Abstract
A comparison of sample preparation based on the quick, easy, cheap, effective, rugged and safe (QuEChERS) method for analysis of pesticide residues in strawberries by LC–MS/MS was made using different sorbents in the clean-up by dispersive solid-phase extraction (d-SPE). Some sorbents were laboratory-made, prepared by depositing poly(methyloctadecylsiloxane) (PMODS), poly(methyloctylsiloxane) (PMOS), aminopropyl-terminated poly(dimethylsiloxane) (APPS) and copolymer of (52–48 %)dimethyl-(48–52 %)methylphenyl-siloxane (DMMPS) onto silica supports. The commercial sorbent primary–secondary amine (PSA) and mixtures of two sorbents, primary–secondary amine and poly(methyloctadecylsiloxane), were also used in the experiments. The performances of the sorbents were evaluated by parameters such as color of the final extract, gravimetric measurement, recovery and matrix effect at the fortification level of 100 ng g−1 of the pesticide mixture in strawberries. The recoveries were in the range 70–120 %, and the RSD values were lower than 20 % for most of the pesticides using the modified QuEChERS method with different sorbents in the clean-up step. The strawberry extracts were cleaned more efficiently with the use of primary–secondary amine sorbent, which has the function of removing sugars, organic acids and especially pigments. The sample preparation method was efficient, and LC–MS/MS determination was optimal because of high selectivity and good detectivity for the multiresidue analyses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strawberries (Fragaria × ananassa Duch.) are fruits produced and appreciated in various regions of the world. They have great economic impact, high nutritional values and very important functional properties such as anti-inflammatory, antioxidant, anti-neurodegenerative and anticarcinogenic activities [1–3].
Since 2002, strawberry crops have led the ranking of the foods most contaminated with pesticide residues in Brazil, because they have high incidences of pests and diseases that affect the various stages of the production [4, 5]. This contamination is highly troublesome because the safety interval, the period from harvest to sale, is usually less than 5 days and there is high consumption, especially in natura, by people of all ages [6].
Pesticides are substances that belong to many different chemical groups and are widely used in agriculture to increase productivity, improve quality and prolong the storage life of food crops [7]. Unfortunately, not all farmers follow good agricultural practices, leading to an increasing concern about food safety and environmental contamination [8]. Thus, regulations have been established in most countries worldwide to set maximum residue limits (MRLs) of pesticides in foods. In order to observe these requirements, an efficient method to monitor multiresidue pesticides is of upmost importance.
Nowadays, many pesticides are more polar and less volatile and/or thermally labile, consequently, LC–MS/MS is replacing GC methodologies as the method of choice for pesticide residue analyses [7, 9].
Most traditional methods for determination of pesticides in foods are time-consuming, expensive, restricted to a single class of pesticides, need large amounts of sample, have high LOQ and low percent recoveries [10, 11]. Anastassiades et al. [12] developed the QuEChERS method for the determination of different pesticides in foods that involve extraction followed by liquid–liquid partitioning and a clean-up step using d-SPE. The main advantages of this methodology are speed, simplicity, good recoveries for pesticides having different physicochemical properties that are present in low concentrations in complex matrices, besides the significant reduction of volume of organic solvents, not including chlorinated ones, thus contribute favorably to Green Chemistry.
The QuEChERS method has undergone various modifications and enhancements over the years, most of them aimed at improving recovery and stability of some pesticides. These modifications are versions of the original, unbuffered method, first published in 2003, or the AOAC Official Method from 2007, which uses acetate buffering, or the European Committee for Standardization (CEN) Standard Method EN 15662 from 2008, which uses citrate buffering [13, 14].
The clean-up step in d-SPE, introduced by Anastassiades et al. [12], uses only PSA sorbent. Currently, diverse sorbents as octadecylsiloxane (C18), graphitized carbon black (GCB), graphene, multi-walled carbon nanotubes (MWCNTs) and others have been evaluated in the QuEChERS method, to improve clean-up and recovery during in analysis of pesticides [15–18].
