Abstract
Sexually and socially selected signals are predicated to express the present and past condition of individuals and thereby their capacity to cope with environmental challenges. The concentration of corticosterone in feathers (CORTf) has been validated as a marker of activation of the hypothalamic-pituitary axis in birds during feather development. Measurements of CORTf can thus express the physiological stress of migratory birds during non-breeding periods of the annual cycle when feathers moulted in the wintering range are analysed. Thus, negative trends of ornament expression with CORTf may be predicted. Unpigmented plumage patches may constitute signals of phenotypic quality and are used in many species in social interactions by both sexes. Pied Flycatchers of both sexes Ficedula hypoleuca show white forehead and wing patches during the breeding season, of which the forehead and part of the wing patch (tertials) are moulted on the wintering grounds before spring migration. These patches are used as signals in social and sexual interactions and their expression has been previously related to several indicators of physiological status. We collected tertials of females during nestling provisioning in a Spanish population and determined their CORTf in the laboratory. CORTf was thus measured in some of the prenuptially moulted feathers used as signals of social dominance by breeding females. According to AICc model selection, CORTf turned up in all the best models explaining forehead patch size and in most best models for wing patch size when controlling for age and size. Both forehead and wing patch sizes were negatively related to CORTf, although the trend for wing patch size was not significant. This indicates that breeding females expressing large unpigmented patches experienced low levels of stress during the prenuptial moult in their winter quarters, or that strongly signalling individuals present a lower level of activation of the HPA axis.
Zusammenfassung
Das Ausmaß der weißen Flecken im Gefieder von weiblichen Trauerschnäppern (Ficedula hypoleuca) korreliert negativ mit der Kortikosteronkonzentration in teilweise pigmentfreien Federn.
Soziale und für das jeweilige Geschlecht selektierte Signale sollen die gegenwärtige und vorangegangene Verfassung von Individuen anzeigen und damit ihre Fähigkeit, mit den Herausforderungen der Umwelt fertigzuwerden. Die Konzentration von Kortikosteron in den Federn (CORTf) wurde bei Vögeln während der Federentwicklung als Marker für die Aktivierung der Hypothalamus-Hypophysen-Achse validiert. Messungen des CORTf können daher den physiologischen Stress von Zugvögeln im Jahreszyklus außerhalb der Brutzeiten ausdrücken, wenn man im Überwinterungsgebiet bei der Mauser ausgefallene Federn analysiert. Somit können möglicherweise negative Trends im gesamten Federkleid vorhergesagt werden. Unpigmentierte Stellen im Gefieder können Signale der phänotypischen Qualität darstellen und werden bei vielen Arten in sozialen Interaktionen von beiden Geschlechtern verwendet. Trauerschnäpper (Fidecula hypoleuca) beider Geschlechter zeigen während der Brutzeit weiße Stirn- und Flügelflecken; die Federn an Stirn und Teilen der Flügel mausern im Winterquartier vor Beginn des Frühjahrszuges. Die Flecken werden in sozialen Interaktionen und zwischen den Geschlechtern als Signal eingesetzt, und ihr Ausprägungsgrad wurde bislang mit mehreren Indikatoren für die physiologische Verfassung in Verbindung gebracht. Wir sammelten die Federn von Weibchen einer spanischen Population während des Nestbaus und bestimmten deren CORTf im Labor. Somit wurde CORTf in Federn aus der Mauser vor der Begattung gemessen, die von brütenden Weibchen als Zeichen ihrer sozialen Dominanz benutzt werden. Bei der Prüfung des Alters und der Körpergröße tauchte unter den AICc-Modellen die CORTf zur Erklärung der Größe des Stirnflecks in allen bestgeeigneten Modellen auf, zur Erklärung der Größe der Flügelflecken in den meisten bestgeeigneten Modellen. Die Größe sowohl der Stirn- als auch der Flügelflecken korrelierte negativ mit CORTf, letztere allerdings nicht signifikant. Dies deutet darauf hin, dass brütende Weibchen mit großen pigmentfreien Flecken während der ersten Mauser im Winterquartier einem niedrigen Stresslevel ausgesetzt waren, oder dass starke Signale aussendende Einzeltiere ein niedrigeres Aktivitätsniveau der Hypothalamus-Hypophysen-Achse aufweisen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexually and socially selected signals are predicated to express the present and past condition of individuals and thereby their capacity to cope with environmental challenges (Andersson 1994; West-Eberhard 1979). Glucocorticoid hormones mediate relationships between an organism and its environment and their activity could be related to signaling strength. The main glucocorticoid in birds, corticosterone (CORT), has been related to the capacity to sustain environmental and nutritional stress (Wingfield 2013; Sapolsky et al. 2000). Potential or actual adverse environmental conditions requiring reallocation of resources or functions to immediate survival usually result in activation of the hypothalamic-pituitary axis (HPA) and thus in elevated CORT (Wingfield 2013; but see Dickens and Romero 2013). Usually CORT has been measured in plasma of wild birds immediately or sometime after capture (Sapolsky 1982; Wingfield et al. 1982; Wingfield and Romero 2001), although concentration in faeces and other biological materials including feathers has also been estimated (e.g.,Möstl et al. 2005; Bortolotti et al. 2008). Measuring corticosterone in feathers (CORTf) has become a useful method for studying responses to environmental conditions during moult (Romero and Fairhurst 2016), although some problems have been noted with this technique (e.g., Harris et al. 2016). CORTf has been experimentally shown to be the best feather steroid marker for detecting nutritional stress in young (Will et al. 2019) and adult birds (Grunst et al. 2015). In general, CORTf has shown negative associations with fitness-related traits (Harms et al. 2015; Lodjak et al. 2015; Mougeot et al. 2016).
