Abstract
A novel approach for the determination of six fungicides (triadimefon, procymidone, hexaconazole, myclobutanil, diniconazole and iprodione) in fruit samples is presented. Analytes were extracted using the dispersive liquid–liquid microextraction technique and determined by GC–ECD. Parameters affecting the dispersive liquid–liquid microextraction performance, such as the kind and volume of extraction and dispersive solvents, extraction time and salt concentration, were studied and optimized. Under the optimum extraction conditions, the linearities of the method were obtained in the range of 0.5–20.0 μg kg−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 1.0–40.0 μg kg−1 for myclobutanil and iprodione, with the correlation coefficients ranging from 0.9902 to 0.9995. The enrichment factors ranged from 685 to 820 and the extraction recoveries ranged from 81.3 to 98.4%. The relative standard deviations varied from 3.1 to 7.8%. The limits of detection of the method were in the range of 0.02–0.12 μg kg−1. Results showed that the method we proposed can meet the requirements for the determination of target fungicides in fruit samples. Several compounds considered in this study were found in fruit samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungicides represent one of the most relevant groups of pesticides applied to orchards. These compounds are sprayed directly on fruit and leaves to prevent the attack of fungi, which reduce the yield of fruit [1]. Some species of fungicides have been widely used as fruit preservatives in the storage and transport process. However, residues of these compounds were believed to be one of the most important pollution sources in food production and might pose potential threat to public health. Thus, a simple, rapid and sensitive method to evaluate and monitor these compounds, at trace levels, in fruit matrices is required.
Sample preparation plays an important role in the field of pesticide residue analysis. Solid phase extraction (SPE) is the most common method of extracting fungicides and renders high extraction yields [2, 3]. However, the SPE technique is time-consuming, labor-intensive and expensive. Solid phase microextraction (SPME) has been also applied to the determination of fungicides [4–6]. Although SPME normally provides a higher selectivity than SPE, the matrix of sample reduces significantly its extraction efficiency [7, 8]. These limitations are also common to stir-bar sorptive extraction (SBSE), the applicability of which is restricted to low polar fungicides, showing a high affinity for the polydimethylsiloxane sorbent [9]. Some of these drawbacks have been overcome by the dispersive liquid–liquid microextraction (DLLME) technique, first introduced by Assadi et al. [10]. This method consists of two steps: (1) The first is the injection of an appropriate mixture of extraction and disperser solvent into aqueous sample containing analytes. In this step the extraction solvent was dispersed into the aqueous sample as very fine droplets and analytes were enriched into it. Because of the infinitely large surface area between extraction solvent and aqueous sample, the equilibrium state was achieved quickly and extraction was independent of time. (2) The second step is the centrifugation of cloudy solution: After centrifugation, the determination of analytes in sedimented phase can be performed by instrumental analysis. Rapidity, high enrichment factor, simplicity of operation and low cost are some of the advantages of this method. Since its introduction, DLLME has been used for the determination of chlorobenzenes [11], chlorophenols [12], phenols [13], organophosphorus and plasticizers [14], triazine herbicides [15], amitriptyline and nortriptyline [16], polybrominated diphenyl ethers [17], anilines [18] and halogenated organic compounds [19] in liquid samples. To the best of our knowledge, there were only three reports on extraction of pesticides from fruit samples using DLLME procedure [20–22].
However, none of the published papers have reported the determination of fungicides in fruit samples by DLLME. In this study, the selected fungicides (triadimefon, procymidone, hexaconazole, myclobutanil, diniconazole and iprodione) were recommended by the Chinese Agricultural Ministry and have been extensively applied to fruits in mainland China. The aim of the present work was to assess the suitability of DLLME coupled with GC–ECD for the determination of these six fungicides in four kinds of fruits. In addition, the effects of different parameters on the efficiency of DLLME method were investigated.
Experimental Method
Standards and Reagents
Triadimefon, hexaconazole, myclobutanil, diniconazole, procymidone and iprodione were supplied from the Agro-Environmental Protection Institute of Ministry of Agriculture (Tianjin, China). The working standard solution was prepared daily by appropriate dilution of the stock solution. Carbon tetrachloride (CCl4), tetrachloroethane (C2H2Cl4), chlorobenzene (C6H5Cl), chloroform (CHCl3), acetonitrile, acetone and tetrahydrofuran were of HPLC grade. Pear, strawberry, apple and grape samples were purchased from local supermarkets.
