Abstract
Depolymerized real lignin products are very complex mixtures. Comprehensive assessment of the decomposition efficiency and characterizing of depolymerized products still remain extremely challenging. In this study, based on depolymerization solution of commercially available sodium lignosulfonate under mild conditions, an improved method was well established for characterization of real lignin depolymerized products using GC–MS, which enabled the detection of main 37 lignin-based aromatic products. The effects of acid-catalyzed temperature, time and catalyst/lignin ratio on the depolymerization products were systematically investigated. The result revealed that ~ 25 wt% of lignosulfonate was depolymerized into lignin-derived aromatic products under optimized mild acid-catalyzed reaction conditions of 130 °C for 60 min with a catalyst/lignin ratio of 2.334. Most of the identified products were common commercial compounds, while the obtained bisphenols were potential compounds for new applications such as bio-based polymer building blocks. Preliminary studies also highlight that the depolymerization behavior seems to present selectivity to some extent during these specified acid-catalyzed reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignin is an amorphous tridimensional polymer of three primary phenylpropane subunits: sinapyl (3, 5-dimethoxy 4-hydroxycinnamyl), coniferyl (3-methoxy-4-hydroxycinnamyl), and p-coumaryl (4-hydroxycinnamyl) alcohols, predominantly joined by β-aryl ether linkage [1]. Since lignin is the richest renewable bioresource containing high-volume of aromatic repeat units, significant efforts have been undertaken to develop methods and processes to utilize lignin as the feedstock for the large-scale industrial production of aromatic compounds to reduce dependence on petroleum and coal. However, the catalytic conversion of lignocellulose have made great progress [2,3,4], most of those lignin depolymerization techniques reported low yields of small aromatic compounds and using harsh conditions with high temperature and pressure due to the three-dimensional amorphous and complicated aggregated structure of real lignin; while a number of studies had been conducted mainly on small lignin model compounds, many of them failed to be applied effectively for real lignin depolymerization. This also hampers the development of standard protocols for comprehensive assessment of conversion efficiency and depolymerization products [5,6,7,8,9,10,11,12,13].
At present, the key commercial lignin products such as lignosulfonate and Kraft lignin are extracted from lignocellulosic delignification waste stream in the wood pulp processes. Since the lignin extracted in the pulping processes is considered as a low-quality and low-value-added by-product, only about 2 percent of it (1 million tons) is recovered and used for cement concrete, dispersing, and binding applications, etc.; the rest is incinerated on-site as a low-value fuel for the production of process steam and energy [5, 14]. While lignosulfonate is considered less prone to catalytic valorization owing to its high average molecular weight (MW ~ 50 kDa) and numerous sulfonate groups [15], there are a limited number of reports on the depolymerization of lignosulfonate. The most frequent studies are to oxidize lignosulfonate in alkaline medium with O2 or with metal salt catalysts or under harsh conditions to produce few phenolic compounds. The more notable one is to produce an artificial vanilla, a widespread flavoring agent in 2 to 8% yield [16,17,18,19,20,21]. In an attempt to add value to this mass abundant, renewable and low-cost industrial by-product, in our previous report, we proposed a simple one-step method for the depolymerization of lignosulfonate under mild conditions [22]. We reported for the first time an efficient size exclusion chromatography (SEC) method for the separation and analysis of lignin depolymerization products [23], which enabled the detection of low-MW depolymerization products, which had molecular masses of approximately 720, 490, and 260, indicating that lignosulfonate was almost completely converted into oligomers or small molecules. Since no mass spectral libraries have been built for LC–MS analysis and identification of lignin-derived aromatic compounds, screening and identification of depolymerization products was also developed by the self-build online screening database via ultra-high performance liquid chromatography tandem quadrupole time-of-flight Mass spectrometry (UPLC-QTOF-MS) in MSE data acquisition mode, nine aromatic compounds had been proposed therein [21,22,23,24].
