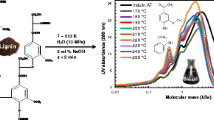
Abstract
The depolymerization of lignin into aromatic monomers is a pivotal strategy for achieving high added value. Base-catalyzed depolymerization (BCD) of lignin has proven effective and offers the potential advantage of cost-effectiveness. Additionally, the high alkalinity of black liquor containing lignin from the pulp and paper industry allows for seamless integration in the utilization of NaOH base catalysts into these sectors. To gain insights into the application of BCD, miscanthus organosolv lignin is subjected to the process at 0–7% NaOH loading and temperatures of 280–320 °C for 5–90 min. The resulting bio-oil is characterized using various techniques, including elemental analysis (EA), matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), Fourier-transform infrared (FTIR), 31P nuclear magnetic resonance (NMR), and gas chromatography-mass spectrometry (GC-MS). This study focuses on bio-oil production and compound formation during the BCD reaction, specifically focusing on selectively obtaining C6 monoaromatic phenolic compounds, such as catechol and phenol. Catechol exhibits its highest concentration at 68.6 mg/g under NaOH 3% at 300 °C for 30 min. As the NaOH content increases, phenol becomes predominant under NaOH 5%, reaching 49.6 mg/g. NaOH catalysts demonstrate their ability to produce valuable phenol monomers from lignin, enabling increased utilization of lignin at a low cost.
Graphical Abstract

Highlights
The depolymerization of lignin was achieved using base catalysis.
Base catalysts aid in converting lignin into monoaromatic compounds.
Bio-oils mainly comprise guaiacol, catechol, and phenolic compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignin, the most abundant natural resource, represents a sustainable polymer with potential applications as an alternative feedstock for liquid fuels and petroleum-based aromatic chemicals [1,2,3]. The increasing interest in lignin utilization has led to extensive research into its valorization, encompassing biofuels, carbon fibers, polymers, and other aromatic compounds [2, 4].
Lignin is a complex amorphous polymer composed of three monolignols: p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) [5, 6]. The structure of lignin varies based on the biomass type: hardwood, softwood, or herbaceous. Hardwood lignins consist of syringyl and guaiacyl units, while softwood lignins primarily comprise guaiacyl units. Herbaceous lignin contains all three types of units [6, 7]. These monolignols form ether (C-O) bonds (β-O-4, α-O-4, 4-O-5) and C-C bonds (β-β, β-5, β-1, 5–5) with β-O-4 ether bonds being predominant [8, 9].
Herbaceous biomass exists as agricultural residues and energy crops, and the miscanthus used in this study is a promising crop developed as a raw material for Korean-style bioenergy. It was developed at the Bioenergy Crop Research Institute of the National Institute of Crop Science, Rural Development Administration, which is a high yielding crop biomass that grows rapidly, low-maintenance, and easy-to-grow [10, 11]. Goedae Uksae 1 is a type of Miscanthus sp., that has been reported to grow to a height of 4 m and a stem thickness of 10 mm and produce a high biomass yield of 30 tons/ha. Additionally, it contains more than 20% lignin and has a higher lignin content compared to another herbaceous biomass [12]. High-value-added products from lignin are usually produced from wood-based biomass, with only a few studies employing herbaceous biomass. In addition, there has been no research on the depolymerization of lignin in Geodae-Uksae 1.
Miscanthus lignin typically contains a high proportion of G and S units, resulting in relatively high carbon content and durability. These characteristics are advantageous when using Miscanthus as a biomass energy source. The chemical composition of Miscanthus lignin can vary depending on the species, growth environment, and harvest time [13, 14].
To achieve high yields in lignin conversion, cleavage of the ether bond is necessary while preserving the aromatic ring, avoiding lignin re-condensation [5, 15]. Various depolymerization technologies, including combustion, gasification, pyrolysis, and hydrothermal liquefaction (HTL) [3, 16], can be employed. HTL, an environmentally friendly technology utilizing water as a solvent, eliminates the need for biomass drying and simplifies the process, thereby reducing costs [6, 17]. However, increased temperature and time may lead to simultaneous lignin depolymerization and repolymerization, resulting in coke formation [16]. A base catalyst was used as an alternative to inhibit lignin repolymerization and coke formation. Base-catalyzed depolymerization (BCD) of lignin cleaves the ether bonds and functional groups of the aromatic ring [8]. Additionally, because of the alkalinity of the black liquor (lignin-containing waste liquor) generated in the chemical pulp industry, base catalysts are easily accessible for lignin depolymerization and can ultimately be integrated into the pulp industry [18]. Additionally, lignin decomposition typically necessitates expensive metal catalysts and hydrogen. Metal catalysts, including palladium, platinum, ruthenium, and nickel, are used in hydrogenolysis reactions and require high-pressure hydrogen. These systems are highly efficient, but the high cost of metal catalysts and difficulties in their reuse are significant drawbacks [8, 19]. In addition to metal catalysts, there are acid and organic catalyst systems. Acid-catalyzed reactions can effectively cleave the ether bonds in lignin. Strong acids such as sulfuric acid, phosphoric acid, and hydrochloric acid are used to promote lignin depolymerization, usually under low pH conditions. However, the corrosiveness of acid catalysts can pose durability issues for the reaction equipment [20]. Recently, research on lignin depolymerization using organic catalysts has been actively conducted. Organic catalysts can selectively cleave bonds by targeting specific functional groups and are relatively cheaper and more environmentally friendly than metal catalysts [21, 22]. Considering these factors, this study presents an effective depolymerization method using inexpensive and recyclable NaOH, which does not require metal catalysts or external hydrogen gas, while minimizing corrosiveness.