For some years, our research laboratory has developed new materials for analytical purposes, such as stationary phases for LC [19–21] and SPE sorbents [22–25] with good results. The latter have been used successfully in the extraction of pesticides from food, biological and environmental samples. These new materials are based on polymer immobilization onto pure silica or metalized silica supports.
In this work, the aim was to evaluate different sorbents made in our laboratory, including poly(methyloctadecylsiloxane) (PMODS), poly(methyloctylsiloxane) (PMOS), aminopropyl-terminated poly(dimethylsiloxane) (APPS) and (52–48 %)dimethyl-(48–52 %) methylphenyl-siloxane (DMMPS) immobilized onto silica, comparing them with commercial primary–secondary amine (PSA), alone or as a mixture of PMODS and PSA, for use in clean-up by d-SPE in a modified QuEChERS method for analysis of pesticide residues in strawberries. The target analytes were selected based on the maximum residue limits established by ANVISA (Agência Nacional de Vigilância Sanitária) also were included non-authorized pesticides found with great frequency in strawberry samples analyzed in Brazil [4]. Some of these pesticides have limits established by other regulatory agencies such as the Codex Alimentarius and the EU Pesticides database. These pesticide multiresidues were determined by LC–MS/MS at 100 ng g−1 level of fortification by evaluating some parameters such as color, gravimetric measurement, recovery and matrix effect.
Experimental
Chemicals and Materials
The reagents and solvents were of analytical grade or HPLC grade, respectively. Acetonitrile, chloroform, dichloromethane, methanol, n-hexane and n-pentane were from Tedia (Fairfield, NJ, USA), toluene from J.T. Baker (Ecatepec, Mexico), formic acid from Synth (Diadema, Brazil), Bondesil PSA (40 µm) from Varian (Palo Alto, CA, USA), anhydrous magnesium sulfate from Vetec (Rio de Janeiro, Brazil), sodium citrate tribasic dihydrate and sodium hydrogencitrate sesquihydrate from Sigma-Aldrich (St. Louis, MO, USA) and sodium chloride from Ecibra (São Paulo, Brazil). Ultrapure water was obtained from a Milli-Q system from Millipore (Bedford, MA, USA) with 18.2 MΩ cm−1 conductivity.
The silica as support for preparing of the d-SPE materials was from Acros Organics (Pittsburgh, PA, USA) with particle size 0.035–0.070 mm with 60 Å pore size. The polymers PMODS, PMOS and APPS were from United Chemical Technologies (Bristol, PA, USA), DMMPS was from Aldrich (St. Louis, MO, USA). The schematic structures of the polymers are represented in Fig. 1.
The pesticide standards used in this work (Supporting Information Table S1) were obtained from Pestanal (Steinheim, Germany), Fluka (Steinheim, Germany), Chem Service (West Chester, PA, USA) or Dr. Ehrenstorfer Gmbh. (Augsburg, Germany). All 29 pesticide standards presented purities higher than 97 %. The individual stock solutions were prepared at 1.0 mg mL−1, except for carbendazim and simazine (0.2 mg mL−1) because of their low solubility, by dissolution in methanol. All solutions were stored at −18 °C.
The pesticide-free strawberries produced through organic agriculture were purchased from a market of organic products in Campinas, Brazil.
Instrumental and Chromatographic Conditions
Pesticide residue determinations were performed with a Micromass Quattro micro™ API Mass Spectrometer (Waters, Wythenshawe, UK) coupled to an Alliance 2690 LC (Waters, Milford, MA, USA). The chromatographic separations were carried out with a Nova-Pak C18 (150 mm × 3.9 mm i.d., 4 µm) analytical column coupled to a Nova-Pak C18 (20 mm × 3.9 mm i.d., 4 µm) guard column, both from Waters, that were maintained at 30 °C. The mobile phase consisted of 0.1 % of formic acid in water (solvent A) and methanol (solvent B). Gradient elution was applied at a flow rate of 0.4 mL min−1 as follows: initial conditions of 30 % B, increased linearly to 95 % B, returning to the initial conditions at 30 min with re-equilibration to the initial conditions in 5 min. The injection volume was 20 µL.
