Abstract
The objectives of this study were to investigate the molecular mechanisms of retention of selected fungicides and to classify stationary phases used for RP-HPLC. Chromatographic measurements were made using eight physicochemically diverse HPLC columns which were evaluated for analysis of nine biologically active compounds (strobilurins and triazoles). QSRRs were derived for the logarithms of the retention factors normalized to a hypothetical zero percent organic modifier in the mobile phase, log k W. Structural descriptors of the pesticides were used to describe their chromatographic retention behaviour under the given LC conditions. The next step was the correlation between retention data, log k W, and the structural descriptors of the analytes from molecular modelling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that the chromatographic retention factor can be regarded as chemical structure-dependent property which is constant for any analyte under defined separation conditions including mobile phase composition, stationary phase, and temperature [1, 2]. Understanding the chromatographic behaviour of pesticides at a molecular level and the possibility of reliable prediction of the retention factor under given RP-LC conditions can be practically useful in improving confidence in identification of pesticides and increasing the number of correctly identified pesticides in metabolomics analysis [3]. Understanding the chromatographic behaviour of pesticides could also enable comparison of the physicochemical similarities and dissimilarities of the stationary phases used.
Quantitative structure–retention relationships (QSRR), which are the most extensively studied manifestations of linear free-energy relationships (LFER), are statistically derived relationships modelling analyte retention as a function of properties (descriptors) related to analyte molecular structure and the physicochemical properties of both the stationary and mobile phases [4]. By use of QSRR the chromatographic column can be regarded as a “free-energy transducer”, relating the different chemical potentials of solutes resulting from differences in their structures to chromatographic retention [5]. QSRR assist in understanding of the molecular mechanism of separation operating in a given chromatographic system and optimization of this process [6]. QSRR studies can be useful for predicting the retention for a new analyte, and for evaluating properties other than chromatographic physicochemical properties of analytes, for example lipophilicity and dissociation constants. It is possible to use QSRR methods to estimate the relative biological activity of a series of analytes and to identify the most useful (regarding properties) structural descriptors of the analytes [4, 7–9]. This is one method of quantitatively comparing the separation properties of different types of chromatographic column.
The validity of the QSRR approach as a potential tool for optimizing the separation of complex mixtures has been largely proved by accurate prediction of solute retention for many compound classes and different chromatographic systems [4, 10–13]. Further, QSRR studies can significantly contribute to clarification of the molecular mechanism of chromatographic retention [6, 14, 15].
In these studies two main types of QSRR have been used. The first consists in regressing logarithms of retention factors (log k) against the logarithms of n-octanol–water partition coefficients (log P). A variety of RP-HPLC stationary phases have been compared in this way, usually with the objective of identifying chromatographic systems reproducing the n-octanol–water partition system. The second type of QSRR equation describes log k values in terms of quantum chemical structural descriptors from computational chemistry [16]. Special attention has been devoted to investigation of stationary phase–mobile phase–analyte interactions for fungicides. Moreover, an attempt has been made to characterize stationary phase properties on the basis of the mechanism of retention of the compounds analyzed.
The objectives of this work were to investigate the molecular mechanism of retention of two groups of fungicides, strobilurins and triazoles, and to classify different types of stationary phases used for reversed-phase chromatography.
Experimental
Equipment and Chromatographic Measurements
The retention factors of the fungicides were investigated by using HPLC equipment comprising pumps (CM 3500 and 3200), a variable wavelength diode-array detector (DAD; SM 5000), and a manual injection valve (TSP, Riviera Beach, FL, USA). LCtalk HPLC version 2.03.02 (TSP) was used for data collection.
Two other high-performance liquid chromatography systems were equipped with a pump (Varian 9012), a variable wavelength UV–visible detector (Gynkotek HPLC a Softron Company, Germering, Germany), an autosampler (Alcott 718Al, Alcott Chromatography, Norcross, GA, USA), and Clarity Station software (DataApex, Praha, Czech Republic) for data collection and instrument control.
HPLC Columns
Chromatographic measurements were performed with the columns listed in Table 1 .
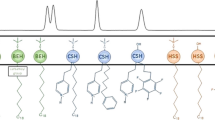
The structures of these stationary phases are illustrated schematically in Fig. 1.
HPLC analysis was performed at room temperature under isocratic conditions. The retention behaviour of the analytes was investigated as a function of mobile phase composition: ranging from 30 to 90% (v/v) acetonitrile in water in steps to 10%. The “dead time” (t 0) of each column was measured by injecting acetonitrile into the system. The flow rate was 1 mL min−1 (except for the PGC and Synergi columns—0.5 mL min−1). The experiments were performed at a detection wavelength of 215 nm. Fungicide samples were dissolved in acetonitrile–water mixture (50:50 v/v).