The valorization of lignin can be achieved through the production of C6 monoaromatic phenolic compounds, such as phenol and catechol, via lignin depolymerization. Catechol, existing in various monomer forms, including pyrocatechol, 3-methylcatechol, 4-methylcatechol, propylcatechol, and 4-ethylcatechol [12], emerges as a promising candidate for utilization as a platform chemical in the synthesis of precursors for polymers and fine chemicals [16]. Phenol, a primary component of lignin depolymerization, comprises phenol, 4-methoxy phenol, and 2-ethyl phenol [12].
In this study, Miscanthus lignin obtained from organosolv pulping was employed to produce monoaromatic phenolic compounds in water, utilizing sodium hydroxide (NaOH) as a catalyst. The chemical degradation mechanisms were systematically investigated, and the conversion of specific phenolic components or aromatic rings was evaluated. Furthermore, emphasis was placed on enhancing the selectivity for catechols and phenols. A diverse array of analytical tools, including elemental analysis (EA), Fourier transform infrared (FT-IR) spectroscopy, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), phosphorous nuclear magnetic resonance (31P NMR) spectroscopy, and gas chromatography–mass spectrometry (GC–MS), was applied for comprehensive characterization and quantification.
Materials and Methods
Materials
The lignin utilized in this study was acquired following the organosolv extraction method for Miscanthus, as previously detailed in relevant literature [12]. Miscanthus sacchariflorus strain Geodae-Uksae 1 was obtained from the Bioenergy Crop Research Center in Muan-gun, Jeollanam-do, Korea. NaOH (97%) and ethyl acetate (99.8%) were procured from Sigma-Aldrich. Ethyl ether (98%) was obtained from TCI Chemicals. Aromatic monomers (phenol, p-cresol, 2-ethyl phenol, 2,4-xylenol, 4-isopropylphenol, 4-hydroxy benzaldehyde, 4′-hydroxyacetophenone, guaiacol, 4-ethyl phenol, 4-ethyl guaiacol, vanillin, apocynin, syringol, and acetosyringone) and acetic acid used for gas chromatography (GC) calibration were purchased from Sigma-Aldrich, Alfa Aesar, and TCI Chemicals. All chemicals were utilized as received.
Base-Catalyzed Lignin Depolymerization
The reactor system comprised a high-pressure vessel in a batch type stainless steel autoclave reactor (KHT Engineering Co., Ltd, Korea) with a 200-mL inner volume, capable of operating within a range of up to 130 bar and 350 °C, and equipped with a magnetically coupled mechanical drive (pitched blade type impeller) and a reactor control system. Upon loading the reactor with a NaOH solution (120 mL) and lignin (6.0 g), the base-catalyzed depolymerization (BCD) process was initiated. The reaction was conducted for durations ranging from 5 to 90 min at five distinct catalyst concentrations (0, 1, 3, 5, and 7 wt %) and varied reaction temperatures (280, 290, 300, 310, and 320 °C). A mixing speed of 150 rpm was maintained in all the experiments. Subsequent to the completion of the reaction, the reactor was cooled, and the resulting reaction products were recovered.
Separation and Recovery of each Fraction
HCl (Sigma-Aldrich) was introduced into the reaction solution to adjust the pH to 1.5. Acidification was performed to eliminate free radicals within the resultant product. Subsequently, ethyl acetate (Sigma-Aldrich) was added to retrieve the organic fraction from the adequately acidified reactant. The solid phase (coke) was then isolated from the mixture through filtration using a 5-µm filter paper. Following the removal of ethyl acetate from the recovered organic fraction, diethyl ether (Sigma-Aldrich) was employed for the recovery of low-molecular-weight compounds.
The organic fraction, augmented with diethyl ether, underwent centrifugation to segregate into solid and liquid fractions. Diethyl ether was then eliminated from the liquid fraction through distillation under reduced pressure, resulting in the production of bio-oil. The residual solid fraction was dissolved in tetrahydrofuran (THF) (Sigma-Aldrich) and subjected to evaporation to recover residual lignin for subsequent analysis. A schematic illustrating the stages of product creation, separation, and analysis is presented in Fig. 1.
The yields of the BCD products were determined from the dry weight of lignin and defined as weight percentages using the following equation (Eq. 1):
where mBio-oil, mResidual lignin, and mCoke are the masses of the bio-oil, residual lignin, and coke recovered from the extraction process, respectively.