For MS/MS detection, the mass spectrometer was equipped with an ESI source operated in both positive- and negative-ionization modes. The acquisition mode was multiple reaction monitoring (MRM). The conditions of source were capillary voltage set at 3.5 kV, the temperature was kept at 130 °C, while the desolvation temperature was held at 450 °C, nitrogen was used as cone and desolvation gas at flow rates of 60 and 750 L h−1, respectively. The collision gas (argon) pressure was set at approximately 2.5 × 10−3 mbar. Instrument control and data analysis were performed using MassLynx 4.0 software from Waters. The retention times, precursor and product ions, cone voltages and collision energies for each analyte are presented in Table S1 (supplementary material).
Preparation of Laboratory-Made Sorbents
A quantity of polymer dissolved in an appropriate organic solvent was added to the silica utilized as support, previously dried at 120 °C for 24 h. This mixture was stirred at room temperature and then placed in a fume hood for slow evaporation of the solvent at room temperature over several days. The sorbents were then placed in stainless steel tubes (150 mm × 10 mm i.d.) fitted with frits and connectors and submitted to different conditions for immobilization using thermal treatments. Next, the stainless steel tubes were connected to 510 pump (Waters) for the extraction of non-immobilized polymer by passing organic solvent at 0.5 mL min−1. The sorbents were removed from the tubes and dried with flowing nitrogen for use in d-SPE in the modified QuEChERS method.
For PMODS and APPS loadings of 40 % (w/w) of the polymers were deposited onto the silica surface and immobilized by thermal treatment at atmospheric pressure, as described by Pozzebon et al. [22] and Melo et al. [23, 26], respectively. For PMOS, the loading was 35 % (w/w) and thermal immobilization was under flowing nitrogen, as described by Vigna et al. [24]. The DMMPS was prepared with a loading of 50 % (w/w) and immobilized with thermal treatment at atmospheric pressure, according to Jardim et al. [20].
The laboratory-made and commercial sorbents were submitted to elemental analysis for determination of the percentage of carbon and nitrogen on a CHN-2400 Analyser (Perkin-Elmer, USA).
Sample Preparation
Pesticides were extracted from strawberries using a modified QuEChERS method, based on a version of CEN 15662 with acidification of the final extract [13, 27]. A representative 10 g portion of homogenized sample of strawberries was placed in a polypropylene centrifuge tube (50 mL), and 10 mL acetonitrile was added to the tube. The mixture was stirred (Phoenix, model AP-56, Araraquara, Brazil) for 1 min. Afterwards, 4 g of anhydrous magnesium sulfate, 1 g sodium chloride, 1 g sodium citrate tribasic dihydrate and 0.5 g sodium hydrogencitrate sesquihydrate were added to this mixture and the tube was vigorously shaken for 1 min. After centrifugation (Hettich Zentrifugen, model Rotofix 32A, Tuttlingen, Germany) at 5,000 g for 15 min, the organic layer was transferred into a clean-up tube (15 mL) containing 150 mg anhydrous magnesium sulfate and 25 mg of one of the following sorbents per mL of extract. (1) PMODS; (2) PMOS; (3) APPS; (4) DMMPS; (5) PSA; (6) 25 mg PMODS and 25 mg PSA (mixture 1) and (7) 12.5 mg PMODS and 12.5 mg PSA (mixture 2). The mixture was shaken for 1 min and centrifuged for 5 min.
After the clean-up step, 10 µL 5 % formic acid in acetonitrile was added per mL of supernatant, to protect any sensitive pesticides from degradation at high pH [12, 13, 27]. Finally, the solvent was evaporated under a nitrogen stream. The residue was redissolved in 1 mL methanol and a volume of 20 µL was injected in the LC–MS/MS system.
Recovery Tests
The recoveries of the extractions were determined using samples spiked at 100 ng g−1 with the pesticides, based on analysis of three replicates with each sorbent type.
Gravimetric Measurements
The co-extracted matrix components or clean-up efficiencies can be evaluated by gravimetric measurements [13, 28].