Chemicals
Pesticide standards were obtained from Department of Pesticide Residues, Plant Protection Institute, Poznań, Poland. Acetonitrile (ACN; HPLC-grade) was from Sigma–Aldrich (Steinheim, Germany) and from Merck (Darmstadt, Germany). Water was prepared with a Milli-Q water-purification system (Millipore, Bedford, MA, USA). The structures of the fungicides analyzed, all from the strobilurin and triazole groups, are illustrated in Fig. 2.
Structural Descriptors of Analytes
The logarithms of the n-octanol–water partition coefficients (log P) for test analytes can be found in the literature (http://www.alanwood.net/pesticides/class_fungicides.html). Quantum chemical and standard additive/constitutive structural descriptors of the fungicides were generated by use of the molecular modelling software HyperChem (HyperCube, Waterloo, Canada). The following properties were significant in QSRRs: molecular mass, square of total dipole moment (μ 2), highest electron excess on a single atom in analyte molecule (δ min), and solvent (water)-accessible molecular surface area (SAS). The software performed geometry optimization by the semi-empirical method (AM1).
The structural descriptors of the test analytes considered in the final QSRR equations are listed in Table 2.
Data Analysis
QSRR equations were derived by Excel from Microsoft Office 2007 and by means of multiple regression analysis (MRA) by use of Statistica software (StatSoft, Tulsa, USA) run on a personal computer. Regression coefficients (R 2), standard errors of the estimate (s), the values of the F test of significance (F), and significance levels of each term and the whole equations (p) were calculated.
Results and Discussion
The main objective of the study was comparison of analytical columns on the basis of retention of the fungicides.
Changes in RP-LC retention data accompanying changes in mobile phase composition are usually in good agreement with the linear solvent strength (LSS) model of Soczewinski–Wachtmeister [17–19]. The equation of this model has the form:
where log k W is the value of log k extrapolated to 100% water as mobile phase, that is, to φ = 0, and S is a constant characteristic of a given analyte and a given RP-LC system.
In this model, the logarithm of the retention factor for a given analyte, log k, is related to the volume fraction of organic modifier in the binary aqueous mobile phase. For QSRR studies, the retention data used correspond to a given mobile phase composition. In RP-LC, it is normally log k W which corresponds to pure water as the hypothetical mobile phase [14]. Extrapolation to pure water is a convenient means of standardization of chromatographic hydrophobicity.
It was decided to consider log k W data instead of individual isocratic log k data in the QSRR studies. log k W is basically an abstract quantity that is more reliable than any arbitrarily selected isocratic log k. Experimental values of log k W for the nine fungicides on eight modern reversed-phase columns with acetonitrile as organic modifier in aqueous mobile phase are listed in Table 3.
Finally, the effect of the structural properties of the molecules on their retention factors was investigated by QSRR analysis. Multiple linear regression was used to find the quantitative relationship between the calculated structural descriptors of test analytes (Table 2) and the retention data (Table 3).
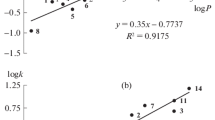
One of the most frequently applied QSRR equations, and the simplest, is the relationship between retention data and the partition coefficients of substances. Usually logarithms of the n-octanol–water partition coefficient (log P) is used [4, 20]. The correlated data are shown in Fig. 3.
Partition of a compound between aqueous and organic phases determines both permeation through biological membranes and retention in reversed-phase liquid chromatography. Therefore, QSRR might be used for assessment of the hydrophobicity of xenobiotics (in this example pesticides). It is known there is almost a correlation between log k W and n-octanol–water partition coefficient, log P. Dissimilarities between the two partition systems are evident. The log k W data determined in this work were linearly regressed against log P of the test analytes. The coefficients k 1 and k 2 (SD) of the regression equations of the general form:
and are listed in Table 4 with the numbers of data points used to derive the regressions (n), the correlation coefficients (R 2) and the standard errors of the estimate (s), the values of the F-test of significance (F), and significance of the whole equations (p).
Table 4 shows that all the log k W data sets correlate significantly with log P (except for SG-CN). The high log k W versus log P correlations confirm the general similarity of the n-octanol–water partition system and the partition chromatographic system. Taking the above values of the regression coefficients k 2 into account and considering the acetonitrile–water system, the stationary phases can be well ordered in accordance with their decreasing hydrophobicity (Fig. 4). This regression coefficient reflects the extent to which the analyte is surrounded by the stationary phase.