Analytical Methods
EA of the depolymerization products involved carbon, hydrogen, and nitrogen and was conducted using the classical total oxidation method with a Thermo Quest system model EA 1112. Oxygen concentration was determined based on the percentage difference. MALDI-TOF MS was employed to verify the molecular weight distribution of the bio-oil and assess the degree of BCD. A 10 g/L solution of 2,5-dihydroxy benzoic acid (DHB) (Sigma-Aldrich) in a methanol/water mixture (volume ratio of 8:2) served as the matrix. Analyses were performed in positive mode. FT-IR spectroscopy measurements of the bio-oil were conducted at room temperature using an FT-IR instrument (Tensor 27, Bruker). The FT-IR spectra, comprising 32 scans, were collected for wavenumbers ranging from 4000 to 400 cm− 1. The background spectrum of the solvent was acquired at the beginning of each measurement. For 31P NMR measurements, a standard phosphorylation procedure was applied to analyze the bio-oil and residual lignin samples. A dried solvent mixture of pyridine (Sigma-Aldrich) and N,N-dimethylformamide (DMF) (Sigma-Aldrich) (1:1, v/v) was used to dissolve the dried lignin (30 mg) at room temperature overnight under continuous stirring. Stock solutions of the internal standard (N-hydroxy-5-norbornene-2,3-dicarboxylic acid imide, 20 mg/mL, Sigma-Aldrich) and the relaxation reagent (chromium(III) acetylacetonate, 5 mg/mL, Sigma-Aldrich) were separately prepared using pyridine for dissolution. Aliquots (100 µL) of these solutions were added to the lignin mixture. Before analysis, 100 µL of the derivatization reagent (deuterated chloroform (Sigma-Aldrich)/2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (Sigma-Aldrich); 4:1, v/v) was added, and the mixture was transferred into a 5-mm OD NMR tube. 31P NMR spectra were obtained on an Avance 500 MHz NMR spectrometer using a standard phosphorus pulse program with a delay time of 10 s and 512 scans. The bio-oil obtained from lignin depolymerization was dissolved in acetonitrile (AN), and the sample was analyzed by GC (7890 A)-MS (5975 C inert MSD with Triple-Axis Detector, Agilent) using a capillary RTi-5 MS column (30 m × 250 μm; 0.25 μm film thickness, Restek, USA). Helium served as the carrier gas at a flow rate of 1 mL/min. The samples (1 µL) were injected at a split ratio of 30:1. The column oven was initially held at 45 °C for 10 min, then heated to 250 °C at a rate of 3 °C/min, and finally held at 250 °C for 50 min. The injector, interface, and ion-source temperatures were 250, 280, and 230 °C, respectively. The mass spectra of the products were acquired in the m/z range 29–600 Da. Standards were employed to obtain quantification of the compounds in bio-oil and based on the analysis procedure developed by the National Renewable Energy Laboratory (NREL) [23]. The peak areas of the compounds were calculated in relation to the peak area of the internal standard, and the concentrations of the compounds were expressed in mg/g. Calibration was performed using three internal standards: isoamyl ether, 1-octanol, and methyl laurate (Sigma-Aldrich). The main standards employed were: phenol, guaiacol, p-cresol, m-cresol, 4-Methyl guaiacol, 2-ethyl phenol, 2,4-xylenol, 4-ethyl phenol, 4-Ethyl guaiacol, 4-Isopropylphenol, catechol, syringol, 4-methlyl catechol, 4-methlyl syringol, vanillin, 4-ethlyl catechol, apocynin, 4-Hydroxy benzaldehyde, 4-Hydroxy acetophenone, syringaldehyde, acetosyringone (Sigma-Aldrich).
Results and Discussion
Effects of Time, Temperature, and Concentration of NaOH on Lignin Depolymerization
The depolymerization of organosolv lignin was observed to vary based on temperature, time, and NaOH solvent concentration. Under all reaction conditions, lignin degradation products were categorized into three groups: bio-oil, residual lignin, and coke. Gases were generated during the reaction and released into the atmosphere through the relief valve of the reactor without recovery or analysis.
Table 1 summarizes the material inputs and outputs for the hydrothermal liquefaction of lignin and the subsequent acidification and solvent separation processes. Lignin was treated with NaOH solutions of different concentrations (0–7%) and subjected to varying temperatures (280 °C to 320 °C). The table details the amounts of lignin, NaOH solution, HCl, and other materials used, as well as the quantities of coke, organic phase, bio-oil, and residual lignin produced under each condition. This comprehensive material balance highlights the effect of NaOH concentration and temperature on the efficiency and yield of the hydrothermal liquefaction process.
Figure 2(a–c) illustrates the product yields obtained from lignin depolymerization, considering reaction times ranging from 5 to 90 min, NaOH concentrations from 0 to 7%, and temperatures from 280 to 320 °C.
A study investigating the impact of reaction time on lignin depolymerization was conducted at 300 °C with 5% NaOH as the reaction solvent for durations of 5, 15, 30, 60, and 90 min. As depicted in Fig. 2(a), bio-oil production increased with time, reaching its maximum yield (43.19 wt%) at 30 min, and then slightly decreased at 60 and 90 min thereafter. The bio-oil yields were 34.62, 37.44, 42.31, and 42.74 wt% at reaction times of 5, 15, 60, and 90 min, respectively. This increase in bio-oil yield was attributed to the further degradation of lignin and reaction intermediates, either through hydrolysis or the fragmentation of C-C bonds via radicals [9, 18, 24]. Regarding coke, there was a slight increase with prolonged residence time, rising by 2.5% from 5 to 90 min. Our findings align with previously reported results [3, 25].
Figure 2 (b) illustrates the light oil yield based on NaOH concentration. The experiment was conducted at 300 °C for 30 min, with NaOH concentrations of 0%, 1%, 3%, 5%, and 7%. The trial conducted solely with water, designated as NaOH 0%, resulted in a bio-oil yield of 16.31 wt%, representing the lowest achieved yield. The maximum yield (43.19 wt%) was observed with 5% NaOH. As depicted in Fig. 2 (b), bio-oil yield demonstrated an increasing trend with the addition of NaOH. The presence of this base positively influenced bio-oil yield, indicating that a higher hydroxyl ion concentration facilitates lignin degradation. However, concerning coke formation, the highest coke yield (30.86 wt%) was observed in the experiment conducted with 0 wt% NaOH. As the NaOH concentration increased, there was a significant gradual decrease in coke yield. Coke yields were 19.68 wt% with 1% NaOH and 13 wt% with 5% NaOH. These findings suggest that the introduction of a base into the reaction mixture impedes coke formation.