The sample extracts were divided into two equal parts, one for testing without the clean-up step and the other after d-SPE clean-up of the modified QuEChERS method. The extracts were dried by a nitrogen stream, and the tubes were then heated at 110 °C for 1 h, where the weight difference between the evaporated extracts without and with clean-up was recorded to estimate the co-extracted matrix components (%).
Matrix Effect
The matrix effect (ME) has been used to demonstrate the ionization efficiency of the analytes, which is represented by the detector responses of the post-extraction spiked samples compared with solutions of the same pesticides in pure solvent. This phenomenon can be represented by increased, decreased or no signal values that show signal enhancement, signal suppression or no ME, respectively [29, 30]. In the current study, the ME was evaluated using the matrix extract with each sorbent type used in the d-SPE step.
Results and Discussion
The QuEChERS method has been successfully applied for many types of matrices, and one of the reasons for this versatility is the use of different sorbents in the clean-up step, which has the function of minimizing the interferences. The d-SPE has some advantages such as saving time, solvent and sorbent and has more reproducible recoveries in comparison with cartridge SPE clean-up [10].
Sorbent Types
In multiresidue analysis, there are pesticides with hydrophobic or hydrophilic characteristics in a complex matrix. Therefore, the combination with one or more sorbents having different interactions could be more effective for clean-up relative to a single sorbent.
The sorbent PSA is a silica-based material with a bonded ethylenediamine-N-propyl phase containing both primary and secondary amine groups. Its bidentate structure has a high chelation effect and weak anion exchange character. As a result, this sorbent removes many organic acids, sugars, fatty acids, pigments and some other matrix co-extractives that form hydrogen bonds [10, 12, 31]. Strawberries have many compounds with these characteristics in their composition.
The polymers PMODS and PMOS are both polysiloxanes, but PMODS has stronger apolar character, causing stronger interaction with non-polar compounds, resulting in good extractions for these types of interferences.
The phenyl sorbent has a potential orthogonal chromatographic selectivity compared to traditional alkyl phases due to its capacity to undergo π–π interactions with aromatic compounds. This interaction is a type of electron donor–acceptor interaction, originating from π-electron systems in two unsaturated functional groups through either intermolecular or intramolecular interactions. The π–π interaction is important when the phase is electron rich and the analyte is electron poor [32]. In practice, no studies have evaluated the role of phenyl ligand density in interaction processes, but according to some papers, the retention process of phenyl type phases is very complex and involves a combination of lipophilic, π–π and dipole–dipole interactions [33, 34].
The APPS polymer has functions as a mixed-mode sorbent with apolar characteristics due to the poly(dimethylsiloxane) chains and polar characteristics because of the aminopropyl termination, allowing it to act as a reversed phase, a weak anion exchanger and a normal phase [23, 26].
The elemental analysis for C (%) evaluates the degree of effective coverage of polymer on the support that is available to perform interactions, while the amount of N (%) indicates the presence of amino groups, which have polar character and may act as a weak anion exchanger. The percentages ranged from 6.51 to 21.06 for carbon and 0.08 to 2.72 for nitrogen. From analysis of these sorbents, PMODS is the most non-polar and PSA is the most polar.
Evaluation of d-SPE Clean-up
Some parameters such as the gravimetric measurements, the colors, the chromatographic interferences, the ME, the recoveries and others, represent one way to determine the degree of clean-up of final extract of samples by different methods [12, 13, 28].
Comparison of Color of Strawberry Extracts After Clean-up
The extracts from strawberries showed differences in color, ranging from red to orange to yellow, as a function of the sorbent used in the clean-up by d-SPE. The adsorption ability for pigments of strawberries was much stronger when PSA alone was used compared to the other sorbents, because the extract was light yellow or almost colorless. Mixtures 1 or 2, PMODS and PSA sorbents, also would be good options for clean-up. PMODS and PMOS are the most common types of sorbents, they have non-polar characteristics and preferential interactions with the hydrophobic compounds. Consequently, clean-up with these sorbents was not as efficient as the PSA sorbent and the colors of the extracts were red and orange, respectively. The extracts using APPS and DMMPS sorbents showed orange and red color, respectively, and also had a turbid aspect that indicated that co-extracts were not removed efficiently during the clean-up step.