Gas chromatography tandem mass Spectrometry (GC–MS) mass spectral libraries enable the profiling of low-MW volatile molecules. In this study, we describe an efficient method for the separation and identification of real lignosulfonate depolymerized products using GC–MS, whereby, a detailed characterization of lignin depolymerization behavior under various reaction parameters and their effect on depolymerization was then explored intensively. Consequently, this preliminary work lays the foundation for fine-tuning the reaction parameters for producing valuable products with decent yields under mild acid-catalytic depolymerization conditions.
Experimental
Chemicals and reagents
Sodium lignosulfonate (Mw ~ 50,000 Da) was purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Analytical grade hydrochloric acid, sulfuric acid and phenol were purchased from Xilong Chemical Corporation (China). HPLC grade acetonitrile was purchased from Fisher Scientific Hampton, NH, U.S.A.). 2, 6-dimethoxyphenol (greater than 98% purity) was purchased from Energy Chemical (China).
Gas chromatography–mass spectrometry (GC–MS) analysis
The Agilent Technologies 7890A-5975C gas chromatograph-mass spectrometer was used. Helium was used as the carrier gas, the injection volume was 1 μL, and the inlet temperature was set to 290 °C. The column temperature program was set as follows: maintain at 50 °C for 3 min, raise to 160 °C at a rate of 5 °C/min, maintain at 160 °C for 8 min, increase to 290 °C at a rate of 5 °C/min, and finally, hold at 290 °C for 5 min. The MS ion source setting was as follows: electron impact voltage, 70 eV; ion source temperature, 230 °C; quadrupole temperature, 150 °C; solvent delay, 3 min; and full scan mode with scanning range from 30 to 500 amu. The NIST08 library was used for MS compound identification with the probability match more than 80%. The quantification of standard curves was performed in single-ion monitoring mode.
Lignosulfonate depolymerization and sample preparation
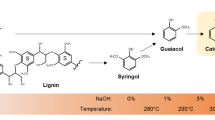
50 g of phenol solvent was added to a round bottom flask equipped with a condenser and a magnetic stir bar, which was stirred in an oil bath. When the bath temperature reached to 50 °C, a mixture of sulfuric acid and hydrochloric acid (10 mL) with mol ratio of H2SO4/HCl: 1:3) was added first, and then 12.5 g of brown lignosulfonate powder was added. The reaction mixture was stirred at 110 °C for ~ 30 min, some white solid precipitate clearly observed during reaction due to the formation of salt in situ [20,21,22,23]. It is worth to notice that lignosulfonate (SL) is quite soluble in water but not soluble in organic solvents such as phenol, methanol, acetone and acetonitrile. However, the depolymerized lignin solution (DLS) is very miscible with the aforementioned organic solvents. For preparation sample detected by GC–MS analysis in this paper, 1 g of the depolymerized lignin solution was extracted with 5 mL acetonitrile. After extraction step, the soluble fraction, containing the majority of the lignin derived mass was obtained and defined as “depolymerized soluble products” (DSP). The acid-catalyzed lignosulfonate depolymerization reaction and the product separation process are illustrated in Fig. 1. Control experiments were conducted without added the sulfuric acid/hydrochloric acid mixture, lignosulfonate was not able to be dissolved and depolymerized in the phenol solution throughout the 30 min reaction time. The brown color powder was separated by high-speed centrifuge filtration, and the acetonitrile soluble fraction was used as the control blank sample for GC–MS analysis. All extracted solution samples hydrated with anhydrous sodium sulfate prior to injection.
Results and discussion
Detection and identification of lignosulfonate depolymerization products using GC–MS
Depolymerized real lignin products especially under harsh conditions are very complex mixtures, for a comprehensive assessment of the depolymerization efficiency and understanding of decomposition components still remains extremely challenging [3, 23,24,25]. Therefore, we believe that it is very important to build an efficient method for symmetrically characterizing depolymerized products, which could facilitate investigating the effects of variations on depolymerization and thus the mechanism. For the depolymerized solution of lignin under these mild conditions, our gel-permeation chromatography results confirmed that the high molecular mass of lignosulfonate was depolymerized to small molecular weight species in higher yield for the first time. Here the volatile chemicals in aforementioned acetonitrile soluble samples were systematically investigated by GC–MS.