The reaction temperature plays a crucial role in lignin depolymerization, and its optimization was investigated through varying temperatures (280, 290, 300, 310, and 320 °C) during a 30-min reaction in 5% NaOH. Figure 2 (c) illustrates the impact of temperature on product yield, revealing that the maximum bio-oil yield (43.19 wt%) occurred at 300 °C. Bio-oil yields at 280, 290, 310, and 320 °C were 34.61, 36.06, 38.13, and 35.36 wt%, respectively. The results indicate an increase in bio-oil yield from 280 to 300 °C, followed by a decrease as the temperature exceeded 300 °C. Generally, elevated temperatures are associated with a synergistic effect on lignin decomposition, leading to increased bio-oil yields. The observed wide range of lignin depolymerizations at high temperatures suggests overcoming the activation energy required to break bonds. Initially, bio-oil yields increased with temperature until reaching a maximum, beyond which further temperature increases inhibited yields. Two primary reasons account for this behavior, distinct from prior studies. Firstly, secondary decomposition and gas reactions occurred at high temperatures, resulting in gaseous product formation [26]. Exceeding the critical temperature led to oil decomposition into gases. Secondly, free radical reactions’ repolymerization contributed to residual lignin formation [9]. Despite reaching 320 °C, there was no significant increase in residual lignin. At 300 °C, residual lignin decreased from 28.80 wt% (280 °C) to 16.82 wt%, with negligible changes thereafter. This suggests that the low bio-oil yield was not due to repolymerization but rather the decomposition of oil into gaseous products.
Characterization of Products
Elemental Analysis
The elemental compositions of the bio-oil and residual lignin resulting from the depolymerization process were compared to those of the raw organosolv lignin, as presented in Table 2. The organosolv lignin exhibits an elemental composition of 67.1% carbon, 5.6% hydrogen, 26.0% oxygen, and trace amounts of 0.3% nitrogen on a dry and ash-free basis. Minor variations in elemental compositions were noted for the bio-oil and residual lignin samples, contingent upon the reaction conditions. The elemental compositions of the bio-oil and residual lignin displayed an increased carbon content but decreased oxygen content compared to the organosolv lignin feed. This indicates a notable enhancement in terms of the presence of elements conducive to fuel production. The reduction in oxygen content suggests the formation of volatiles through decarbonylation (loss of CO), decarboxylation (loss of CO2), or dehydration (loss of H2O) [18, 24]. The hydrogen content in the bio-oil exhibited an increase with the rising concentration of NaOH, reaching a maximum of 6.9% at 7% NaOH, while a lower hydrogen content of 6.3% was observed in the absence of NaOH. The NaOH-induced reaction facilitated the hydrogenation of the bio-oil, with a more pronounced effect observed as the temperature increased from 280 to 320℃. At 280℃, the bio-oil comprised 69.7% carbon and 23.7% oxygen, evolving to 73.0% carbon and 20.1% oxygen at 320 ℃. The observed increase in carbon content is attributed to the hydrolysis of lignin macromolecules, considered the primary reaction responsible for this phenomenon [18].
The O/C and H/C atomic molar ratios of organosolv lignin, bio-oil, and residual lignin were determined through elemental analysis. The starting material, raw organosolv lignin, exhibited O/C and H/C molar ratios of 0.29 and 1.00, respectively. Hydrodeoxygenation was evident in all bio-oils resulting from lignin depolymerization, leading to reduced O/C ratios and increased H/C ratios compared to lignin. The H/C ratios ranged from 1.08 to 1.14, suggesting a potential prevalence of aromatic rings [3]. Notably, an observable trend of decreasing O/C ratios with rising temperature was identified. At 320 °C, a more significant deoxygenation extent (O/C ratio of 0.21) was observed compared to 280 °C (O/C ratio of 0.26). In contrast, the O/C and H/C ratios of residual lignin decreased with increasing base catalyst content and temperature. This trend may be attributed to continuous repolymerization, condensation of lignin, and intramolecular dehydration reactions during BCD [16].
MALDI-TOF MS Analysis
The molecular weights of oligomers in the bio-oils post depolymerization were determined via MALDI-TOF MS (Figs. 3 and 4). Fragments identified by MALDI-TOF MS were attributed to the same molecule at m/z around the main peak [27, 28]. These molecules span various molecular weights (200–800 m/z) and are categorized as monomers, dimers, trimers, or tetramers. Molecules with molecular weights below 253 m/z are monomers, while those within 253 and 378 m/z are dimers associated with two aryl groups and additional side chains, such as hydroxyl, methoxyl, and alkyl groups [25, 26, 29]. Molecules within 378 and 510 m/z are trimers with three aryl groups. Oligomers exceeding 511 m/z are tetramers with three or more aryl groups [25, 26, 29]. Figure 3 illustrates the molecular weight distribution with increasing NaOH concentrations. With the rise in NaOH concentration, signals within the 200–378 m/z range increased noticeably, particularly at 5% NaOH. This suggests that NaOH promotes the degradation of oligomers into aromatic monomers. However, at 7% NaOH, the signal intensity from 200 to 378 m/z decreased, while the signal intensity from the m/z range of 379–510 increased significantly, likely due to conversion to repolymerized oligomers through repolymerization in NaOH 7%. The influence of reaction temperature on the mass distribution of oligomers is depicted in Fig. 4. With the increase in reaction temperature from 280 to 300 °C, signal intensities assigned to monomers and dimers (200–378 m/z) notably enhanced. These findings indicate that bio-oil was depolymerized into smaller molecules at higher temperatures. Subsequently, the intensity and density of peaks in the mass spectra obtained from 300 to 320 °C are nearly identical. When lignin was depolymerized in NaOH 5% at 300 °C for 30 min, it indicated more efficient depolymerization compared to other reaction conditions.
