Abstract
Recently, a new and fast equilibrium-based solvent microextraction technique termed vortex-assisted liquid–liquid microextraction was developed. In this technique, the dispersion of the extraction solvent is enhanced by vortex mixing. The aim of the present review is to discuss the applications of vortex agitation in solvent-microextraction procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modern trends in analytical chemistry are toward the simplification and miniaturization of sample preparation procedures, as well as the minimization of solvent and reagent consumption. The sample preparation step, which leads to the enrichment and purification of the analytes, is one of the most important steps of any chemical analysis procedure. Traditional extraction techniques are laborious and consume a large amount of organic solvent. In the last two decades, miniaturized solvent extraction procedures, also called liquid-phase microextraction (LPME), have attracted much attention by many researchers. The principle of LPME protocols is based on the traditional liquid–liquid extraction, whereby the organic phase is substantially reduced.
From the introduction of the first works on LPME in 1996 [1, 2], different approaches of LPME such as SDME [3, 4], HF-LPME [5], DLLME [6] and SFODME [7], among others, were developed.
In particular, DLLME is a very simple and rapid extraction method, based on the use of a ternary component solvent system, which has been applied to the extraction and preconcentration of both organic and inorganic compounds from aqueous samples. DLLME has attracted more and more attention, due to its superior advantages of high enrichment factor, high recovery, and high extraction speed, low cost and easy operation [8, 9].
Basically, DLLME is based on the fast injection of a mixture of extracting and disperser solvents into the aqueous solution to form a cloudy ternary component solvent (aqueous solution/extracting solvent/disperser solvent) system; after centrifugation, the enriched analyte in the sedimented phase is withdrawn and determined generally by chromatography or spectrometry methods.
The main drawbacks associated with DLLME have been the difficulty to automate and the necessity of using a third component (disperser solvent), which commonly decreases the partition coefficient of analytes into the extractant solvent.
Recently, in the year 2010, Yiantzi et al. [10] introduced a new microextraction method termed vortex-assisted liquid–liquid microextraction (VALLME), whereby dispersion of low density extraction solvent into water is obtained through using vortex mixing, a mild emulsification procedure. The fine droplets can rapidly extract target analytes from water because of the shorter diffusion distance and larger interfacial area. After centrifugation, the floating extractant phase restores its initial single-drop shape for the following instrumental analysis.
Recently, Andruch et al. presented an interesting review where the authors principally discuss the application of ultrasonic irradiation, and in minor extension, the application of vortex agitation in solvent microextraction procedures [11].
This paper reviews the applications of this new procedure for sample preparation based on the vortex agitation. We present a compilation of these applications and summarize the principal characteristics of newly developed methods.
Applications
Table 1 gives a list of various VALLME procedures that have been reported in the literature, and Table 2 shows the characteristics of the corresponding methods of analysis.
Organic Compounds
Liquid Chromatography
For the first time, dispersion of the extractant phase into the aqueous was achieved using vortex mixing, a mild emulsification procedure [10]. The method was successfully applied to the determination of BPA, OP and NP at trace levels in water samples. The fine droplets formed could extract target analytes towards equilibrium faster because of the shorter diffusion distance and larger specific surface area. The single microdrop could easily be collected with the help of a microsyringe and used for HPLC analysis.
Vortex-assisted extraction combined with DLLME was applied by Leng et al. [12] for the extraction of PAHs in sediment samples prior to analysis by HPLC with FLD. This procedure extends and improves the application of DLLME to solid samples. This method gives a comparable detection limit with other previous studies, and provides good linearity and repeatability, as well as good extraction efficiency. Also, the extraction and preconcentration of PAHs in sediment samples could be achieved within 3 min, which indicated that the method was much simpler and rapid compared with previous study.
A one-step method based on VALLME and OPA/2-ME derivatization combined with HPLC–FLD for the determination of eight primary amines in high-amino acid-containing water samples was developed by Chang et al. [13]. In addition to shortening the pretreatment time and concentrating analytes, this method has two major advantages: the excellent stabilization of OPA derivatives of amines and the elimination of amino acid interference.
In a work realized by Wang et al. [16], a new and easy one-step in-syringe device for VALLME was proposed and applied for the analysis of three fungicides in real aqueous samples. This research uses a 5-mL syringe as the extraction vessel to carry out extraction, separation, preconcentration and injection procedures in one step, without the need for any offline processes such as centrifugation to collect the recovered extractant, thus expanding the level of automation of VALLME.