It is important to emphasize that comparison of the colors can suggest clean-up efficiency, but it is not possible to determine with accuracy that one extract had, really, less co-extracted compounds than another.
Evaluation of the Recoveries of Pesticides
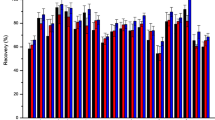
Figure 2 shows the recoveries (%) of the method developed for the extraction of pesticide residues in strawberries, using laboratory-made and commercial sorbents for clean-up in d-SPE. Considering the acceptability criteria to be recoveries between 70 and 120 %, with RSD below 20 %, according to SANCO guidelines [35], all pesticides were found in this recommended range, except for abamectin when using PMODS and DMMPS, and methamidophos with PSA, but the low RSD values for both pesticides indicate that the method is reproducible.
Commonly, lower recoveries of pesticides may be related to factors such as irreversible sorption on sorbents, degradation of analytes, high solubility in some component of the sample, little or no liquid–liquid partitioning and also loss of analyte in the evaporation step.
In the case of the problematic pesticides, methamidophos, an organophosphorus pesticide, is extremely water soluble and very polar, therefore it may not partition completely into the organic phase because the strawberries have a high water content (>90 %). Abamectin, an avermectin pesticide, has high lipophilicity, consequently it may not completely partition into the acetonitrile solvent or may have been retained on the PMODS and DMMPS sorbents that have less polar character than PSA sorbent.
Evaluation of Clean-up of Extracts by Gravimetric Measurements
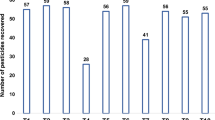
The effectiveness of the clean-up on the removal of matrix components from extracts with different sorbents used in d-SPE was evaluated by gravimetric measurements. The results are shown in Fig. 3.
The d-SPE clean-up using 25 mg PSA sorbent per mL of extract removed 89 % of co-extractives by weight, ensuring greater efficiency of clean-up from the strawberry extracts, compared to the other sorbents. The two mixtures of sorbents can also be considered as good alternatives, but the addition of PMODS did not improve the clean-up efficiency and the use only one type of sorbent is easier and quicker in sample preparation.
The PMODS, PMOS and DMMPS sorbents showed similar clean-up results. The APPS sorbent extracted very little co-extracts, consequently providing a low clean-up of interferences.
The QuEChERS method is very flexible and adjustments such as different sorbents have little impact on pesticide recoveries in strawberries, but may influence the evaluation of the clean-up of extracts by the gravimetric measurements. Removal of co-extractives is mainly related to the interactions between the sample and the sorbent, but extraction solvent, sample composition and pesticides also have great influence in the clean-up step. The different polarity sorbents have retention mechanisms as hydrophobic interactions (van der Waals and London dispersion forces), hydrophilic interactions (hydrogen bonding, π–π interactions, dipole–dipole interactions and others) or ion exchange (electrostatic interactions). The favorable interactions will allow more removal of a broad range of compounds from strawberries, consequently, it results in reduction of co-extracts that interfere in the analytical method (e.g., S/N, LOQ, ME and less chances to have false positive results) and affect the equipment maintenance.
Matrix Effects
In analysis using LC–MS/MS, the ME is a problem due to the interactions between analyte and co-extractives of matrix that elute at the same time, therefore competing with the analyte during the ionization process, causing loss of method accuracy, reproducibility and detectivity, leading to incorrect analytical determinations [30, 36].
Sample preparation is essential, but there are other alternatives to minimize the ME, such as use of matrix-matched calibration standards, sample dilution, reduction of the injected volume, appropriate internal standards, improving LC separation efficiency and even modification of the mass spectrometric conditions [30, 36, 37]. It is important to note that these alternatives may also decrease the analytical response and this can seriously hamper the determination of low concentrations of analytes.