Instrumentation
The instrumental analysis was conducted with an Agilent Mode 6890N GC system equipped with electron capture detection (ECD) system. Agilent Chemstation was used for instrument control and data analysis. Analytes were separated on an Agilent DB-1 type capillary column (30 m × 0.32 mm i.d., df 0.25 μm, Agilent Technologies) operated at a constant nitrogen flow of 0.8 mL min−1. The oven temperature was programmed as follows: started at 120 °C, first ramp at 10 °C min−1 until 200 °C, then ramp at 0.5 °C min−1 to 210 °C, finally ramp at 5 °C min−1 to 220 °C and held for 2 min. Injector and detector were set at 250 and 280 °C, respectively. The total analytical time was 32 min. The identification of the analytes was confirmed based on the retention time.
Sample Preparation
As much as 200 g of fruit sample was homogenized by a food processor. Then 20 g of previously homogenized sample was weighed and transferred into centrifuge tubes. After centrifugation for 5 min at 12,000 rpm, the supernate was filtered through a 0.45-μm membrane into a 10-mL volumetric flask with doubly distilled water to the volume for the DLLME procedure.
DLLME Procedure
A 5.0-mL of fruit sample solution previously obtained was placed in a 10-mL glass test tube with conical bottom, and 1% (w/v) of sodium chloride (NaCl) was added to the glass tube. The organic solution containing 0.8 mL acetonitrile as dispersive solvent and 14.0 μL C2H2Cl4 as extraction solvent was rapidly injected into the sample solution. Then the sample was gently shaken for 30 s, and a cloudy solution was formed in the test tube. In this step, the analytes in sample solution were extracted into the fine droplets of C2H2Cl4 rapidly. In order to separate the organic phase from the aqueous phase, the sample was centrifuged for 5 min at 3,200 rpm. After this process, the dispersed fine droplets of C2H2Cl4 were sedimented at the bottom of the test tube. The sedimented phase (6 ± 0.5 μL) was transferred to a small sample vial and 1 μL of sedimented phase was injected to GC for further instrument analysis.
Results and Discussion
Optimization of Dispersive Liquid–Liquid Microextraction
The effects of various experimental parameters were studied and optimized, including the kind and volume of extraction and dispersive solvent, extraction time and salt addition. To evaluate the extraction efficiency under different conditions, extraction recovery and enrichment factor were used. Equations 1 and 2 were used for calculation of enrichment factor (EF) and extraction recovery (R).
where C sed, C 0, V sed and V 0 are the concentration of analyte in sedimented phase, initial concentration of analyte in aqueous sample, volume of sedimented phase and volume of aqueous sample, respectively.
Selection of Extraction Solvent
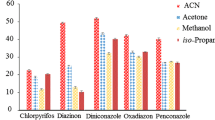
Extraction solvents were selected on the basis of higher density than water, extraction capability of interested compounds, immiscibility with water but miscibility in the dispersive solvent and good gas chromatography behavior. Based on these considerations, C6H5Cl, CCl4, C2H2Cl4 and CHCl3 were selected as potential extraction solvents. A series of sample solutions was tested by using 0.8 mL of acetonitrile containing different volumes of extraction solvent to achieve 10.0 μL volume of sediment phase. Thereby, 18.0, 25.0, 28.0 and 32.0 μL of C2H2Cl4, CCl4, C6H5Cl and CHCl3 were used, respectively. As shown in Fig. 1, C2H2Cl4 had the highest extraction efficiency in comparison to the other tested solvents. Consequently, C2H2Cl4 was selected as the optimal extraction solvent.
Effect of different extraction solvents on enrichment factors of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; dispersive solvent (acetonitrile) volume, 0.8 mL; sedimented phase volume, 10 ± 0.5 μL; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Selection of Dispersive Solvent
Miscibility of dispersive solvent in organic phase and aqueous phase is the main point for selection of dispersive solvent. Accordingly, acetonitrile, acetone, methanol and tetrahydrofuran were evaluated for this purpose. Figure 2 indicates the enrichment factors using acetonitrile, acetone and tetrahydrofuran as dispersive solvents. According to the results, acetonitrile was chosen as dispersive solvent in this work.