FT-IR
The FT-IR analysis revealed various functional groups in the bio-oil. Figure 5(a) illustrates the FT-IR spectra of bio-oils obtained using NaOH-based catalysts at different concentrations. A broad peak at 3435 cm− 1 indicates the presence of OH functional groups in the aromatic and aliphatic groups of the bio-oil. The bands at 2938 and 2841 cm− 1 correspond to the asymmetric and symmetric vibration bands of C-H, respectively. The intensity of the asymmetric stretching band at 2938 cm− 1 increased with higher NaOH concentrations. The band at 1710 cm− 1 originates from the C = O stretching vibration of aliphatic ketones and carboxylic acid derivatives in bio-oil, with higher intensity observed in bio-oil with increased NaOH concentration. The two peaks at 1580 and 1516 cm− 1 represent the C = C stretching of the syringyl and guaiacyl aromatic rings, respectively, indicating the relatively well-preserved benzene ring structure of lignin in the depolymerized bio-oil, mainly present in phenol and its derivatives [29]. The peaks at 1452 cm− 1 are attributed to the bending stretching of methyl (–CH3) groups, while phenolic OH groups are denoted by absorption bands at 1365 cm− 1. The bands between 1000 and 1300 cm− 1 represent C-O functional groups, suggesting the presence of phenol and aromatic ester or alcohol functional groups in the bio-oil. This implies that the primary chemical component of the lignin depolymerization products is phenol [29]. The presence of aromatic C–H bending is indicated by signals located at 834 and 750 cm-1. Figure 5(b) displays the FT-IR spectrum of the bio-oil as a function of temperature. It was observed that most spectra, according to temperature, exhibited similar functional groups as the bio-oils according to NaOH concentration. The spectra displayed vibration bands at 3335, 2938, 2841, 1710, 1580, 1516, 1452, 1300–1000, 834, and 750 cm− 1 stretching frequency.
31P NMR Analysis
By examining the 31P NMR spectrum, distinct hydroxyl groups within the bio-oil and residual lignin obtained from lignin depolymerization were identified (Table 3). The samples were acquired following in situ derivatization with 2-chloro-4,5,5-tetramethyl-1,3,2-dioxaphosphorane (TMDP). Quantitative analysis of the lignin functional groups was performed using 31P NMR to ascertain the respective concentrations of aliphatic (R-OH), phenolic (Ph-OH), and carboxylic (R-COOH) units. Specifically, in the Ph-OH category, syringyl-OH and guaiacyl-OH resonate within the range of 143.3–142.0 ppm and 140.4–138.7 ppm, respectively, while the p-hydroxylic-OH range spans from 138.7 to 136.5 ppm. C5-substituted condensed ph-OH is evident in the range of 145.0–140.4 ppm. The integrated region for aliphatic OH groups is 151.0–145.0 ppm. Furthermore, the carboxyl-OH group is determined based on the range of 136.5–133.3 ppm, with the internal standard exhibiting a range of 153.0–151.0 ppm.
According to the 31P NMR results, organosolv lignin predominantly comprised guaiacyl (G) units with a minor presence of syringyl (S)- and p-hydroxyphenyl (H)-type phenolic structures. A notable alteration was observed, leading to a reduction in the signal of aliphatic hydroxyl groups during the depolymerization of lignin. This decline in aliphatic-OH groups signifies oxidative cleavage of aliphatic side chains from phenolic units, attributed to the dehydration reaction. This process results in the generation of new phenolic hydroxyl groups and carboxylic acids, corresponding to small aldehydes such as formic acid and acetic acid, aligning with earlier studies [27, 30]. The newly formed carboxyl and phenol hydroxyl groups encompassed P-hydroxylic-OH, guaiacyl-OH, syringyl-OH, and carboxyl-OH groups, surpassing the levels found in organosolv lignin. Furthermore, the reduced concentrations of C5 substituted/condensed phenolic hydroxyl groups may be ascribed to an increase in guaiacyl, p-hydroxyphenyl, and syringyl hydroxyl group concentrations. Notably, NaOH facilitated the hydrogenolysis of ether bonds, yielding phenolic OH groups and their methyl or phenyl counterparts. The carboxylic acid OH peak intensity significantly increased under all conditions with the presence of NaOH, indicating the formation of new carboxyl groups through oxidative cleavage, repolymerization, or other chemical transformations. In the assessed conditions, the number of phenolic OH groups increased with NaOH concentration up to 5%, suggesting ether cleavage yielding smaller molecular weight compounds. However, with a further increase in NaOH concentration to 7%, the number of phenolic OH groups decreased, while concentrations of aliphatic OH groups and C5 substituted/condensed phenolic hydroxyl groups increased. The 31P NMR results align with MALDI-TOF MS analysis, revealing the regeneration of large molecule oligomers in NaOH 7%, indicative of repolymerization. Under conditions of 300℃ and NaOH 5%, phenolic OH increased by 57% (from 3.56 to 7.58 mmol/g), signifying enhanced ether linkage cleavage.