CORTf denotes conditions during moult of ornamental plumage in conditions which may be similar or different from the mating period when signals are exhibited (Jenkins et al. 2013). This variation may relate to migratory strategy and environmental variability in relation to timing of moult. In general, a poor condition during moult may imply impaired ability to cope with the environment at that time, so CORTf should be related to conditions at the time of moult of analyzed feathers (Romero and Fairhurst 2016). For plumage signals to be informative about individual condition or quality in the mating season, CORTf should show a negative association with the ornamental plumage trait that includes the analyzed feathers if condition at moult has some predictive value for breeding condition.
There is yet scant information on the association of CORTf with other than carotenoid-based plumage colours of adult birds. The extent of white plumage patches has been shown to be condition-dependent (D’Alba et al. 2011) and predict reproductive success (Doucet et al. 2005). White patches in otherwise melanised feathers are common across many avian taxa and have been negatively related to feather resistance to mechanical stress (Bonser 1996) and to microbial degradation because of their lack of melanin (Ruiz-de-Castañeda et al. 2012, 2015). Larger white patches or spots may therefore be costly and evolve as honest signals of plumage quality in both sexes (Hegyi et al. 2008; Zanollo et al. 2012). The capacity to moult partly unpigmented feathers may be related to nutritional stress or to the capacity to sustain such stress during moult itself. If large white patches constitute honest signals of stress avoidance capacity, we should expect the extent of such patches to be negatively related to levels of stress during moult as expressed by CORTf.
Some migratory birds moult their nuptial plumage at the winter quarters prior to migrating to the breeding range (Ginn and Melville 1983). Measuring CORTf in these nuptial feathers thus gives an indication of stress experienced in the wintering range before migration in these species. In some species like the Pied Flycatcher Ficedula hypoleuca, both males and females acquire in Africa part of their body plumage, including the forehead, and the tertials, before migration (Lundberg and Alatalo 1992). The extent of the achromatic forehead patch and of the white parts of tertials differs between sexes and populations, with males presenting much larger patches and larger tertials overall than females (in some populations, no female presents a forehead patch, Lundberg and Alatalo 1992) and birds of southern populations presenting larger unpigmented patches than in northern ones (Curio 1960; Alatalo et al. 1984; Lehtonen et al. 2009; Laaksonen et al. 2015; Cantarero et al. 2017). The extent of achromatic plumage patches in females is phenotypically plastic (Morales et al. 2007a; Moreno et al. 2019) and has been related to age (Morales et al. 2007a; Potti et al. 2013), reproductive success (Morales et al. 2007a), social dominance (Cantarero et al. 2017; Plaza et al. 2018), health (Morales et al. 2007a), testosterone levels (Moreno et al. 2014; Cantarero et al. 2017) and oxidative stress (Moreno et al. 2013; López-Arrabé et al. 2014). A large part of the white wing patch is composed by the unpigmented parts of the ornamental tertials (60% on average in our population, Cantarero et al. 2017), while the rest is composed of the white bands on primaries and secondaries which are moulted in the postnuptial moult in the breeding range (Cantarero et al. 2017). While other studies have measured the UV reflectance of these patches as part of its signaling value (e.g., Cantarero et al. 2017), here we will only consider their size.
In the present study, we have measured concentration of CORTf in tertials of females captured during the breeding season while provisioning young at the nest in a Spanish population of Pied Flycatchers. We have also measured in these females the size of the white forehead and wing patches. Thus CORTf is estimated in some of the feathers moulted in Africa prenuptially, the extent of whose white patches is also measured. Our prediction is that females with the largest white patches on forehead and wing in the breeding season will present low levels of CORTf in prenuptially moulted feathers.
Material and methods
General field methods
The study was carried out in 2019 at the Lozoya study site (central Spain, 40°58′N, 3°48′W, 1400–1500 m a.s.l), where a long-term study of Pied Flycatchers is being conducted (Moreno 2015, 2020). The habitat is montane deciduous forest of Pyrenean Oak Quercus pyrenaica, where 100 nest-boxes were installed in an area covering roughly 85 ha (for size and other properties on nest-boxes see Lambrechts et al. 2010). Nest-box occupation has been checked every year since 2001. The breeding period of the species lasts from the middle of April when the first males arrive from migration, to the beginning of July when most chicks have fledged. We clean all nest-boxes every year after breeding is over. Regular checking from April 15 to the end of the breeding period is done to detect the presence and settlement of every flycatcher breeding pair. The number of Pied Flycatcher nests was 41 in the study year.