Trtic-Petrovic and Dimitrijevic investigate the first application of the binary ionic liquid (IL)-VALLME technique, and combine it with HPLC for determination of the selected pesticides in the pesticide manufacturing wastewater sample [18]. The benefits of this method are rapidity, simplicity, easy of the operation, low consumption of IL, simultaneous extraction of the low polar and more polar compounds, direct injection in HPLC, and the environmentally friendly aspect of the method. This work shows that IL-VALLME technique can be treated as a promising alternative to the other extraction techniques as the sample pretreatment before HPLC determination.
The same research group that proposed VALLME for first time developed a simple and fast analytical method for trace level determination of perfluorosulfonates in water samples, based on VALLME and coupled to HPLC–single quadrupole MS [21]. For this study, PFOS anion was chosen by the authors as the model analyte, as it is the most commonly occurring contaminant. This analytical procedure does not require the use of certain sample preparation apparatus, which has been repeatedly reported to act as a source of procedural contamination or uncertainty.
A novel VALLME method combined with UFLC was established by Sun et al. for the preconcentration and determination of three aromatic amines in water samples [22]. Compared with other microextraction techniques, this method provided satisfactory performance, such as low detection limits, short extraction time and good repeatability values. This is a low organic solvent consumption method in which 1-hexyl-3-methylimidazolium hexafluorophosphate [C6MIM][PF6] was used as the extraction solvent without any other organic solvent.
A new extraction method, based on dispersive nano-solid material-ultrasound assisted microextraction, was used by Khodadoust et al. for the preconcentration of the bendiocarb and promecarb pesticides in the water samples prior to HPLC [24]. The authors used NiZnS nanoparticles, loaded on activated carbon (NiZnS-AC), as the new adsorbent for extraction of analytes in aqueous media. In this method, nano-particles are dispersed in aqueous media by vortex and ultrasonic device for adsorption of target analyte, then adsorbed analyte is eluted and determined by HPLC–UV.
Gas Chromatography
In a study presented by Jia et al. [25], the purpose was to introduce a new solvent microextraction method for the extraction of pesticides in water samples using the volumetric flask as the extraction vessel. The dispersion system formed under vortex assistance is thermodynamically unstable, and cleared into two phases quickly in the volumetric flask after the extraction process. In order to efficiently collect the small volumes of extraction solvent floating on the surface of water, a pipet tip (diameter: 7 mm) is adhered on the neck of the volumetric flask. By using this simple home-designed device, the application of low-density solvents in other DLLME methods is also possible.
A new micro-extraction technique, named low-density magneto fluid dispersive liquid–liquid microextraction, has been developed by Shen et al.; it permits a wider range of solvents and can be combined with various detection methods [26]. Compared with the existing low-density solvents micro-extraction methods, no special devices and complicated operations were required during the whole extraction process. Dispersion of the low-density magneto fluid into the aqueous sample was achieved by using vortex mixing, so disperser solvent was unnecessary. The extraction solvent was collected conveniently with an external magnetic field placed outside the extraction container after dispersing. Then, the magnetic nanoparticles were easily removed by adding precipitation reagent under the magnetic field.
Very recently, Wu et al. [27] develop a temperature-controlled ultrasound-assisted and vortex-assisted liquid–liquid microextraction as a fast and efficient approach for the extraction of nine OPPs followed by GC-FPD. The goal of this study was to develop a rapid and effective microextraction method that overcomes the drawbacks of ultrasound-assisted emulsification-microextraction and VALLME and that combines the advantages of these methods. Temperature-controlled ultrasound-assisted and vortex-assisted liquid–liquid microextraction was developed by the authors and employs ultrasonication and vortex agitation.
Ozcan [29] developed a VALLME procedure followed by GC–MS for the determination of OCPs. After the determination of the most suitable extraction solvent and rotational speed of the vortex, parameters such as vortex extraction time, solvent volume and ionic strength of the sample were optimized by using a 23 factorial experimental design.