There is no criterion for the percentage of ME in guidelines of analysis of pesticide residues in foods, but most papers in this area use values greater than ±20 % as a threshold value for this parameter [29, 38]. Figure 4 shows the ME, represented as percentage of the number of pesticides analyzed in this work with the different sorbents, expressed by signal enhancement or suppression when higher than ±20 % and considered not to have significant ME when lower than ±20 %.
Considering the results, signal enhancement was not shown in any case. The mixture of sorbents, PSA and PMODS, had less ME compared to other sorbents. PMODS, PMOS and PSA alone showed similar results of distribution of ME. APPS presented signal suppression for all pesticides, which indicates that its structure did not help as efficiently as the other sorbents in removing interferences in the clean-up step. DMMPS also showed a high percentage of pesticides with signal suppression, and may be related to the origin of the turbidity in the extract, due to the presence of interferences, which also occurred with the APPS sorbent.
For the most polar pesticides, acephate and methamidophos, that are eluted first (Table S1 in supplementary material), the negative ME is higher, ranging from −92.8 to −49.9 % and −94.9 to −79.0 %, respectively. The phenomenon causing more ME could be explained by high amounts of the matrix interferences from the strawberries that co-elute with pesticides at the start of the chromatographic run.
On the other hand, at the end of the chromatographic run, ME with low signal was observed because of smaller amounts of interferences with longer retention times. The ME ranged from −23.3 to +0.2 % for prochloraz and from −24.8 to −2.3 % for fenazaquin. Not surprisingly, a good chromatographic separation is essential to help minimize the ME in any analysis.
Conclusions
The QuEChERS method has great merit in solving many problems of traditional methods of pesticide analysis, mainly the larger number of analytes extracted with good recovery and clean-up.
Although it seems impossible to eliminate the presence of interferences of samples completely, it can be minimized, mainly by the clean-up step of sample preparation. Some parameters such as the color of the extract, recovery, gravimetric measurements and ME must be evaluated for the best choice of sorbent in clean-up by d-SPE, because the amount of co-extracted interferences can affect the performance of the analytical method and equipment maintenance.
In this study, PSA presented the best results, when compared to the other sorbents, for almost all of the parameters analyzed, but the use of the mixture of two sorbents, PSA and PMODS, also offered good results in determination of pesticide residues in strawberries. More experiments involving the use of mixtures of sorbents in sample preparation with the QuEChERS method should be studied, because of its low matrix effect and excellent recovery values in the range accepted by most guidelines, such studies may also aid in understanding the possible interactions that occur in the retention mechanism in the clean-up step.
References
Häkkinen SH, Törrönen AR (2000) Food Res Int 33:517–524
Seeram NP, Lee R, Scheuller HS, Heber D (2006) Food Chem 97:1–11
Giménez G, Andriolo J, Godoi R (2008) Cienc Rural 38:273–279
ANVISA (2013), Agrotóxicos e Toxicologia, Programa de Análise de Resíduos de Agrotóxicos em Alimentos. http://portal.anvisa.gov.br/wps/portal/anvisa/home. Accessed 10 Dec 2013
Oshita D, Jardim ICSF (2012) Sci Chromatogr 4:52–76. doi:10.4322/sc.2012.005
Hu R, Hennion B, Urruty L, Montury M (1999) Food Addit Contam A 16(3):111–117