Capture and sampling
Females were captured in their nest boxes while feeding 7–8 days nestlings (fledging takes place 16–19 days after hatching, Moreno 2020) during daytime, using conventional nest-box traps set at the entrance (Moreno 2020). The trap was removed once both adults were trapped or after a maximum of 1 h. All females were ringed or identified by their rings. Tarsus length was measured with a digital calliper and wing length with a stopped ruler following Svensson (1984). Digital photographs of the forehead patch, if present, and of the right white wing patch were taken from above at distances of 10 cm from the bird with a ruler aligned beside the head or wing for reference. The wing was completely extended when taking the photograph. All photographs were taken during the morning hours with the same digital camera and following the method described in previous studies (Moreno et al. 2014; Cantarero et al. 2017; Plaza et al. 2018). Surfaces of the forehead and wing patches were estimated with Adobe Photoshop CS5 v.11.0. following Sirkiä et al. (2015). We also estimated the total area of the tertials and of their white parts separately. We could establish the exact age of individuals only if they had been ringed as nestlings. Age for unringed birds was estimated assuming they were 2 years when captured for the first time as breeders in the area, as half of recruits in the population are first captured as breeders at this age (Moreno et al. 2015). After measurements and before release, the three tertials on each wing were removed with scissors at the rachis. Many males and some females overlap moult of wing feathers and nestling-provisioning (Morales et al. 2007b), so the manipulation can be considered mild. Moreover, tertials are less important for flight than primaries and secondaries.
Corticosterone extraction
A methanol-based extraction technique was used to extract CORTf, since steroid hormones are generally soluble in polar alcohols such as methanol (Pötsch and Moeller 1996). All glassware used for CORT extraction was silanized using 1% v/v dichloromethylsilane ((CH3)2SiCl2) in hexane (C6H14), rinsed twice with methanol, and air-dried. Silanization prevents CORT from adhering to glassware and was shown to significantly reduce losses of CORT during the extraction process (Bicudo et al. 2020; Kouwenberg et al. 2015).
To measure CORTf levels, we followed the protocol of Bortolotti et al. (2008) with some modifications as described below. Concentrations were normalized by mass of feathers allowing for the representation of CORT levels per unit mass (pg/mg) (Freeman and Newman 2018), and controlling that we obtained a small range of pooled feather masses per individual (11.2 ± 1.9 (Mean ± SD) mg) to avoid potential confounding effects on CORTf (Romero and Fairhurst 2016). Although Bortolotti et al. (2008) suggested that CORT values should be corrected for the length of the feathers rather than mass, all the feathers from the same individual were pooled before extraction and the sum of lengths could be estimated less reliably than the pooled mass (Koren et al. 2012; Lendvai et al. 2013; Sepp et al. 2018). The calamus was removed and the feather vanes and the rachis cut into small pieces (< 5 mm) with scissors. All feathers per individual were pooled and weighed in tared silanized glass tubes. To these were added 6 ml of methanol, after which the tubes were capped with a rubber plug crossed with two syringe needles and parafilm to avoid evaporation, and incubated for 30 min in an ultrasound water bath at room temperature. The tubes were left overnight (around 19 h: from 15:00 to 10:00) in a shaking (60 rpm) water bath at 50 °C. They were then decanted into a syringe. Tubes were then washed with 2 additional ml of methanol, vortexed and to them added the previous extract, after which they were filtered using a nylon syringe filter (0.45 µ). They were placed in a heated block at 50ºC under a stream of nitrogen until evaporation which took approx. 50 min. The dried extracts were then resuspended in 150 µl of steroid-free serum (DRG, Germany) and vigorously vortexed for 10 min. The reconstituted samples were frozen at –20° in microtubes.
Corticosterone quantification
To measure CORTf concentrations we used an enzyme immunoassay (EIA-4164 DRG, Germany) following kit protocol. Inter-assay variability was assessed using the coefficient of variation (CV) of two known controls. Samples, controls and standards were run in duplicate across nine assays with a mean intra-assay CV of 14.65% and interassay CV of 7.321%. All samples were above detection limits (1.1 pg/ml). Serial dilutions of an extraction pool were included in the assay to test the linearity with the correlation line between expected and observed concentration of CORT in the dilutions using the set ratios performed (y = 1.382×—0.709, r2 = 0.969). A Synergy HT Multi-Mode Microplate Reader (Biotek, USA) at 450 nm was used. All samples, standards and controls were measured in duplicate to calculate intra- and inter-plate % CV.
Assay plates can differ in factors, such as temperature, which in turn can affect the amount of hormone found in each feather (Bosholn et al. 2020). There was a significant association of CORTf with plate (F2,32 = 4.07, p = 0.026). There was no significant association of CORTf with the mass of the tertials analysed (r33 = 0.028, p = 0.873).