Slopes (k 2 values from Table 1) of the log k W versus log P relationships for the nine columns tested. The log k W data considered were determined by use of acetonitrile–water mobile phases
To summarize, from Fig. 4 and Table 4 the most hydrophobic (in log P terms) columns seem to be PGC and Puro C18e. The least lipophilic column is SG-CN. The other columns have similar hydrophobic properties.
The next step was correlation of retention data, log k W , with structural descriptors of the analytes from molecular modelling (total dipole moment μ, electron excess charge of the most negatively charged atom δ min, water-accessible molecular surface area SAS). This model was previously developed by Kaliszan and co-workers [7, 16, 20]. The QSRR equation using structural data from molecular modelling of the analytes is:
The coefficients k 1, k 2, k 3, and k 4 (SD) are listed in Table 5, with the numbers of data points used to derive the regressions (n), the correlation coefficients (R 2) and standard errors of the estimates (s), the values of the F test of significance (F), and the significance levels (p) of each term and the whole equations.
Most of the regression coefficients are statistically significant and, even more important, all the equations are statistically significant. However, the multiple correlation coefficients (R 2) for the Supelco C18 and SG-Chol columns are more statistically significant than those for the other columns. All equations obtained are acceptable statistically and for the purpose of comparing columns. It must, however, be noticed that for all the columns except SG-CN the regression coefficients for the SAS were not statistically significant. However, the physical meaning of the equations characterized in Table 5 is clear. Only for the SG-CN column is there a net negative input to retention from the SAS, which is evidently related to the ability of the analytes to participate in dispersive interactions. These attractive London-type interactions are stronger between an analyte and the small molecules of the mobile phase than between the same analyte and the bulky ligand of the stationary phase. The k 4 regression coefficient is therefore negative. The effects on retention of specific polar intermolecular interactions are reflected by the coefficients k 2 and k 3 (Table 5) for all the columns. Coefficient k 2 proves that the net effect on retention of the moment dipole, μ, is negative. This seems reasonable, because the dipole–dipole and dipole-induced dipole attractions are stronger between an analyte and the polar molecules of the mobile phase than between the same analyte and the polar ligands of the stationary phase. An analogous explanation is valid for coefficient k 3 (Table 5). The coefficients k 2 (Table 5) differentiate the phases from each other well. According to k 2, the most polar of the phases studied is SG-CN (Table 1). The Lichro C18, Supelco C18, and SG-Chol are less polar. The least polar are PGC, Puro C18e, Synergi, and Gemini C18.
Intercorrelations were checked between log k W for the Puro C18e column and the retention data for the test solutes determined on the five columns: SG-CN, PGC, SupelcoC18, Gemini C18, and SG-Chol (Fig. 5). The Puro C18e column was selected as the reference column because it contained the most hydrophobic bonded stationary phase, because of replacement of accessible silanol groups by trimethylsilyl groups (endcapping). Correlations between log k W (Puro C18) and SG-Chol, Gemini C18, and Supelco C18 are evidently higher than for PGC and SG-CN. This confirms the expected similarity of the hydrocarbonaceous phases octadecyl-silica and cholesterol-silica.
Similarities and dissimilarities of the LC stationary phases were considered by principal-components analysis. All the experimental log k W data for the nine solutes used for the column comparison are listed in Table 3. These values were used for statistical evaluation. According to the eigenvalue-one criterion, only principal components (PCs) with eigenvalues greater than unity are regarded as important. This criterion is based on the fact that the average eigenvalue of the autoscaled data is just unity. The scree-plot shows that only two factors fulfilled this criterion. As is apparent from Table 6, most information about individual column properties (more than 99% of total data variance) can be described by the two first PCs.
Principal-component loadings are presented in Fig. 6 and correspond to the correlation coefficient of the particular variable. The main differentiating loading is the PC1 which goes from negative to positive values from PGC to Nucleosil CN, and is approximately zero for the other columns. This can be explained by considering the structures of the phases enlightened in Fig. 1 and reported in Table 1. The fact that these two phases have close values for PC2 is less significant, because PC2 explains only 1.68% of the variance compared with 97.54% explained by PC1 (Table 6). Those packing materials seem to be different from the others.