GC-MS Analysis
GC-MS was employed to analyze bio-oil samples based on NaOH concentration and temperature, as presented in Table 4. Table 4 provides a detailed account of the quantities of 21 monomeric compounds detected in the bio-oil through GC-MS analysis. The concentration was confirmed by the content (mg) of monomer compounds per 1 g of bio-oil. As previously noted, lignin comprises three primary monomer units. The predominant products observed in the lignin bio-oil were guaiacyl, syringyl, and p-hydroxyphenyl derivatives, with significant variations in the guaiacyl derivatives. Results indicated that the components with the highest yields under all conditions were catechol, phenol, guaiacol, and 4-ethyl phenol.
Figure 6 was confirming the distribution of initial lignin change to aromatic compounds, and categorizes the compounds into four groups: syringyl (S)-, guaiacyl (G)-, p-hydroxyphenyl (H)-type phenols, and other types of compounds. Compounds possessing two methoxy and hydroxyl groups, such as syringol, acetosyringone, syringaldehyde, and 4-methylsyringol, are classified as S-type phenolics. G-type phenolics include guaiacol, 4-ethylguaiacol, creosol, vanillin, and apocynin, characterized by one methoxy and one hydroxyl group. H-type phenolics consist of hydroxyl-only compounds, including phenol, p-Cresol, 2,4-Xylenol, 4-Ethylphenol, 4-Isopropylphenol, 4-Hydroxy benzaldehyde, and 4-Hydroxy acetophenone. All compounds not falling into S, G, or H types are classified as other types.
In Fig. 6(a), the impact of NaOH concentration on phenolic compounds is evident. S-type phenolics measured 5.02 mg/g in the absence of NaOH (0% NaOH) and rapidly decreased with NaOH addition. Similarly, syringol decreased with increasing NaOH concentration: 2.97, 1.66, 1.00, 0.58, and 0.40 mg/g at 0%, 1%, 3%, 5%, and 7% NaOH, respectively. Conversely, the guaiacol compound increased significantly at 1% NaOH, measuring 1.62, 8.72, 3.74, 3.50, and 0.33 mg/g at 0%, 1%, 3%, 5%, and 7% NaOH, respectively. G-type phenolics peaked at 12.09 mg/g with 1% NaOH. Guaiacol production results from the cleavage of the β-O-4 bond in the long amorphous polymer or methoxy group hydrolysis of syringol [16], supported by the increase in guaiacol and decrease in syringol at 1% NaOH. At 3% NaOH, guaiacol decreased, while catechol reached the highest level at 27.95 mg/g. These findings align with the hypothesis that catechol forms by cleaving a methyl group from the methoxy group on the aromatic ring of guaiacol [18, 28]. Subsequently, catechol converts into phenol as a secondary degradation product [17, 31]. Confirmation comes from the observed decrease in catechol and increase in phenol at 5% NaOH. With increasing NaOH concentration, guaiacol decomposes to produce catechol, losing one OH group to form phenol. At a higher NaOH concentration of 7%, not only phenol but also abundant monoaromatics decreased, indicating their repolymerization into dimers, trimers, and oligomers. MALDI-TOF MS results confirmed the presence of dimers, trimers, and oligomers in the bio-oil containing 7% NaOH. Moreover, with increasing NaOH concentrations, 4-ethylguaiacol decreased, while 4-ethylcatechol and 4-ethylphenol increased. 4-ethylcatechol results from the hydrolysis of the methoxy group in 4-ethylguaiacol, and 4-ethylphenol suggests the dehydroxylation of 4-ethylcatechol [18, 28]. The amount of base influences the phenolic compounds produced through depolymerization, significantly impacting their properties and abundance. Typically, compound decomposition increases with temperature. Figure 6(b) illustrates the effect of temperature increase from 280 to 320 °C. Monoaromatic compounds gradually increased up to 300 °C and decreased from 310 °C. Catechol and phenol gradually increased with temperature, peaking at 300 °C (24.95 and 21.42 mg/g of lignin, respectively). Concurrently, the decrease in syringol and guaiacol indicated hydrolysis under basic conditions, converting monomers into smaller products, as targeted. Most formed catechols and phenols exhibit greater stability against self-condensation than guaiacol, inhibiting the formation of char and gaseous components [17, 32]. The greater stability of catechol and phenol compared to guaiacol against self-condensation can be explained by their molecular structures and the effects of their substituents. Catechol and phenol exhibit greater stability against self-condensation compared to guaiacol due to the effects of their substituents on electron density and molecular interactions. Catechol and phenol have electron-donating substituents that increase electron density and stability, particularly catechol with its two hydroxyl groups providing additional stability. In contrast, guaiacol’s methoxy group increases reactivity and the likelihood of self-condensation due to its strong electron-donating effect and bulkiness.
Conclusions
The organosolv lignin derived from Miscanthus underwent depolymerization under basic catalytic conditions in an environmentally friendly process utilizing water as a solvent. The results demonstrate that lignin can be preferentially decomposed into monoaromatic phenolic compounds based on the NaOH loading. The maximum yield of bio oil containing monoaromatic compounds was achieved at 5% NaOH, 300 °C, and 30 min, reaching 43.19 wt%. Experimental outcomes validate the demethoxylation of syringol and guaiacol to produce guaiacol and catechol, respectively. Additionally, catechol was observed to undergo dehydroxylation, forming phenol.