Statistical analyses
We hypothesized that the size of the white patches should be related to CORTf when controlling for age and size of the female. As plumage traits we included the size (cm2) of the forehead patch and the first Principal Component extracted from the following variables related to the size of the wing patch: total area of the white patch including flight feathers (cm2), total area of the tertials including melanised parts (cm2), size of the white parts of tertials (cm2), proportion of the total wing patch made up by the white parts of tertials and the proportion of tertials that were white. The first proportion estimates the relative importance of tertials as part of the signal made up by the flicking wing, while the second addresses the relative importance of the achromatic part of the feathers in which CORTf is measured. The main axis (WPC1) explained 54.4% of the total variation and showed strong negative associations with total wing patch area (r = − 0.95) and tertial white patch area (r = − 0.97), and less strong with total tertial area (r = − 0.67) and proportion white on tertials (r = − 0.64). It can be defined as an inverse index of investment in wing patches.
Both forehead patch size and WPC1 were normally distributed (Kolmogorov–Smirnov, p > 0.20). Model selection in the GLIM module of the STATISTICA package was conducted independently for the two plumage patches (normal distribution, identity link function), using AICc (AIC corrected for small sample sizes) to estimate the relative strength of the associations with individual female traits including CORTf. To estimate body size, we used the first PC derived from wing length and tarsus length as these variables were positively correlated (r33 = 0.63, p < 0.001). PC1 Size accounted for 81% of the variation in both size estimates and showed positive correlations with both tarsus and wing length (r = 0.90 in both cases). The independent traits included in GLIM analyses were thus age, SizePC1 and CORTf. Two and three-way interactions between independent variables were included in model selection. Correlations between the independent variables were lower than 0.27 and non-significant. Only models showing differences in AICc lower than 2 with the best model (that with the lowest AICc value) will be presented in "Results". The significance of the models was estimated through the p value associated with the associated Log-likelihood ratio χ2. The sign of the associations of the size of the two patches with CORTf was obtained from linear correlation analysis.
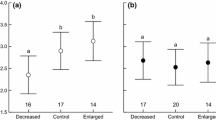
To control for effects of plate, model selection without interactions was conducted on data on age, size and CORTf from the two most common plates separately (n = 19 and n = 14, respectively).
Results
The mean mass of tertials analyzed was 11.57 ± SD1.56 mg. The mean concentration of CORT in tertials was 24.25 ± SD10.81 pg/mg (n = 35).
The ten best models (deltaAICc < 2) for forehead patch size incorporated CORTf (Table 1). In seven of these, interactions between factors were included, with CORTf interacting with other variables in five of them. The fourth best model included only CORTf. In contrast, body size was included in five best models and age only in one. All selected models according to AICc were significant (Table 1). For the most common plate, the three best models included CORTf. For the second most common plate, two of the three best models included CORTf and it were the first and second best models.
CORTf was included in the 2 models with lowest AICc and in 15 of the 21 best models (deltaAICc < 2) for WPC1 (Table 2). Of the 18 models including interactions between variables, 14 included interactions involving CORTf (Table 2). In contrast, age and body size were included in only 8 resp. 6 best models (Table 2). The model with lowest AICc included CORTf and was very close to significance (Table 2). For the most common plate, one of the three best models included CORTf. For the second most common plate, three of the four best models included CORTf, including the model with lowest AICc.
CORTf was negatively associated with forehead patch size (Fig. 1). CORTf was positively although not significantly associated with WPC1 (r33 = 0.20, p = 0.27), which means a negative association with wing patch size as WPC1 is an inverse index of wing patch size (see above).
Discussion
Our results indicate that the size of the white forehead patch of females is negatively related to levels of CORT in tertial feathers moulted on the wintering grounds. However, although CORTf turns up in the best models explaining the size of both patches, the association of CORTf with the size of the white wing patch is clearly weaker, suggesting that other unmeasured factors may be involved in wing feather moult.
Several studies have related the coloration of plumage ornaments to levels of CORT in adult birds (Kennedy et al. 2013; Henderson et al. 2013; Saino et al. 2013; Henschen et al. 2018; Angelier et al. 2018; Berg et al. 2019; Windsor et al. 2019), or after experimental post-natal exposure (Dupont et al. 2019; Fairhurst et al. 2015), although structural colours have in some cases shown less association with CORT (Sarpong et al. 2019). Some of these studies include measurements of CORTf. Thus, Grunst et al. (2015) showed in Yellow Warblers Setophaga petechia that carotenoid hue and chroma correlated negatively with CORTf, while phaeomelanin-based pigmentation was unrelated to CORTf. Kennedy et al. (2013) showed a negative trend of CORTf with the brightness of the red epaulets of male museum specimens of Red-winged Blackbirds Agelaius phoeniceus, although not with hue and chroma of these ornaments. On the other hand, Fairhurst et al. (2014) showed a positive association of carotenoid pigmentation and feather quality with CORTf in male Common Redpolls Acanthis flammae. A similar result was obtained by Lendvai et al. (2013) in carotenoid-pigmented House Finch Heamorhous mexicanus males. As shown by these disparate results, CORTf can be negatively or positively related to signal intensity for carotenoid-based signals.