In general, the columns play a central role in the GC–MS analysis. Since GC–MS technique has some limitations on analyzing nonvolatile oligomers and polar compounds such as acids, qualitative analysis of the volatile products from lignin depolymerization was usually reported to use kind of nonpolarity column such as HP-5 MS type [16–119, 26.27]. In this paper, considering the very complex depolymerized mixtures of real lignosulfonate, Agilent DB-1701 capillary column with low/medium polarity (bonded with 14% cyanopropyl-phenyl)-methylpolysiloxane) was used to separate and analyze the unknown depolymerized compounds since this column has higher inertness and very lower column bleed compared to a HP-5 ms nonpolar column bonded with (5%-phenyl)-methylpolysiloxane phase. According to the proposed GC–MS method in experimental section, the GC–MS total ion chromatogram of the DSP sample was processed within a longer retention time (RT) of 60 min in full scan data acquisition mode to ensure detection of each targeted compound. Furthermore, a single ramp temperature program were suggested in this experimental method, and the final oven temperature of 290 °C was set to get better resolution of peaks than 300 °C. Chromatographic separation of the complex depolymerized lignin sample was possible due to the high efficiency of DB-1701 column. More peak signals were detected on the DB-1701 column compared to the HP-5 MS, especially when the retention time were locked between 25 and 40 min, as shown in Fig. 2, suggesting that the improved inertness performance provided by the DB-1701 column resulted in the most sharpest peaks. It could be concluded that DB type column is more ideal for the analysis of lignin depolymerized mixtures with good mass spectral integrity. In contrast, except a phenol solvent signal eluted first and formed a peak from 10 to 12 min, any product signal was not observed in the total ion chromatogram of the control blank sample (see Figure S1 for details). It confirmed that lignosulfonate can be quickly depolymerized at this mild acid-catalytic condition.
Before analyses for a series lignosulfonate depolymerized samples under specific parameter conditions, except for a control blank sample, single standard sample of phenol, 2, 6-dimethoxy- (identified in all samples) was commonly used to evaluate performance of GC–MS for the mentioned method through comparing the retention times, resolution and peak height. Furthermore, 4-methyl-2, 6-dimethoxyphenol standard (not identified in any samples, not listed in this paper) was also used as an internal standard to improve the reliability. The chromatograms for this standards are shown in Figure S2. All identified compounds must maintain a RT error of < 0.1 min. The NIST08 library was used for MS identification with a minimum probability match of 80%. Total 37 key monomeric components were identified and listed in sequence of RT, as shown in Tables 1, 2 and 3. Among them, 22 compounds could be detected by each column, while other 15 of them only be separated and detected with DB-1701 column (see compounds name labeled with an asterisk in tables). It was noticeable that those compounds labeled with asterisk included most of the low/mid polar compounds such as phenolic -aldehydes -ketones, -esters and -acids detected, illustrating that DB-1701 capillary column have high separation efficiency for weakly or medium polar phenolic compounds, thus, DB-1701 column is more suit for detecting the resultant depolymerized products of lignosulfonate.
37 identified compounds can be categorized into mono-phenols (13), bisphenols (7), phenolic –aldehydes (2), phenolic –ketones (4), phenolic–acids (5) and phenolic-esters (6) with 42.38%, 51.38%, 0.27%, 1.94%, 2.31% and 1.72% of relative abundance (calculated by peak area), respectively. A pie chart with the percentages of each class of compounds is shown in Fig. 3. A combined yield of up to 93% of the total identified volatile aromatics are lignin-derived monophenols and bisphenols, while less than 7% of that were phenolic -aldehydes, -ketones, -esters and acids. Most of compounds are simple aromatics containing phenolic hydroxyl or lignin-specific phenolic methoxy functional group, suggesting those compounds are generated by directly breaking down the phenylpropanoid building blocks of lignin, therefore, the phenolic structural units remain intact under mild acidolysis conditions. A few other phenolic -aldehydes, -ketones, -acid, -ester and olefinic compounds seem to be produced through hydrolysis or oxidation, which might be used to investigate the reaction mechanisms in future work. These lignin-derived (C7–C16) aromatic compounds have one or two benzene rings and contain phenolic hydroxyl or lignin-specific phenolic methoxy functional groups, are valuable phenolic intermediates for pharmaceuticals and fine chemicals, which can be used directly to prepare well-defined resins (e.g., epoxy) and bio-based nanocomposites [27,28,29]. Furthermore, many of these natural depolymerized compounds detected herein were first reported, especially, more added valuable bisphenols, preferably obtained through this mild acid-catalytic lignosulfonate depolymerization procedure, retaining most of the functionality presented in the natural feedstock [27].