Syringol and guaiacol were most abundant at 0% and 1% NaOH, while catechol and phenol emerged as the predominant monoaromatics at 3%, 5%, and 7% NaOH. The employment of NaOH as a base catalyst represents a potentially economical and effective strategy for the selective production of monoaromatic compounds, including catechol and phenol, through lignin depolymerization.
Data Availability
The datasets generated during and/or analyzed during the current study are available on request from the authors.
References
Kong, X., Liu, C., Fan, Y., Li, M., Xiao, R.: Depolymerization of technical lignin to valuable platform aromatics in lower alcohol without added catalyst and external hydrogen. Fuel Process. Technol. 242, 107637 (2023). https://doi.org/10.1016/j.fuproc.2022.107637
Xue, J., Wang, D., Li, X., Li, G., Wang, Z., Liu, Y., Li, X.: A tandem strategy of mild preoxidation-hydrogenolysis for efficient depolymerization of lignin. Mol. Catal. 549, 113529 (2023). https://doi.org/10.1016/j.mcat.2023.113529
Ghoreishi, S., Barth, T., Hermundsgård, D.H.: Effect of reaction conditions on Catalytic and Noncatalytic Lignin Solvolysis in Water Media investigated for a 5 L Reactor. ACS Omega. 4, 19265–19278 (2019). https://doi.org/10.1021/acsomega.9b02629
Otromke, M., Shuttleworth, P.S., Sauer, J., White, R.J.: Hydrothermal base catalysed treatment of Kraft lignin - time dependent analysis and a techno-economic evaluation for carbon fibre applications. Bioresource Technol. Rep. 6, 241–250 (2019). https://doi.org/10.1016/j.biteb.2019.03.008
He, D., Yu, M., Xu, J., Du, B., Zhou, J., Wang, X.: Thiourea dioxide as a green reductant for selective depolymerization of lignin to guaiacol. Ind. Crops Prod. 194, 116176 (2023). https://doi.org/10.1016/j.indcrop.2022.116176
Pérez, E., Abad-Fernández, N., Lourençon, T., Balakshin, M., Sixta, H., Cocero, M.J.: Base-catalysed depolymerization of lignins in supercritical water: Influence of lignin nature and valorisation of pulping and biorefinery by-products. Biomass Bioenerg. 163, 106536 (2022). https://doi.org/10.1016/j.biombioe.2022.106536
Ong, V.Z., Yong, K.J., Wu, T.Y.: Production of aromatic monomers at one atmospheric pressure through depolymerization of lignin using combined alkaline solution and aqueous ChCl:Urea. Ind. Crops Prod. 192, 115911 (2023). https://doi.org/10.1016/j.indcrop.2022.115911
Zhou, N., Thilakarathna, W.P.D.W., He, Q.S., Rupasinghe, H.P.V.: A review: Depolymerization of lignin to Generate High-Value Bio-products: Opportunities, challenges, and prospects. Front. Energy Res. 9 (2022). https://doi.org/10.3389/fenrg.2021.758744
Hashmi, S.F., Meriö-Talvio, H., Hakonen, K.J., Ruuttunen, K., Sixta, H.: Hydrothermolysis of organosolv lignin for the production of bio-oil rich in monoaromatic phenolic compounds. Fuel Process. Technol. 168, 74–83 (2017). https://doi.org/10.1016/j.fuproc.2017.09.005
Kang, K.E., Han, M., Moon, S.-K., Kang, H.-W., Kim, Y., Cha, Y.-L., Choi, G.-W.: Optimization of alkali-extrusion pretreatment with twin-screw for bioethanol production from Miscanthus. Fuel. 109, 520–526 (2013). https://doi.org/10.1016/j.fuel.2013.03.026
Lim, G.H., Chae, J.S., Cha, Y.-L., Kang, Y.C., Roh, K.C.: Giant-miscanthus-derived activated carbon and its application to lithium sulfur batteries. Carbon Lett. 30, 477–484 (2020). https://doi.org/10.1007/s42823-019-00117-w
Kim, G.H., Um, B.H.: Fractionation and characterization of lignins from Miscanthus via organosolv and soda pulping for biorefinery applications. Int. J. Biol. Macromol. 158, 443–451 (2020). https://doi.org/10.1016/j.ijbiomac.2020.04.229
Lourenço, A., Pereira, H.: Compositional Variability of Lignin in Biomass. In: Lignin - Trends and Applications. InTech (2018)
Arnoult, S., Brancourt-Hulmel, M.: A review on Miscanthus Biomass production and composition for Bioenergy Use: Genotypic and environmental variability and implications for breeding. Bioenergy Res. 8, 502–526 (2015). https://doi.org/10.1007/s12155-014-9524-7
Zhang, Y., Jia, S., Wang, X., Deng, H., Xu, W., Shi, J.: Bimetallic polyoxometalates catalysts for efficient lignin depolymerization: Unlocking valuable aromatic compounds from renewable feedstock. Int. J. Biol. Macromol. 253, 127363 (2023). https://doi.org/10.1016/j.ijbiomac.2023.127363
Zhu, H., Du, B., Bai, Y., Pan, Z., Sun, Y., Wang, X., Zhou, J.: Base-catalyzed depolymerization of lignin into phenols: Methoxy groups’ secondary reactions triggered phenol regulation and repolymerization. Biomass Convers. Biorefinery. 13, 13009–13021 (2023). https://doi.org/10.1007/s13399-021-02190-6