Black, grey, and white plumage (termed achromatic plumage) has been shown to have a role in the context of status signaling (Senar et al. 2000; Gonzalez et al. 2002). The extent of achromatic plumage patches has been related to social dominance and mating preferences in both male and female birds (Mennill et al. 2003; Woodcock et al. 2005; Santos et al. 2011; Crowhurst et al. 2012) and to conspicuousness to predators (Slagsvold and Dale 1996). Due to the highly conspicuous contrast between melanistic and white body regions, achromatic plumage may be an efficient mode of visual communication. Unpigmented feather patches may increase conspicuousness towards predators (Dale and Slagsvold 1996) or be more easily degradable by bacteria because of their lack of melanin (Ruiz-de-Castañeda et al. 2012). They could thus be costly to maintain and therefore represent honest signals of individual quality. If achromatic plumage indicates phenotypic quality, white feather patches could also be used as signals in social interactions during the breeding and non-breeding seasons (Santos et al. 2011). Because individuals of both sexes risk injury during agonistic encounters, these signals could be socially costly to maintain and therefore possibly favored by social selection in both sexes (Lyon and Montgomerie 2012). According to this scenario, we could expect the extent of white plumage patches to relate to competitive potential and aggressive predisposition.
Female Pied Flycatchers exhibit a conspicuous white patch on the wing based on white edges of tertials, and secondary coverts and white bands on some secondaries and primaries (Lundberg and Alatalo 1992). There is a large degree of variation in the extent of the white wing patch of females, ranging from highly conspicuous badges to barely noticeable feather edges (Moreno et al. 2014; Cantarero et al. 2017). Some females also exhibit a white forehead patch in some populations (Potti 1993; Moreno et al. 2013). Wing patches are exhibited in social interactions by repeatedly flicking the folded or partly folded wings (Curio 1959, 1978). Females with larger wing patches breed earlier and have a higher hatching success (Morales et al. 2007a). The extent of the wing patch in females is also positively linked to testosterone levels during incubation (Moreno et al. 2014; Cantarero et al. 2017) and is related to oxidative stress (Moreno et al. 2013; López-Arrabé et al. 2014). The extent of the forehead patch is linked to social dominance in female-female competitive interactions (Morales et al. 2014; Moreno 2015). Moreover, there is strong female intrasexual competition for nest cavities during the incubation stage (Moreno 2015) as indicated by the intense aggression of territorial females towards female intruders during initial breeding stages (Breiehagen and Slagsvold 1988; Lifjeld and Slagsvold 1989; Morales et al. 2014; Cantarero et al. 2015; Moreno et al. 2016). It is therefore reasonable to consider the extent of the white forehead and wing patches as indicators of individual quality in female Pied Flycatchers.
The size of achromatic patches is determined during the yearly partial moult in the wintering areas when body feathers and tertials are moulted prenuptially (Lundberg and Alatalo 1992). We could expect that poor condition at the time of moult in Africa before migration may affect levels of plasma CORT as revealed by CORTf in those feathers. By analyzing CORTf in tertials we could thus gauge the stress experienced by birds at the time of prenuptial moult. We found that CORTf was always included in the best models including age, body size and CORTf as explanatory variables of the size of the forehead patch and in most best models addressing the size of the wing patch. We also identified a significant negative association of CORTf with the size of the forehead patch. However, the models were more weakly related to the size of the wing patch, and its association with CORTf was not significant. The wing patch is composed of white patches on feathers moulted both on the breeding grounds after the previous reproductive season and on the wintering grounds (tertials). Thus, we should expect that CORTf in tertials should be more indirectly related to the extent of the wing patch. On the other hand, the white patch probably functions as a signaling unit during wing flicking (Curio 1959) making separate analyses of its constituents inappropriate.
Results of a single-year observational study need to be interpreted with care. It is possible that the adrenocortical function (HPA) of individuals with large patches is in general lower compared to that of individuals presenting smaller patches and is not necessarily reflecting environmental conditions experienced by individuals during feather growth (Romero et al. 2009; Lattin et al. 2012). For example, conditions during early ontogenetic development might affect multiple traits such as HPA, tarsus size, and/or white patch size (Spencer et al. 2009; Fairhurst et al. 2015). In this scenario, the size of white patches would reflect genetic or ontogenetic factors involved in HPA activation.
To conclude, our results suggest that larger achromatic patches are developed in the winter quarters in females with low CORT concentration in plasma and therefore experiencing a reduced physiological stress (Romero and Fairhurst 2016). Females in nuptial plumage could thus be expressing through the extent of their achromatic patches their physiological state before migrating to the breeding grounds. The capacity to cope physiologically when preparing for the presumably stressful prenuptial migration may be a crucial variable to evaluate in social, including sexual, interactions.