Effect of different dispersive solvents on enrichment factors of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; extraction solvent (C2H2Cl4) volume, 14 μL; dispersive solvent volume, 0.8 mL; sedimented phase volume, 6.0 ± 0.5 μL; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Effect of Extraction Solvent Volume
To examine the effect of extraction solvent volume, 0.8 mL of acetonitrile containing different volumes of C2H2Cl4 (14.0, 18.0, 22.0 and 26.0 μL) was subjected to the same DLLME procedures. Figures 3 and 4 present the plots of extraction recoveries and enrichment factors versus volume of extraction solvent C2H2Cl4, respectively. It was obvious that extraction recoveries for most of the analytes varied slightly, but enrichment factors decreased by increasing the volume of C2H2Cl4. As a consequence, 14.0 μL C2H2Cl4 was selected to obtain high enrichment factor, good recovery and low detection limit in the subsequent experiments.
Effect of different volumes of C2H2Cl4 on extraction recoveries of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; dispersive solvent (acetonitrile) volume, 0.8 mL; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Effect of different volumes of C2H2Cl4 on enrichment factors of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; dispersive solvent (acetonitrile) volume, 0.8 mL; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Effect of Dispersive Solvent Volume
To obtain optimized volume of acetonitrile, 0.3, 0.5, 0.8, 1.0 and 1.2 mL of acetonitrile containing the corresponding volume of C2H2Cl4 were studied to attain the constant volume of the sedimented phase (6.0 ± 0.5 μL). As seen in Fig. 5, enrichment factors increased with the increase of volume of acetonitrile when it was less than 1.0 mL, but decreased after the volume of acetonitrile exceeded 1.0 mL. Therefore, 1.0 mL was chosen as the optimum volume of the dispersive solvent.
Effect of different volumes of acetonitrile on enrichment factors of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; extraction solvent (C2H2Cl4) volume, 14 μL; sedimented phase volume, 6.0 ± 0.5 μL; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Effect of Extraction Time
The effect of extraction time was examined in the range of 3–30 min with constant experimental conditions. According to Fig. 6, the variations in enrichment factors were not remarkable. It was revealed that after formation of a cloudy solution, the surface area between extraction solvent and fruit sample phase was infinitely large. Thereby, transition of analytes from fruit sample phase to extraction solvent was fast. Subsequently, equilibrium state was established rapidly so that the extraction time was very short. In this study, 5 min was suitable for the procedure.
Effect of extraction time on enrichment factors of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; extraction solvent (C2H2Cl4) volume, 14 μL; dispersive solvent (acetonitrile) volume, 0.8 mL; sedimented phase volume, 6.0 ± 0.5 μL; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Effect of Salt Concentration
The salting-out effect is an important parameter in DLLME. Generally, addition of salt decreases the solubility of target compounds in the aqueous sample and enhances their partitioning into the organic phase. For investigating the influence of ionic strength on extraction efficiency of DLLME, various experiments were performed by adding different amounts of NaCl (0–10%, w/v). The increase in ionic strength led to a decrease in C2H2Cl4 solubility in aqueous phase, which increased the volume of sedimented phase but decreased the enrichment factor. As seen in Figs. 7 and 8, enrichment factors decreased when the salt addition exceeded 1%, but extraction recoveries were almost constant. As a result, a salt concentration of 1% was utilized.
Effect of salt addition on enrichment factors of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; extraction solvent (C2H2Cl4) volume, 14 μL; dispersive solvent (acetonitrile) volume, 0.8 mL; sedimented phase volume, 6.0 ± 0.5 μL; extraction time, 5 min; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Effect of salt addition on extraction recoveries of six fungicides obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; extraction solvent (C2H2Cl4) volume, 14 μL; dispersive solvent (acetonitrile) volume, 0.8 mL; sedimented phase volume, 6.0 ± 0.5 μL; extraction time, 5 min; concentration of each pesticide, 2 ng mL−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 4 ng mL−1 for iprodione and myclobutanil
Analytical Performance
To investigate the applicability of the proposed method, several factors including enrichment factor, linearity range and limit of detection were evaluated. The performance of this technique is shown in Table 1. Linearities of calibration curves were observed at the range of 1.0–40 μg kg−1 for myclobutanil and iprodione, and 0.5–20 μg kg−1 for the other four kinds of fungicides, with correlation coefficients in the range of 0.9902–0.9995. The limits of detection, based on signal-to-noise ratio (S/N) of 3, ranged from 0.02 to 0.12 μg kg−1, which was very low using ECD. The enrichment factors ranged from 685 to 820. The extraction recoveries were evaluated by extracting the spiked fruit samples at two different concentration levels of six fungicides. Each treatment was carried out five times successively. The results are presented in Table 2. Acceptable recoveries and repeatability could be obtained. These results demonstrated that the different fruit matrices used in this experiment had little effect on DLLME efficiency. Chromatograms of non-spiked and spiked pear sample obtained by using DLLME are shown in Fig. 9.