Alhassan, Y., Hornung, U., Bugaje, M.: I.: Lignin Hydrothermal Liquefaction into Bifunctional Chemicals: A Concise Review. In: Biorefinery Concepts, Energy and Products. IntechOpen (2020)
Hashmi, S.F., Pitkänen, L., Usvalampi, A., Meriö-Talvio, H., Ruuttunen, K., Sixta, H.: Effect of metal formates on hydrothermolysis of organosolv lignin for the production of bio-oil. Fuel. 271, 117573 (2020). https://doi.org/10.1016/j.fuel.2020.117573
Roy, R., Rahman, M.S., Amit, T.A., Jadhav, B.: Recent advances in Lignin depolymerization techniques: A comparative overview of traditional and greener approaches. Biomass. 2, 130–154 (2022). https://doi.org/10.3390/biomass2030009
Wu, Z., Hu, L., Jiang, Y., Wang, X., Xu, J., Wang, Q., Jiang, S.: Recent advances in the acid-catalyzed conversion of lignin. Biomass Convers. Biorefinery. 13, 519–539 (2023). https://doi.org/10.1007/s13399-020-00976-8
Kaur, P., Singh, G., Arya, S.K.: Tandem catalytic approaches for lignin depolymerization: A review. Biomass Convers. Biorefinery. 14, 6143–6154 (2024). https://doi.org/10.1007/s13399-022-02980-6
Klapiszewski, Ł., Szalaty, T.J., Jesionowski, T.: Depolymerization and Activation of Lignin: Current State of Knowledge and Perspectives. In: Lignin - Trends and Applications. InTech (2018)
Christensen, E., Ferrell, J., Olarte, M.V., Padmaperuma, A.B.: Quantification of Semi-Volatile Oxygenated Components of Pyrolysis Bio-Oil by Gas Chromatography/Mass Spectrometry (GC/MS). Laboratory Analytical Procedure (LAP) (2016)
YANG, T., LIU, Z., LI, B., ZHANG, H.: Experimental study on preparation of bio-oil by hydrothermal liquefaction of three kinds of lignin. J. Fuel Chem. Technol. 51, 1084–1095 (2023). https://doi.org/10.1016/S1872-5813(23)60345-7
Kumar, M.M., Prabhudesai, V.S., Vinu, R.: Lignin depolymerization to Guaiacol and Vanillin Derivatives via Catalytic Transfer Hydrogenolysis using Pd-Lewis Metal Oxide supported on activated Carbon catalysts. Mol. Catal. 549, 113474 (2023). https://doi.org/10.1016/j.mcat.2023.113474
Shao, L., Zhang, Q., You, T., Zhang, X., Xu, F.: Microwave-assisted efficient depolymerization of alkaline lignin in methanol/formic acid media. Bioresour. Technol. 264, 238–243 (2018). https://doi.org/10.1016/j.biortech.2018.05.083
Hasegawa, I., Inoue, Y., Muranaka, Y., Yasukawa, T., Mae, K.: Selective production of Organic acids and depolymerization of lignin by Hydrothermal Oxidation with Diluted Hydrogen Peroxide. Energy Fuels. 25, 791–796 (2011). https://doi.org/10.1021/ef101477d
Katahira, R., Mittal, A., McKinney, K., Chen, X., Tucker, M.P., Johnson, D.K., Beckham, G.T.: Base-catalyzed depolymerization of Biorefinery Lignins. ACS Sustain. Chem. Eng. 4, 1474–1486 (2016). https://doi.org/10.1021/acssuschemeng.5b01451
Verziu, M., Tirsoaga, A., Cojocaru, B., Bucur, C., Tudora, B., Richel, A., Aguedo, M., Samikannu, A., Mikkola, J.P.: Hydrogenolysis of lignin over Ru-based catalysts: The role of the ruthenium in a lignin fragmentation process. Mol. Catal. 450, 65–76 (2018). https://doi.org/10.1016/j.mcat.2018.03.004
Paananen, H., Eronen, E., Mäkinen, M., Jänis, J., Suvanto, M., Pakkanen, T.T.: Base-catalyzed oxidative depolymerization of softwood kraft lignin. Ind. Crops Prod. 152, 112473 (2020). https://doi.org/10.1016/j.indcrop.2020.112473
Wahyudiono, Kanetake, T., Sasaki, M., Goto, M.: Decomposition of a lignin model compound under Hydrothermal conditions. Chem. Eng. Technol. 30, 1113–1122 (2007). https://doi.org/10.1002/ceat.200700066
Fernández-Rodríguez, J., Erdocia, X., Sánchez, C., González Alriols, M., Labidi, J.: Lignin depolymerization for phenolic monomers production by sustainable processes. J. Energy Chem. 26, 622–631 (2017). https://doi.org/10.1016/j.jechem.2017.02.007
Funding
This study was carried out with the support of ‘R&D Program for Forest Science Technology (Project No. 2023473C10-2325-EE02)’ provided by Korea Forest Service (Korea Forestry promotion Institute). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. NRF-2022R1F1A1069984).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization: Ga Hee Kim, Youn Il Kim; Methodology: Ga Hee Kim, Youn Il Kim; Formal analysis and investigation: Youn Il Kim; Writing - original draft preparation: Ga Hee Kim, Youn Il Kim; Writing - review and editing: Ga Hee Kim; Funding acquisition: Byung Hwan Um; Resources: Byung Hwan Um; Supervision: Byung Hwan Um.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, G.H., Kim, Y.I. & Um, B.H. Base-Catalyzed Depolymerization of Organosolv Lignin into Monoaromatic Phenolic Compounds. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02655-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02655-5