Pico Y, Blasco C, Font G (2004) Mass Spectrom Rev 23:45–85
Kmellár B, Abrankó L, Fodor P, Lehotay SJ (2010) Food Addit Contam A 27(10):1415–1430
Hollosi L, Mittendorf K, Senyuva HZ (2012) Chromatographia 75:1377–1393
Wilkowska A, Biziuk M (2011) Food Chem 125:803–812
Lambropoulou DA, Albanis TA (2007) Anal Bioanal Chem 389:1663–1683
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) J AOAC Int 86(2):412–431
Lehotay SJ, Son KA, Kwon H, Koesukwiwat U, Fu W, Mastovska K, Hoh E, Leepipatpiboon N (2010) J Chromatogr A 1217:2548–2560
Lehotay SJ, Anastassiades M, Majors RE (2010) LCGC N Am 28(7):504–516. http://www.chromatographyonline.com/lcgc/Column%3A+Sample+Prep+Perspectives/QuEChERS-An-Interview-with-the-Inventors/ArticleStandard/Article/detail/680292
Koesukwiwat U, Lehotay SJ, Mastovska K, Dorweiler KJ, Leepipatpiboon N (2010) J Agric Food Chem 58:5950–5958
Gonzalez-Curbelo MA, Hernandez-Borges J, Ravelo-Perez LM, Rodriguez-Delgado MA (2011) Food Chem 125:1083–1090
Zhao P, Wang L, Luo J, Li J, Pan C (2012) J Sep Sci 35:153–158
Liu X, Guan W, Hao X, Wu X, Ma Y, Pan C (2014) Chromatographia 77:31–37
Jardim ICSF, Collins KE, Collins CH (2004) Microchem J 77:191–198
Jardim ICSF, Maldaner L, Lourenço J, Fioravanti LMA, Collins CH (2010) J Sep Sci 33:2917–2929
Goraieb K, Collins CH (2013) Chromatographia 76:899–908
Pozzebon JM, Queiroz SCN, Melo LFC, Kapor MA, Jardim ICSF (2003) J Chromatogr A 987:381–387
Melo LFC, Collins CH, Jardim ICSF (2004) J Chromatogr A 1032:51–58
Vigna CRM, Morais LSR, Collins CH, Jardim ICSF (2006) J Chromatogr A 1114:211–215
Faria AM, Maldaner L, Santana CC, Jardim ICSF, Collins CH (2007) Anal Chim Acta 582:34–40
Melo LFC, Collins CH, Jardim ICSF (2005) J Chromatogr A 1073:75–81
CVUA Stuttgart, QuEChERS: a mini-multiresidue method for the analysis of pesticide residues in low-fat products. http://quechers.cvua-stuttgart.de/pdf/reality.pdf. Accessed 15 Dec 2013
Cunha SC, Lehotay SJ, Mastovská K, Fernandes JO, Beatriz M, Oliveira PP (2007) J Sep Sci 30:620–632
Pizzutti IR, Kok A, Hiemstra M, Wickert C, Prestes OD (2009) J Chromatogr A 1216:4539–4552
Trufelli H, Palma P, Famiglini G, Cappiello A (2011) Mass Spectrom Rev 30:491–509
Prestes OD, Friggi CA, Adaime MB, Zanella R (2009) Quim Nova 32(6):1620–1634
Euerby MR, Petersson P, Campbell W, Roe W (2007) J Chromatogr A 1154:138–151
Croes K, Steffens A, Marchand DH, Snyder LR (2005) J Chromatogr A 1098:123–130
Markopoulou C, Tweedlie T, Watson D, Skellern G, Reda H, Petersson P, Bradstock H, Euerby M (2009) Chromatographia 70(5/6):705–715
European Commission DG-SANCO (2010) Method validation and quality control procedures for pesticide residues analysis in food and feed, SANCO/10684/2009, 01/01/2010. http://ec.europa.eu/food/plant/protection/resources/qualcontrol_en.pdf. Accessed 05 Nov 2013
Lehotay SJ, Mastovska K, Lightfield AR, Gates RA (2010) J AOAC Int 93(2):355–367
Kruve A, Künnapas A, Herodes K, Leito I (2008) J Chromatogr A 1187:58–66
Kmellar B, Fodor P, Pareja L, Ferrer C, Martinez-Uroz MA, Valverde A, Fernandez-Alba AR (2008) J Chromatogr A 1215:37–50
Acknowledgments
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, no. 2006/57897-9) for financial support and fellowships that made this research possible. They also thank C.H. Collins for helpful discussion and suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oshita, D., Jardim, I.C.S.F. Comparison of Different Sorbents in the QuEChERS Method for the Determination of Pesticide Residues in Strawberries by LC–MS/MS. Chromatographia 77, 1291–1298 (2014). https://doi.org/10.1007/s10337-014-2726-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2726-5