The scope of the work carried out by Zacharis et al. [30] was to extend the applicability of the VALLME by determining 12 OPPs in environmental water and wine samples using GC–MS by using a low density organic solvent. From the green analytical chemistry point of view, the usage of lighter-than-water organic solvents is preferable to chlorinated ones, while additional dispersing reagents are avoided.
In a study described by Ozcan [31], the VALLE method combined with GC–MS for the determination of seven PCBs at trace levels in environmental samples was developed. Under optimized extraction conditions, the VALLME method provides high recovery and repeatability. Compared to the other methods, this method requires only a small volume of sample and consumption of toxic organic solvent, and a shorter extraction time. In addition, the developed method is viable, rapid, and easy to use for the qualitative and quantitative analyses of PCBs in waters. Additionally, minimum organic solvent consumption in the developed method diminishes environmental pollution and extra operational costs for waste treatment.
Recently, diverse protocols based on the use of different combination of VA with DLLME and other extraction systems have been proposed. In this sense, a vortex-assisted μ-solid phase extraction (μ-SPE) combined with low density solvent-based DLLME (VA-μ-SPE-LDS-DLLME) was developed for the GC–MS determination of PEs in environmental water samples [33]. The objective of the combined approach developed by Guo and Lee was to improve overall extraction time and analyte preconcentration, and simultaneously, to enable extraction from more complex aqueous samples. In the first step, with the assistance of vortex, the time required for μ-SPE was only several minutes, and was relatively much faster than the conventional μ-SPE procedure, which involves stirring and usually takes 20–40 min. In the second step, the analytes were further concentrated by LDS-DLLME. High extraction efficiency was obtained. In another study, an ultrasound-vortex-assisted dispersive liquid–liquid microextraction (USVADLLME) procedure coupled with GC-FID or GC–IT/MS was proposed for rapid analysis of six phthalate esters [34]. The results obtained using this analytical procedure based on USVADLLME and GC-FID or GC–IT/MS analysis allows for investigating PEs in matrices with large alcohol content.
Metal Ions
At this moment, the application of VALLME for the preconcentration of inorganic ions is being investigated very little. In this way, Chamsaz et al. employed VALLME for the determination of trace amounts of cadmium by FAAS [39] and GFAAS [40]. In these works, IL, 1-hexyl-3-methylimidazolium hexafluorophosphate ([Hmim][PF6]), was used as an extractant solvent. Cd2+ was complexed with APDC, and then entered into fine IL droplets by the assistance of vortex agitator system. These determinations of cadmium by the VALLME method have better dynamic range, less toxicity (using IL as extraction solvent) and simplicity, compared to some reported methods in the literature. The same investigation group reported another determination of cadmium by FAAS, where cadmium preconcentration was mediated by chelation with the 8-hydroxyquinoline (oxine) reagent and an IL, 1-octyl-3-methylimidazolium hexafluorophosphate ([Omim][PF6]) was chosen as the extraction solvent to extract the hydrophobic complex [41].
In other research, Leng et al. [43] developed an analytical method for trace level determination of mercury species (methylmercury MeHg+, ethylmercury EtHg+ and inorganic mercury Hg2+) in sediment samples based on VALLME and coupled to HPLC-CVAFS. Using l-Cysteine as extraction solvent, this method is sensitive, simple and rapid, as well as environmentally friendly.
A novel and simple ionic liquid-linked dual magnetic microextraction was developed by Yilmaz and Soylak [45] for fast and effective determination of lead combined with FAAS. In this report, [C4mim][PF6] was used to extract complex of Pb with pyrrolidine dithiocarbamate in the DLLME step without organic solvents.In the D-μ-SPE step of the microextraction method, Fe3O4 magnetic nanoparticles were used to extraction of the IL and complex. Because of using MNPs and IL in the IL- DMME, the procedure can be described as environmentally friendly. No specific equipment, or time-consuming and complex processes other than conventional preconcentration methods are required. The main advantage of the method is that it can be applied to water, plant and hair samples without matrix interferences.