References
Alatalo RV, Lundberg A, Ståhlbrandt K (1984) Female mate choice in the Pied Flycatcher Ficedula hypoleuca. Behav Ecol Sociobiol 14(4):253–261
Andersson M (1994) Sexual selerction. Princeton Univ Press, Princeton
Angelier F, Parenteau C, Trouve C, Angelier N (2018) The behavioural and physiological stress responses are linked to plumage coloration in the rock pigeon (Columbia livia). Physiol Behav 184:261–267. https://doi.org/10.1016/j.physbeh.2017.12.012
Berg ML, Knott B, Ribot RFH, Buchanan KL, Bennett ATD (2019) Do glucocorticoids or carotenoids mediate plumage coloration in parrots? An experiment in Platycercus elegans. General Compar Endocrinol 280:82–90. https://doi.org/10.1016/j.ygcen.2019.04.014
Bicudo T, Anciães M, Arregui L, Gil D (2020) Effects of forest fragmentation on feather corticosterone levels in an amazonian avian community. Ardeola 67:229–245
Bonser RHC (1996) The mechanical properties of feather keratin. J Zool Lond 239:477–484
Bortolotti GA, Marchant TA, Blas J, German T (2008) Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Func Ecol 22:494–500. https://doi.org/10.1111/j.1365-2435.2008.01387.x
Bosholn M, Anciães M, Gil D, Weckstein JD, Dispoto JH, Fecchio A (2020) Individual variation in feather corticosterone levels and its influence on haemosporidian infection in a Neotropical bird. Ibis 162(1):215–226
Breiehagen T, Slagsvold T (1988) Male polyterritoriality and female-female aggression in pied flycatchers Ficedula hypoleuca. Anim Behav 36:604–606
Cantarero A, Laaksonen T, Järvistö PE, Gil D, López-Arrabé J, Redondo AJ, Moreno J (2015) Nest defense behaviour and testosterone levels in female Pied Flycatchers. Ethology 121:946–957
Cantarero A, Laaksonen T, Järvistö P, Gil D, López-arrabé J, Moreno J (2017) Testosterone levels in relation to size and UV reflectance of achromatic plumage traits of female pied flycatchers. J Avian Biol 48:243–254
Crowhurst CJ, Zanollo V, Griggio M, Robertson J, Kleindorfer S (2012) White flank spots signal feeding dominance in female diamond firetails, stagonopleura guttata. Ethology 118:63–75
Curio E (1959) Verhaltensstudien am Trauerschnäpper. Zeitschrift Tierpsychol, Beiheft 3:1–118
Curio E (1960) Die systematische Stellung des spanischen Trauerschnäppers. Vogelwelt 81:113–121
Curio E (1978) The adaptive significance of avian mobbing I: teleonomic hypotheses and predictions. Zeitschrift Tierpsychol 48:175–183
D’Alba L, Van Hemert C, Handel CM, Shawkey MD (2011) A natural experiment on the condition-dependence of achromatic plumage reflectance in Black-Capped Chickadees. PLoS ONE 6:8
Dale S, Slagsvold T (1996) Plumage coloration and conspicuousness in birds: experiments with the pied flycatcher. Auk 113:849–857
Dickens MJ, Romero LM (2013) a consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrin 191:177–189
Doucet SM, Mennill DJ, Montgomerie R, Boag PT, Ratcliffe LM (2005) Achromatic plumage reflectance predicts reproductive success in male black-capped chickadees. Behav Ecol 16:218–222
Dupont SM, Grace JK, Brischoux F, Angelier F (2019) Post-natal corticosterone exposure affects ornaments in adult male house sparrows (Passer domesticus). Gen Comp Endocrinol 276:45–51. https://doi.org/10.1016/j.ygcen.2019.02.021
Fairhurst GD, Dawson RD, van Oort H, Bortolotti GR (2014) Synchronizing feather-based measures of corticosterone and carotenoid-dependent signals: what relationships do we expect? Oecologia 174:689–698. https://doi.org/10.1007/s00442-013-2830-5
Fairhurst GD, Damore N, Butler MW (2015) Feather corticosterone levels independent of developmental immune challenges predict carotenoid-based, but not melanin-based, traits at adulthood. Auk 132:863–877. https://doi.org/10.1642/AUK-15-34.1
Freeman NE, Newman AEM (2018) Quantifying corticosterone in feathers: validations for an emerging technique. Conserv Physiol 6:1–9
Ginn HB, Melville DS (1983) Moult in Birds. BTO Guide no. 19: Tring.