Chromatogram of (a) non-spiked and (b) spiked pear sample by using DLLME combined with GC–ECD under optimized conditions. Peak identification: (1) triadimefon, (2) procymidone, (3) hexaconazole, (4) myclobutanil, (5) diniconazole, (6) iprodione. Spiked concentration level: 0.5 μg kg−1 for triadimefon, hexaconazole, diniconazole and procymidone, and 1 μg kg−1 for iprodione and myclobutanil
Real Fruit Sample Analysis
Three batches of apple, grape, pear and strawberry were collected from different local supermarkets. The samples were pretreated as described in “Sample preparation”, extracted using DLLME method, followed by “DLLME procedure” and analyzed by GC–ECD. The results are summarized in Table 3. It is revealed that the recommended method could be applied for the trace analysis of selected fungicides in real fruit samples.
Conclusion
In this research, DLLME combined with GC–ECD has been applied to the simultaneous determination of selected fungicide residues in fruit samples. Compared with other extraction methods, DLLME is a very rapid, simple and inexpensive pretreatment technique with lower consumption of organic solvent. To our knowledge, this is the first report on the application of DLLME for analysis of fungicides in fruit samples, and the present study broadens the applicability of the DLLME technique to more complicated matrices successfully. The results of this study demonstrate that the proposed method provides high enrichment factor, low limit of detection, and acceptable extraction recovery and repeatability. Performance of the proposed method fits the requirements for the determination of selected fungicides in real fruit samples.
References
Rial-Otero R, Cancho-Grande B, Simal-Gándara J (2003) J Chromatogr A 992:121–131
Wu L, Chen MX, Mou RX, Ying XH, Cao ZY (2009) Chin J Instrum Anal 11:351–356
Xu GF, Nie JY, Li J, Li HF (2009) Chin J Pesticide Sci 28:846–848
Correia M, Delerue-Matos C, Alves A (2001) Fresenius J Anal Chem 369:647–651
Zambonin CG, Cilenti A, Palmisano F (2002) J Chromatogr A 967:255–260
Urruty L, Montury M, Braci M, Fournier J, Dournel JM (1997) J Agric Food Chem 45:1519–1522
Urruty L, Montury M (1996) J Agric Food Chem 44:3871–3877
Lambropoulou DA, Konstantinou IK, Albanis TA (2000) J Chromatogr A 893:143–156
Viñas P, Aguinaga N, Campillo N, Hernández-Córdoba M (2008) J Chromatogr A 1194:178–183
Rezaee M, Assadi Y, Millani MR, Aghaee E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116:1–9
Rahnama-Kozani R, Assadi Y, Shemirani F, Milani-Hosseini MR, Jamali MR (2007) Talanta 72:387–393
Fattahi N, Assadi Y, Milani-Hosseini MR, Zeini-Jahromi E (2007) J Chromatogr A 1157:23–29
Fariña L, Boido E, Carrau F, Dellacassa E (2007) J Chromatogr A 1157:46–50
García-López M, Rodríguez I, Cela R (2007) J Chromatogr A 1166:9–15
Nagaraju D, Huang SD (2007) J Chromatogr A1161:89–97
Yazdi AS, Razavi N, Yazdinejad SR (2008) Talanta 75:1293–1299
Li Y, Wei G, Hu J, Liu X, Zhao X, Wang X (2008) Anal Chem 615:96–103
Chiang JS, Huang SD (2008) Talanta 75:70–75
Leong MI, Huang SD (2008) J Chromatogr A 1211:8–12
Zhao E, Zhao W, Han L, Jiang S, Zhou Z (2007) J Chromatogr A 1175:137–140
Fu LY, Liu XJ, Hu J, Zhao XN, Wang HL, Wang XD (2009) Anal Chim Acta 632:289–295
Zhou X, Zang XH, Wang DY, Cui PL, Wang Z (2009) Chin J Anal Chem 37:41–45
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huo, X., Li, Q., Lin, X. et al. Application of Dispersive Liquid–Liquid Microextraction for the Analysis of Six Fungicides in Fruit Samples by GC–ECD. Chromatographia 73, 313–319 (2011). https://doi.org/10.1007/s10337-010-1875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-010-1875-4