Vortex-Assisted Surfactant-Enhanced-Emulsification Liquid–Liquid Microextraction
A novel sample pretreatment technique, based on vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction (VSLLME), followed by GC-FPD, was developed by Yang et al. [47] for the determination of seven OPPs. The addition of a surfactant, which was used as an emulsifier, could enhance the speed of the mass-transfer from aqueous samples to the extraction solvent. The developed VSLLME overcomes several disadvantages of the former LPME methods, while maintaining their advantages. Its main disadvantage was that the extraction solvent must have a higher density than water in order to be sedimented by centrifugation; typically chlorinated solvents such as chlorobenzene, which was potentially toxic to human health and the environment, were used. For this reason, the same investigation group, in two later studies, used a disposable polyethylene pipette as the extraction device in a new VSLLME method that permitted the use of light solvent such as toluene, as the extraction solvent [48, 49]. Low-density and nonchlorine solvents could be used as extraction solvent in this procedure, which overcame the main disadvantage of the former VSLLME method.
In another recent study, LDS–VSLLME with GC–MS was applied for the first time to the fast determination of six PEs in bottled water samples [50]. In this procedure, toluene was employed as the extraction solvent, and CTAB was used as an emulsifier to facilitate the dispersion of organic solvent in the aqueous sample. After a 30 s extraction assisted by vortex agitation, phase separation was achieved by centrifugation. The supernatant (extraction solvent) was collected at the conical bottom of the tube after removing the aqueous sample by a syringe. This method avoids the necessity of a special homemade device for the collection of low-density organic solvents, it being tedious and troublesome to fabricate.
Vichapong et al. [52] have explored and developed an efficient LDS-VSLLME method coupled to HPLC with photodiode array detection for the extraction, preconcentration and analysis of neonicotinoid pesticide residues in surface water and fruit juice samples. This method has potential to be used as an alternative green extraction method for the determination of neonicotinoids in various sample matrices, with good recovery in the range of 85–105 %.
A novel method based on the combination of MEEKC and VSLLME was developed by Li et al. for the determination of five triazine herbicides (simazine, atrazine, ametryn, prometryn, and terbutryn) in water samples [53]. For the first time, MEEKC was combined with VSLLME, and the results indicated that the combination could improve the detection sensitivity of MEEKC, while keeping high separation efficiency. Meanwhile, in the developed VSLLME technique, a surfactant was used as an emulsifier to enhance the dispersion of extraction solvent into aqueous sample caused by vortex mixing without any hazardous dispersant solvents.
Conclusions
The VALLME technique, in which the dispersion of the extraction solvent is enhanced by vortex mixing instead of ultrasound irradiation, was devised [10]. The advantages of this approach are that employing VALLME overcomes the difficulties of DLLME, namely the necessity of using a disperser solvent and the problem of possible analyte degradation resulting from the high temperatures and pressures, as well as the free radicals that are generated when using ultrasound; using a vortex for mixing is more cost-effective than an ultrasonic bath and a great deal less expensive than using an ultrasonic probe, and the dispersion formed under vortex-mixing is thermodynamically unstable, which means the extraction phase that contains the target analyte can be easily separated [21].
The initial VALLME [10] was improved by employing a surfactant, so VSLLME was developed [47]. Using a surfactant in this method as an emulsifier enhanced the extraction efficiency and enabled the extraction time to be decreased.
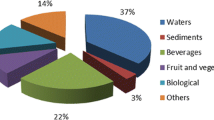
This new and fast sample preparation method termed VALLME has the inherent advantage of achieving equilibrium conditions within only a few minutes. VALLME has thus far proven to be efficient for the rapid extraction of trace amounts of organic pollutants with both high enrichment factors and low detection limits. As can be seen from Fig. 1, the highest number of applications of the VALLME procedure has been in aqueous samples.
The main disadvantage of VALLME has to do with the tested low-density extractants, such as decane, 1-octanol, n-hexane, toluene, and octane. Only 1-octanol can restore its initial single-drop shape after centrifugation and can therefore be effectively collected for analysis. Other tested solvents are left dispersed on the surface of the water and are thus hard to collect.