Gonzalez G, Sorci G, Smith LC, de Lope F (2002) Social Control and Physiological Cost of Cheating in Status Signalling Male House Sparrows (Passer domesticus). Ethology 108(4):289-302
Grunst ML, Grunst AS, Parker CE, Romero LM, Rotenberry JT (2015) Pigment-specific relationships between feather corticosterone concentrations and sexual coloration. Behav Ecol 26:706–715. https://doi.org/10.1093/beheco/aru210
Harms NJ, Legagneux P, Gilchrist HG, Betty J, Love OP, Forbes MR, Bortolotti GR, Soos C (2015) Feather corticosterone reveals effect of moulting conditions in the autumn on subsequent reproductive output and survival in an Arctic migratory bird. Proc R Soc B 282:2014–2085. https://doi.org/10.1098/rspb.2014.2085
Harris CM, Madliger CL, Love OP (2016) Temporal overlap and repeatability of feather corticosterone levels: practical considerations for use as a biomarker. Conserv Physiol 4:cow051. https://doi.org/10.1093/conphys/cow051
Hegyi G, Garamszegi LZ, Eens M, Török J (2008) Female ornamentation and territorial conflicts in collared flycatchers (Ficedula albicollis). Naturwissenschaften 95:993–996
Henderson LJ, Heidinger BJ, Evans NP, Arnold KE (2013) Ultraviolet crown coloration in female blue tits predicts reproductive success and baseline corticosterone. Behav Ecol 24:1299–1305. https://doi.org/10.1093/beheco/art066
Henschen AE, Whittingham LA, Dunn PO (2018) Male stress response is related to ornamentation but not resistance to oxidative stress in a warbler. Func Ecol 32:1810–1818. https://doi.org/10.1111/1365-2435.13104
Jaatinen K, Seltmann MW, Hollmen T, Atkinson S, Mashburn K, Öst M (2013) Context dependency of baseline glucocorticoids as indicators of individual quality in a capital breeder. Gen Comp Endocrinol 191:231–238. https://doi.org/10.1016/j.ygcen.2013.06.022
Jenkins BR, Vitousek MN, Safran RJ (2013) Signaling stress? An analysis of phaeomelanin-based plumage color and individual corticosterone levels at two temporal scales in North American barn swallows, Hirundo rustica erythrogaster. Horm Behav 64:665–672. https://doi.org/10.1016/j.yhbeh.2013.08.006
Kennedy EA, Lattin CR, Romero LM, Dearborn DC (2013) Feather coloration in museum specimens is related to feather corticosterone. Behav Ecol Sociobiol 67:341–348. https://doi.org/10.1007/s00265-012-1454-9
Koren L, Nakagawa S, Burke T, Soma KK, Wynne-Edwards KE, Geffen E (2012) Non-breeding feather concentrations of testosterone, corticosterone and cortisol are associated with subsequent survival in wild house sparrows. Proc R Soc B 279:1560–1566. https://doi.org/10.1098/rspb.2011.2062
Kouwenberg AL, McKay DW, Fitzsimmons MG, Storey AE (2015) Measuring corticostrone in feathers using an acetonitrile/hexane extraction and enzyme immunosassy: feather corticosterone levels of food-supplemented Atlantiv Puffin chicks. J Field Orn 86:73–83. https://doi.org/10.1111/jofo.12090
Laaksonen T, Sirkiä PM, Calhim S, Brommer JE, Leskinen PK, Primmer CR, Adamík P, Artemyev AV, Belskii E, Both C, Bureš S, Burgess MD, Doligez B, Forsman JT, Grinkov V, Hoffman U, Ivankina E, Král M, Krams I, Lampe HM, Moreno J, Mägi M, Nord A, Potti J, Ravussin PA, Sokolov L (2015) Sympatric divergence and clinal variation in multiple coloration traits of Ficedula flycatchers. J Evol Biol 28:779–790
Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bánbura J, Barba E, Bouvier J-C, Camprodon J, Cooper CB, Dawson RD, Eens M, Eeva T, Faivre B, Garamszegi LZ, Goodenough AE, Gosler AG, Grégoire A, Griffith SC, Gustafsson LS, Johnson L, Kania W, Keišs O, Llambias PE, Mainwaring MC, Mänd R, Massa B, Mazgajski TD, Møller AP, Moreno J, Naef-Daenzer B, Nilsson J-A, Norte AC, Orell M, Otter KA, Park CR, Perrins CM, Pinowski J, Porkert J, Potti J, Remeš V, Richner H, Rytkönen S, Shiao M-T, Silverin B, Slagsvold T, Smith HG, Sorace A, Stenning MJ, Stewart I, Thompson CF, Török J, Tryjanowski P, van Noordwijk AJ, Winkler DW, Ziane N (2010) The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol 45:1–26
Lattin CR, Bauer CM, de Bruijn R, Romero LM (2012) Hypothalamus–pituitary–adrenal axis activity and the subsequent response to chronic stress differ depending upon life history stage. Gen Comp Endocrinol 178:494–501
Lehtonen PK, Laaksonen T, Artemyev AV, Belskii E, Both C, Bures S, Bushuev AV, Krams I, Moreno J, Magi M, Nord A, Potti J, Sirkiä PM, Sætre GP, Primmer CR (2009) Geographic patterns of genetic differentiation and plumage colour variation are different in the pied flycatcher (Ficedula hypoleuca). Mol Ecol 18:4463–4476
Lendvai AZ, Giraudeau M, Németh J, Bakó V, K. McGraw KJ, (2013) Carotenoid-based plumage coloration reflects feather corticosterone levels in male house finches (Haemorhous mexicanus). Behav Ecol Sociobiol 2013(67):1817–1824
Lifjeld JT, Slagsvold T (1989) Female nutritional state influences the allocation of incubation feeding by polygynous pied flycatcher males. Anim Behav 38:903–904