Investigation of effects on lignosulfonate depolymerization products using GC–MS
Based on the relative peak area, identified 9 products with more abundant were selected as representative compounds for quantification evaluation of various reaction parameters and their effect on lignin depolymerization behavior. Preliminary study found that higher temperature or concentrated acidic catalyst possibly lead to partial degradation and even carbonization of the native structure, parameters ranges were thus set under relatively mild conditions, which together with 9 compounds name were listed in Table 4. According to the depolymerization and separation process established in experimental section, lignosulfonate depolymerized samples under different parameter conditions were obtained, and characterized using GC–MS equipped with DB-1701 type column.
Catalyst/lignin mol ratio of 0.583, 1.167, 2.334 and 3.500 were selected to investigate of effects of catalyst content on depolymerization. The results are shown in Fig. 4, when depolymerization lignosulfonate was processed at 110 °C for ~ 30 min, the abundance of the 9 depolymerized products increased as the catalyst/lignin ratio increased, illustrating that catalyst content had certain effect on depolymerization reaction, especially for there was a remarkable increase when catalyst/lignin ratio was around 1.167 and 2.334, while tiny increase presented for ester and olefin compounds when catalyst/lignin ratio w Phenol, 2, 6-dimethoxy-,as higher than 1.167. However, the black residue that exhibited good water solubility occurred when catalyst/lignin ratio was 0.583, suggesting that lignosulfonate fail to fully convert into low molecular components; while insoluble char was observed when catalyst/lignin ratio was up to 3.500. It can assume that lignosulfonate was depolymerized efficiently with catalyst/lignin ratio around 2.0–3.0 without carbonization.
The abundance of most depolymerized products increased as the reaction temperature increased from 70 to 130 °C for ~ 30 min when catalyst/lignin ratio was 2.334, as shown in Fig. 5. It was noticeable that there was a sharp increase around 130 °C for some monophenols (NO. 1–3) such as guaiacol, 1,2-benzenediol, 3-methoxy- and phenol, 2, 6-dimethoxy-. While others remained tiny variations from 70 to 110 °C, and bisphenols (NO. 5–8) even decreased when temperature was up to 130 °C, indicating that those compounds could be completely formed at lower temperatures of depolymerization, and easily transferred into new compounds through functionalization reaction at higher temperature possibly, possibly due to the active nature.
Variations of peak area for the nine depolymerized products under different reaction times were shown in Fig. 6. As the reaction time increased from 0.5 h to 1.5 h, there were gradually increase for most products (NO. 1–3, 5–8), it was found that there was a sharp increase for Phenol, 2, 6-dimethoxy- when the reaction time was up to 2 h,, While other 3 ester and olefin compounds (NO. 4, 8 and 9) remain low abundance all the time, and olefin compounds even decreased when reaction time was up to 2 h (NO. 4, 8), further confirmed that some compounds completely formed at the beginning time of depolymerization and at lower temperature as well, and converted into new compounds through further reaction at higher temperature for longer time.