Abbreviations
- APDC:
-
Ammonium pyrrolidinethiocarbamate
- BPA:
-
Bisphenol-A
- CTAB:
-
Cetylthimethyl ammonium bromide
- CVAFS:
-
Cold vapour atomic fluorescence spectrometry
- DAD:
-
Diode array detection
- DLLME:
-
Dispersive liquid–liquid microextraction
- DNSUAME:
-
Dispersive nano-solid material-ultrasound assisted microextraction
- FAAS:
-
Flame atomic absorption spectrometry
- FLD:
-
Fluorescence detection
- FPD:
-
Flame photometric detection
- GC:
-
Gas chromatography
- GC-FID:
-
Gas chromatography-flame ionization detection
- GC-FPD:
-
Gas chromatography-flame photometric detection
- GC–IT-MS:
-
Gas chromatography-ion trap-mass spectrometry
- GC–MS:
-
Gas chromatography-mass spectrometry
- GFAAS:
-
Graphite furnace atomic absorption spectrometry
- HF-LPME:
-
Hollow fiber liquid-phase microextraction
- HPLC:
-
High performance liquid chromatography
- IL:
-
Ionic liquid
- IL-DMME:
-
Ionic liquid-linked dual magnetic microextraction
- LDS:
-
Low density solvent
- LDS-VSLLME:
-
Low density solvent-vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction
- LMF-DMMLE:
-
Low-density magneto fluid dispersive liquid–liquid microextraction
- LPME:
-
Liquid-phase microextraction
- 2-ME:
-
2-Mercaptoethanol
- MEEKC:
-
Microemulsion electrokinetic chromatography
- MNPs:
-
Magnetic nanoparticles
- MS:
-
Mass spectrometry
- NP:
-
Nonylphenol
- OCPs:
-
Organochlorine pesticides
- OP:
-
Octylphenol
- OPA:
-
o-Phthalaldehyde
- OPPs:
-
Organophosphate pesticides
- PAHs:
-
Polycyclic aromatic hydrocarbons
- PCBs:
-
Polychlorinated biphenyls
- PEs:
-
Phthalate esthers
- PFOS:
-
Perfluorooctane sulfonate
- SDME:
-
Single drop microextraction
- SFODME:
-
Solidified floating organic drop microextraction
- SPE:
-
Solid phase extraction
- UFLC:
-
Ultra-fast liquid chromatography
- US:
-
Ultrasound
- USAEME:
-
Ultrasound assisted emulsification microextraction
- USVADLLME:
-
Ultrasound-vortex-assisted dispersive liquid–liquid microextraction
- US-VALLME:
-
Ultrasound-vortex assisted liquid–liquid microextraction
- VA:
-
Vortex-assisted
- VAE:
-
Vortex-assisted extraction
- VALLME:
-
Vortex-assisted liquid–liquid microextraction
- VSLLME:
-
Vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction
References
Liu H, Dasgupta PK (1996) Anal Chem 68:1817–1821
Jeannot MA, Cantwell FF (1996) Anal Chem 68:2236–2240
He Y, Lee HK (1997) Anal Chem 69:4634–4640
de Jager LS, Andrews ARJ (1999) Chromatographia 50:733–738
Pedersen-Bjergaard S, Rasmussen KE (1999) Anal Chem 71:2650–2656
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116:1–9
Khalili Zanjani MR, Yamini Y, Shariati S, Jonsson JA (2007) Anal Chim Acta 585:286–293
Bosch Ojeda C, Sánchez Rojas F (2009) Chromatographia 69:1149–1159
Bosch Ojeda C, Sánchez Rojas F (2011) Chromatographia 74:651–679
Yiantzi E, Psillakis E, Tyrovola K, Kalogerakis N (2010) Talanta 80:2057–2062
Andruch V, Burdel M, Kocúrová L, Sandrejová J, Balogh IS (2013) Trends Anal Chem 49:1–19
Leng G, Lui G, Chen Y, Yin H, Dan D (2012) J Sep Sci 35:2796–2804
Chang WY, Wang CY, Jan JL, Lo YS, Wu CH (2012) J Chromatogr A 1248:41–47
Zhang L, Chen F, Liu S, Chen B, Pan C (2012) J Sep Sci 35:2514–2519
