveria: Host specificity ecology and significance of morpho-molecular characterization in accurate taxonomic classification. J Asia Pac Entomol 20:1204–1212
Javed K, Javed H, Mukhtar T, Qiu D (2019) Efficacy of Beauveria bassiana and Verticillium lecanii for the management of whitefly and aphid. Pak J Agri Sci 56(3):669–674
Koo YD, Ahn JE, Salzman RA, Moon J, Chi YH, Yun DJ, Yun SY, Koiwa LH, Zhu-Salzman K (2008) Functional expression of an insect cathepsin B-like counter-defence protein. Insect Mol Biol 17(3):235–245
Kulu IP, Abadi AL, Afandhi A, Nooraidawati S (2015) Morphological and molecular identification of Beauveria bassiana as entomopathogen agent from Central Kalimantan Peatland Indonesia. Int J Chem Tech Res 8(4):2079–2084
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41
Lee YP, Takahashi T (1966) An improved colorimetric determination of amino acids with the use of ninhydrin. Analytical Biochem 14:71–73
Luangsa-Ard J, Houbraken J, van Doorn T, Hong S, Borman AM, Hywel-Jones NL, Samson RA (2011) Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol Lett 321(2):141–149
Malik CP, Singh MB (1980) Plant Enzymology and Histo Enzymology. Kalyani Publishers, New Delhi, p 286
Mascarin GM (2013) The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (Hemiptera:Aleyrodidae) and their conidial production using solid substrate fermentation. Biol Control 66:209–218
Nahar P, Ghormade V, Deshpande MV (2004) The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: possible edge to entomopathogenic fungi in the biological control of insect pests. J Invertebr Pathol 85:80–88
Pedrini N, Crespo R, Juarez MP (2007) Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Com Biochem Physiol 146:124–137
Pelizza SA, Eliades LA, Scorsetti AC, Cabello MN, Lange CE (2012) Entomopathogenic fungi from Argentina for the control of Schistocerca cancellarta (Orthoptera:Acrididae) nymphs: fungal pathogenicity and enzyme activity. Biocontrol Sci Technol 22(10):1119–1129
Pelizza SA, Medina H, Ferreri NA, Elíades LA, Pocco ME, Stenglein SA, Lange CE (2020) Virulence and enzymatic activity of three new isolates of Beauveria bassiana (Ascomycota: Hypocreales) from the South American locust Schistocerca cancellata (Orthoptera: Acrididae). J King Saud Uni Sci 32(1):44–47
Petrisor C, Stoian G (2017) The role of hydrolytic enzymes produced by entomopathogenic fungi in pathogenesis of insects mini review. Rom J Plant Prot 10:66–72
Rakh RR, Raut LS, Dalvi SM, Manwar AV (2011) Biological control of Sclerotiumrolfsii causing stem rot of groundnut by Pseudomonas Cf Monteilii. Recent Res Sci Technol 3(3):26–34
Reissig J, Strominger J, Leloir L (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 4:959–966
Sadeh D, Nitzan N, Shachter A, Chaimovitsh D, Dudai N, Ghanim M (2017) Whitefly attraction to rosemary (Rosmarinu sofficinialis L) is associated with volatile composition and quantity. PLoS ONE 12(5):e0177483
Sain SK, Monga D, Kumar R, Nagrale DT, Kranthi S, Kranthi KR (2019) Comparative effectiveness of bioassay methods in identifying the most virulent entomopathogenic fungal strains to control Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Egypt J Biol Pest Co 29:31–41
Samuels RI, Santos AV, Silva CP (2011) Enzymology of enthomopathogenic fungi. Microbial Insecticides -Principles and Applications, Nova Science Publishers, Inc, pp.71–92
Sevim A, Donzelli BG, Wu D, Demirbag Z, Gibson DM, Turgeon BG (2012) Hydrophobin genes of the entomopathogenic fungus Metarhizium brunneum are differentially expressed and corresponding mutants are decreased in virulence. Curr Genet 58:79–92
Shah PA, Pell JK (2003) Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol 61:413–423
Shams G, Safaralizadeh MH, Imani S, Shojai M, Aramideh S (2011) A laboratory assessment of the potential of the entomopathogenic fungi B bassiana (Beauvarin®) to control Callosobruchus maculatus (F) (Coleoptera: Bruchidae) and Sitophilus granarius (L) (Coleoptera: Curculionidae). Afr J Microbiol Res 5(10):1192–1196
Tuininga R, Miller J, Shannon U, Daniels T, Richard C, Kirby C (2009) Isolation of entomopathogenic fungi from soils. J Med Entomol 46:557–565
Vey A, Hoagland R, Butt TM (2001) Toxic metabolites of fungal biocontrol agents. In: Fungi as biocontrol agents: progress, problems and potential, (Ed. Butt TM, Jackson C, Magan N) pp. 311–346. CAB eBooks, https://doi.org/10.1079/9780851993560.0311
Vyas P, Deshpande MV (1989) Chitinase production by Myrothecium verrucaria and its significance for fungal mycelia degradation. J Gen Appl Microbiol 35:343–350
Wightman JA, Whitford DNJ (1982) Integrated control of pests of legume seed crops. New Zeal J Exp Agr 10:209–215
Wraight SP, Inglis GD, Goettel MS (2007) Fungi In: Lacey L A Kaya HK (Eds) field manual of techniques in invertebrate pathology: application and evaluation of pathogens for control of insects and other invertebrate pests Springer Dordrecht the Netherlands pp. 223–248.
Zafar J, Freed S, Basir AK, Muzammil F (2016) Effectiveness of Beauveria bassiana against cotton whitefly Bemisia tabaci (Gennadius) (Aleyrodidae: Homoptera) on different host plants. Pak J Zool 48(1):91–99
Zimmermann G, Huger AM, Kleespies RG (2013) Occurrence and prevalence of insect pathogens in populations of the codling moth Cydia pomonella L: a long-term diagnostic survey. Insects 4:425–446
Author information
Authors and Affiliations
Contributions
RVB carried out the experiment, microbial work, and biochemical analysis and working out the results; HPG was responsible for interpretation of data, writing of the manuscript, and liable for the idea and coordination of the experiment. DGH and HJK were responsible for helping in microbial and biochemical analysis. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Guido Favia
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhadani, R.V., Gajera, H.P., Hirpara, D.G. et al. Characterization and bio-efficacy of entomopathogenic Beauveria associated with cuticle-degrading enzymes to restrain sucking pest Bemisia tabaci. Parasitol Res 121, 2019–2031 (2022). https://doi.org/10.1007/s00436-022-07557-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07557-w