Based on above investigation, it can be concluded that acid-catalytic reaction temperature, time and catalyst/lignin ratio all have significant effect on depolymerization efficiency. The mild acid-catalyzed reaction of lignosulfonate afforded phenolic depolymerization products quickly and directly. All nine representative compounds could be detected under different parameter conditions, most of them increased as reaction time, temperature and catalyst/lignin ratio increased, suggesting that lignosulfonate could be selectively depolymerized into specific products. while a few bisphenols or ester compounds could be achieved completely at the very beginning of depolymerization reaction under very mild conditions and decreased at higher temperature for more than 2 h, possibly due to functionalization or recondensation reaction [30]. Obviously, this acid-catalyzed reaction appears to involve a combination of depolymerization under mild conditions and partial functionalization reaction under severity conditions. A large amount of aromatic compounds were eventually produced under optimized mild depolymerization, e.g., acid-catalyzed reaction at 110–120 °C for 1.5–2 h with catalyst/lignin ratio of 2.5–3.0, no insoluble char was observed during the reaction, and phenol, 2, 6-dimethoxy- was most abundant. Since we did not get most standards of the identified products, and considering 93 wt% of the total identified volatile aromatics are lignin-derived monophenols and bisphenols, phenol, 2, 6-dimethoxy- was used to create a standard curve (see figure S3 for details) for quantification evaluation of these approximate products. Based on the relative peak area, 37 identified components was quantified with the total concentration of ~ 20 mg/mL in DSP solution ((a combined yield of up to 25 wt% of the initial lignosulfonate calculated by the mass balance), which was obtained under optimized reaction conditions of 130 °C for 60 min with catalyst/lignin ratio of 2.334.
Conclusion
An efficient method for the analysis of depolymerized lignosulfonate products and depolymerization efficiency has been developed. The total 37 aromatic compounds of the depolymerized lignin solution were well-separated and directly characterized with a GC–MS system equipped with a DB-1701 capillary column. The acid-catalyzed reaction under optimized conditions afforded key volatile components including mono-phenols, bisphenols, phenolic –aldehydes, phenolic –ketones, phenolic–acids and phenolic-esters with the yield of up to 25 wt% of the initial lignosulfonate. This work further confirmed that lignosulfonate was almost completely converted into low MW compounds under optimized acid-catalytic conditions, a new path toward preparing value-added industrially relevant aromatics from low-cost, renewable lignosulfonate is revealed. Aromatic monomers obtained in this work could be a potential material for new applications such as bio-based polymers or polymer building blocks. To fully elucidate the mechanism and provide more data for fine-tuning the conditions for producing higher value products; currently we are working on developing method for isolating and identifying the nonvolatile oligomers from depolymerization solution..
References
Li MF, Sun SN, Xu F, Sun RC (2012) Organosolv fractionation of lignocelluloses for fuels, chemicals and materials: A biorefinery processing perspective. In: Baskar C, Baskar S, Dhillon RS (eds) Biomass conversion The interface of biotechnology, chemistry and materials science. Springer, Berlin, p 341
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem 12:539–554
Ennaert T, Van AJ, Dijkmans J, De CR, Schutyser W, Dusselier M, Verboekend D, Sels BF (2016) Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem Soc Rev 45:584–611
Ruppert AM, Weinberg K, Palkovits R (2012) Hydrogenolysis goes bio: from carbohydrates and sugar alcohols to platform chemicals. Angew Chem Int Ed 51:2564–2601
Smolarski N (2012) High-value opportunities for lignin: Unlocking its potential. Frost & Sullivan, pp 1–15
Da Costa Sousa L, Chundawat SP, Balan V, Dale BE (2009) Cradle-to-grave assessment of existing lignocellulose pretreatment technologies. Curr Opin Biotechnol 20:339–347
Pandey MP, Kim CS (2011) Lignin depolymerization and conversion: a review of thermochemical methods. Chem Eng Technol 34:29–41
Zakzeski J, Bruijnincx PC, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599