Projection of the eight HPLC columns in the space of the first two PCs reveals six of the columns form a group, i.e. PCA of the retention data reveals the very close similarity of the Puro C18e, Synergi, Gemini C18, Lichro C18, Supelco C18, and SG-Chol columns in comparison with the other columns. In this cluster one can, moreover, find two groups: Puro C18e, Synergi, Gemini C18, and Lichro C18, and Supelco C18 and SG-Chol, in which the similarity between columns is even stronger. This assumes the similarity is connected with the hydrophobic character of the columns. It can be concluded that the first component describes the non-polar properties of the fungicides that are able to interact by hydrophobic (e.g. van der Waals) interactions during chromatographic analysis. This can explain the linear relationship between the above mentioned columns. Retention of hydrophobic compounds increases with increasing hydrophobic character of the stationary phase (from Puro C18e to Supelco C18). The most hydrophobic column seems to be Puro C18e, because in this bonded stationary phase accessible silanol groups are replaced by trimethylsilyl groups (endcapping). The Synergi column has octadecyl ligands and embedded polar groups, although the charges on these groups are of no significance for retention of the tested analytes (long alkyl chains shield the charge on the bonded unable groups; Fig. 1). Supelco C18 is not an endcapped column, so its hydrophobic character is less than for the other columns in this cluster, because of the presence of accessible silanol groups on the surface of the stationary phase. The least hydrophobic in this cluster seems to be SG-Chol. It contains cholesterol ligands on the silica matrix surface, which have a shielding effect, and another groups which provide specific inputs to retention because of their polar fragments. It is observed that the SG-CHOL column has intermediate properties and behaves like pseudo-membrane layer.
Conclusions
The QSRR models enable rational interpretation of differences and/or similarities in the molecular mechanisms of chromatographic separation among the HPLC systems. The models can be of help in objective comparison of the separation properties of the stationary phases. Two QSRR models relating standardized retention data obtained on eight modern reversed-phase materials demonstrated the lack of substantial differences in the molecular mechanism of separation.
The objective of use of QSRR analysis for comparison of HPLC stationary phases was to enable objective, quantitative and physical interpretation of results. The biologically active fungicides investigated belong to groups of compounds whose properties make characterization of stationary phases possible. Investigation of the fungicides on a given stationary phase type as a function of different molecular descriptors enables description of individual packing material properties. This combination makes it possible to compare the properties of all the packing materials used in this study. This kind of measurement also enables description of analyte (fungicides) retention mechanisms.
References
Kaliszan R, Baczek T, Cimochowska A, Juszczyk P, Wisniewska K, Grzonka Z (2005) Proteomics 5:409–415
Michel M, Baczek T, Studzinska S, Badzioch K, Jonsson T, Kaliszan R, Buszewski B (2007) J Chromatogr A 1175:49–54
Kaliszan R (1992) Anal Chem 64:619A–631A
Kaliszan R (2007) Chem Rev 107:3212–3246
Romanelli GP, Autino JC, Castro EA (2002) Turk J Chem 26:335–343
Szepesy L (2002) J Chromatogr A 960:69–83
Baczek T, Kaliszan R (2002) J Chromatogr A 962:41–55
Jiskra J, Claessens HA, Cramers CA, Kaliszan R (2002) J Chromatogr A 977:193–206
Baczek T, Kaliszan R, Novotna K, Jandera P (2005) J Chromatogr A 1075:109–115
Ledesma EB, Wornat MJ (2000) Anal Chem 72:5437–5443
Jakab A, Schubert G, Prodan M, Forgacs E (2002) J Chromatogr B 770:227–236
Aschi M, D’Archivio AA, Maggi MA, Mazzeo P, Ruggieri F (2007) Anal Chim Acta 582:235–242
Heberger K (2007) J Chromatogr A 1158:273–305
Kaliszan R (1987) Quantitative structure-chromatographic retention relationships. Wiley, New York
Al-Haj MA, Kaliszan R, Nasal A (1999) Anal Chem 71:2976–2985
Kaliszan R, van Straten MA, Markuszewski M, Cramers CA, Claessens HA (1999) J Chromatogr A 855:455–486
Soczewinski E, Wachtmeister CA (1962) J Chromatogr A 7:311–320
Valko K, Snyder LR, Glajch JL (1993) J Chromatogr A 656:501–520
Snyder LR, Dolan JW (1998) Adv Chromatogr 38:115–185
Al-Haj MA, Kaliszan R, Buszewski B (2001) J Chromatogr Sci 39:29–38
Acknowledgments
This work is supported by the European Social Fund, the Polish National Budget, and the Kujawsko-pomorskie Vovidship Budget—“Stypendia dla doktorantów 2008/2009-ZPORR”. Financial support from Foundation for Polish Science Professor’s Subsidy “Mistrz” is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noga, S., Michel, M. & Buszewski, B. Effect of Functionalized Stationary Phases on the Mechanism of Retention of Fungicides in RP-LC Elution. Chromatographia 73, 857–864 (2011). https://doi.org/10.1007/s10337-011-1931-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-011-1931-8