Sousa TFA, Aniceto MC, Amorin CG, Souto-Lopes M, Pérez-Mongiovi D, Montenegro MCBSM, Araújo AN (2013) Biomed Chromatogr. doi:10.1002/bmc.3089wileyonlinelibrary.com
Wang X, Cheng J, Xiao J, Wang X, Chen M, Cheng M (2013) Anal Methods 5:2034–2040
Lian Y, Qiu X, Yang Y (2013) Food Anal Methods 6:1–9
Trtic-Peteovic TM, Dimitrijevic A (2014) Cent Eur J Chem 12:98–106
Seebunrueng K, Santaladchaiyakit Y, Srijaranai S (2013) Chemosphere. doi:10.1016/j.chemosphere.2013.11.024
Abu-Bakar NB, Makahleh A, Saad B (2014) Talanta 120:47–54
Papadopoulou A, Román IP, Canals A, Tyrovola K, Psillakis E (2011) Anal Chim Acta 691:56–61
Sun XM, Sun Y, Wu LW, Jiang CZ, Yu X, Gao Y, Wang LY, Song DQ (2012) Anal Methods 4:2074–2080
Ge D, Lee HK (2013) J Chromatogr A 1317:217–222
Khodadoust S, Ghaedi M, Hadjmohammadi MR (2013) Talanta 116:637–646
Jia C, Zhu X, Wang J, Zhao E, He M, Chen L, Yu P (2010) J Chromatogr A 1217:5868–5871
Shen Z, He Z, Wang P, Zhou Z, Sun M, Li J, Liu D (2013) Anal Chim Acta 793:37–43
Wu T, Zhao W, Yang Z, Gao H, Zhou Z (2013) J Sep Sci. doi:10.1002/jssc.201300888
Cinelli G, Avino P, Notardonato I, Russo MV (2014) Anal Methods 6:782–790
Ozcan S (2010) Clean Soil Air Water 38:457–465
Zacharis CK, Christophoridis C, Fytianos K (2012) J Sep Sci 35:2422–2429
Ozcan S (2011) J Sep Sci 34:574–584
Zhang Y, Lee HK (2012) J Chromatogr A 1249:25–31
Guo L, Lee HK (2013) J Chromatogr A 1300:24–30
Cinelli G, Avino P, Notardonato I, Centola A, Russo MV (2013) Anal Chim Acta 769:72–78
Lu Y, Zhu Y (2014) Talanta 119:430–434
Lu Y, Zhu Y (2013) J Chromatogr A 1319:27–34
Yu F, Liu C, Guo Y, Yang Y (2014) Spectros Lett. http://dx.doi.org/10.1080/00387010.2013.845576
Monasterio RP, Fernández MA, Silva MF (2013) Electrophoresis 34:1836–1843
Chamsaz M, Eftekhari M, Atarodi A, Asadpour S, Ariani M (2012) J Braz Chem Soc 23:1630–1635
Chamsaz M, Yazdi AS, Dousti F (2013) Asian J Chem 25:7543–7547
Chamsaz M, Atarodi A, Eftekhari M, Asadpour S, Adibi M (2013) J Adv Res 4:35–41
Chamsaz M, Eftekhari M, Eftekhari A, Yekkebashi A (2013) Environ Monit Assess 185:9067–9075
Leng G, Yin H, Li S, Chen Y, Dan D (2012) Talanta 99:631–636
Shah F, Yilmaz E, Kazi TG, Afridi HI, Soylak M (2012) Anal Methods 4:4091–4095
Yilmaz E, Soylak M (2013) Talanta 116:882–886
Zolfonoun E, Salahinejad M (2013) J Radioanal Nucl Chem 298:1801–1807
Yang ZH, Lu YL, Liu Y, Wu T, Zhou ZQ, Liu DH (2011) J Chromatogr A 1218:7071–7077
Yang ZH, Liu DH, Zhao WT, Wu T, Zhou ZQ, Wang P (2013) J Sep Sci 36:916–922
Yang ZH, Wang P, Zhao WT, Zhou ZQ, Liu DH (2013) J Chromatogr A 1300:58–63
Zhang Y, Lee HK (2013) J Chromatogr A 1274:28–35
Leng G, Chen W, Zhang M, Huang F, Cao Q (2013) J Sep Sci. doi:10.1002/jssc.201301033
Vichapong J, Burakham R, Srijaranai S (2013) Talanta 117:221–228
Li RH, Liu DH, Yang ZH, Zhou ZQ, Wang P (2012) Electrophoresis 33:2176–2183
Khan S, Yilmaz E, Kazi TG, Soylak M (2013) Clean Soil Air Water 41:1–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bosch Ojeda, C., Sánchez Rojas, F. Vortex-Assisted Liquid–Liquid Microextraction (VALLME): Applications. Chromatographia 77, 745–754 (2014). https://doi.org/10.1007/s10337-014-2669-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2669-x