Wang H, Tucker M, Ji Y (2013) (2013) Recent development in chemical depolymerization of lignin: a review. J Appl Chem 2013:1–9
Ennaert, T., Beeck, B. O. D., Vanneste, J. , Smit, A. T. & Sels, B. F. (2016). The importance of pretreatment and feedstock purity in the reductive splitting of (ligno)cellulose by metal supported usy zeolite. Green Chem 18: 2095–2105.-
Yu F, Thomas J, Smet M, Dehaen W, Sels BF (2016) Molecular design of sulfonated hyperbranched poly(arylene oxindole)s for efficient cellulose conversion to levulinic acid. Green Chem 18:1694–1705
Galkin MV, Samec JSM (2016) Lignin valorization through catalytic lignocellulose fractionation: a fundamental platform for the future biorefinery. Chemsuschem 9:1544–1558
Rinaldi R, Jastrzebski R, Clough MT, Kennema RJ, Bruijnincx M, Weckhuysen PCA (2016) Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chem Int Ed 55:8164–8215
Arel HS (2017) The effect of lignosulfonates on concretes produced with cements of variable fineness and calcium aluminate content. Constr Build Mater 131:347–360
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624
Bjørsvik HR, Minisci F (1999) Fine chemicals from lignosulfonates. 1. Synthesis of vanillin by oxidation of lignosulfonates. Org Process Res Dev 3:330–340
Bjørsvik HR, Norman K (1999) Fine chemicals from lignosulfonates. 2. Synthesis of veratric acid from acetovanillon. Org Process Res Dev 3:341–346
Pacek AW, Ding P, Garrett M, Sheldrake G, Nienow AW (2013) Catalytic conversion of sodium lignosulfonate to vanillin: engineering aspects. part 1. Effects of processing conditions on vanillin yield and selectivity. Ind Eng Chem Res 52:8361–8372
Han H, Wang Y, Li J, Xue F, Wang H, Zhang Y, Ge Q, Liu Y, Zhang M, Chen Y (2019) Production of oxygen-containing compounds catalytic from depolymerization of calcium lignosulphonate by submicron-scale MgAl solid base. Chem J Chin Univ 40:2322–2331
Khudoshin AG, Lunin VV, Bogdan VI (2011) Conversion of veratrole and sodium lignosulfonate in the suband supercritical water. Russ J Phys Chem B 5:1069–1075
Santos SG, Marques AP, Lima DLD, Evtuguin DV, Esteves VI (2011) Kinetics of eucalypt lignosulfonate oxidation to aromatic aldehydes by oxygen in alkaline medium. Ind Eng Chem Res 50:291–298
Fang H, Cui P, Qian C, Liu J, Liu T, Li D, Hu X (2018) Products separation and analysis of depolymerized lignosulfonate under mild acid-catalyzed conditions. Nat Prod Res Dev 30:176–1781
Qian Ch, Fang HX, Cui P, Cai F, Gao XY, He HL, Hu XP (2019) Rapid determination of lignosulfonate depolymerization products by advanced polymer chromatography. J Sep Sci 42:2289–2297
Cui P, Fang HX, Qian Ch, Chen MH (2020) Detection and Identification of Lignosulfonate Depolymerization Products Using UPLC-QTOF-MS and a Self-Built Database. Chromatographia 83:87–93
Wang YY, Ling LL, Jiang H (2016) Selective hydrogenation of lignin to chemical commodities by a biochar supported Ni-Mo2C catalyst obtained from biomass. Green Chem 18:4032–4041
Shu RY, Xu Y, Ma LL, Zhang Q, Wang TJ, Chen PR, Wu QY (2016) Hydrogenolysis process for lignosulfonate depolymerization using synergistic catalysts of noble metal and metal chloride. RSC Adv 6:88788–88796
Sun ZH, Fridrich B, Santi A, Elangovan S, Barta K (2018) Bright side of lignin depolymerization: toward new platform chemicals. Chem Rev 118:614–678
Zhao S, Abu-Omar MM (2015) Biobased epoxy nanocomposites derived from lignin-based monomers. Biomacromol 16:2025–2031
Zhou J, Zhang H, Deng J, Wu Y (2016) High glass-transition temperature acrylate polymers derived from biomasses, syringaldehyde, and vanillin. Macromol Chem Phys 217:2402–2408
Deuss PJ, Scott M, Tran F, Westwood NJ, de Vries JG, Barta K (2015) Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J Am Chem Soc 137:7456–7467
Funding
This study was funded by Abroad Visiting & Research Program for Young Talents (No. gxfxZD2016236); Anhui Natural Science Foundation: KJHS2019B14; KJ2019A0613. Underground innovation and entrepreneurship training program of China (No. 201810375038).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hong-Xia, F., Peng, C. & Chen, Q. Behavior characterization of lignosulfonate depolymerization products under acid-catalyzed conditions using gas chromatography–mass spectrometry. Chromatographia 84, 109–116 (2021). https://doi.org/10.1007/s10337-020-03988-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03988-8