Lodjak J, Mägi M, Roonit U, Tilgar V (2015) Context-dependent effects of feather corticostrone on growth rate and fledging success of wild passerine nestlings in heterogeneous habitat. Oecologia 179:937–946. https://doi.org/10.1007/s0042-015-3357-8
López-Arrabé J, Cantarero A, Pérez-Rodríguez L, Palma A, Moreno J (2014) Plumage ornaments and reproductive investment in relation to oxidative status in the Pied Flycatcher Ficedula hypoleuca iberiae. Can J Zool 92:1019–1027
Lundberg A, Alatalo RV (1992) The Pied Flycatcher. Poyser, London
Lyon BE, Montgomerie R (2012) Sexual selection is a form of social selection. Phil Trans Roy Soc B Biol Sci 367:2266–2273
Mennill DJ, Doucet SM, Montgomerie R, Ratcliffe LM (2003) Achromatic color variation in black-capped chickadees, Poecile atricapilla: black and white signals of sex and rank. Behav Ecol Sociobiol 53:350–357
Moore FR, Shuker DM, Dougherty L (2016) Stress and sexual signaling: a systematic review and meta-analysis. Behav Ecol 27:363–371. https://doi.org/10.1093/beheco/arv195
Morales J, Moreno J, Merino S, Sanz JJ, Tomás G, Arriero E, Lobato E, Martínez-de la Puente J (2007a) Female ornaments in the pied flycatcher: associations with age, health and reproductive success. Ibis 149:245–254
Morales J, Moreno J, Merino S, Sanz JJ, Tomás G, Arriero E, Lobato E, Martínez-de la Puente J (2007b) Early moult improves local survival and reduces reproductive output in female pied flycatchers. Écoscience 14:31–39
Morales J, Gordo O, Lobato E, Ippi S, Martínez-de la Puente J, Tomás G, Merino S, Moreno J (2014) Female-female competition is influenced by forehead patch expression in pied flycatcher females. Behav Ecol Sociobiol 68:1195–1204
Moreno J (2015) The Incidence of Clutch Replacements in the pied flycatcher Ficedula hypoleuca is related to nest-box availability: evidence of female-female competition? Ardeola 62:67–80
Moreno J (2020) Effects of food availability and parental risk taking on duration of the nestling period: a field experiment. Ardeola 67:29–38
Moreno J, Velando A, Ruiz-De Castañeda R, González-Braojos S, Cantarero A (2013) Oxidative damage in relation to a female plumage badge: evidence for signalling costs. Acta Ethol 16:67–75
Moreno J, Gil D, Cantarero A, López-Arrabé J (2014) Extent of a white plumage patch covaries with testosterone levels in female pied flycatchers Ficedula hypoleuca. J Ornithol 155:639–648
Moreno J, Gil D, Cantarero A, López-Arrabé J (2016) Female aggressiveness towards female decoys decreases with mate T level in the pied flycatcher. Acta Ethol 19:9–14
Moreno J, Cantarero A, Plaza M, López-Arrabé J (2019) Phenotypic plasticity in breeding plumaje signals in both sexes of a migratory bird: responses to breeding conditions. J Avian Biol. https://doi.org/10.1111/jav01855
Möstl E, Rettenbacher S, Palme R (2005) Measurement of corticostrone metabolites in birds’ droppings: an analytical approach. Ann NY Acad Sci 1046:17–34
Mougeot F, Lendvai AZ, Martínez-Padilla J, Pérez-Rodríguez L, Giraudeau M, Casas F, Moore IT, Redpath S (2016) Parasites, mate attractiveness and female feather corticosteronelevels in a socially monogamous bird. Behav Ecol Sociobiol 70:277–283. https://doi.org/10.1007/s00265-015-2048-0
Ouyang JQ, Sharp P, Quetting M, Hau M (2013) Endocrine phenotype, reproductive success and survival in the great tit, Parus major. J Evol Biol 26:1988–1998. https://doi.org/10.1111/jeb.12202
Plaza M, Cantarero A, Cuervo JJ, Moreno J (2018) Female incubation attendance and nest vigilance reflect social signalling capacity: a field experiment. Behav Ecol Sociobiol 72:UNSP24
Potti J (1993) A male trait expressed in female pied flycatchers, Ficedula hypoleuca: the white forehead patch. Anim Behav 45:1245–1247
Potti J, Canal D, Serrano D (2013) Lifetime fitness and age-related female ornament signalling: evidence for survival and fecundity selection in the pied flycatcher. J Evol Biol 26:1445–1457
Pötsch L, Moeller MR (1996) On pathways for small molecules into and out of human hair fibers. J Forensic Sci 41:121–125
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389
Romero LM, Fairhurst GD (2016) Measuring corticosterone in feathers: strengths, limitations, and suggestions for the future. Comp Biochem Physiol, A 202:112–122
Ruiz-de-Castañeda R, Burtt EH, González-Braojos S, Moreno J (2012) Bacterial degradability of an intrafeather unmelanized ornament: a role for feather-degrading bacteria in sexual selection? Biol J Linn Soc 105:409–419
Ruiz-de-Castañeda R, Burtt EH, González-Braojos S, Moreno J (2015) Bacterial degradability of white patches on primary feathers is associated with breeding date and parental effort in a migratory bird. Ibis 157:871–876
Saino N, Canova L, Costanzo A, Rubolini D, Roulin A, Möller AP (2013) Immune and stress response covary with melanin-based coloration in the Barn Swallow. Evol Biol 40:521–531. https://doi.org/10.1007/s11692-013-9228-5
Santos ESA, Scheck D, Nakagawa S (2011) Dominance and plumage traits: meta-analysis and metaregression analysis. Anim Behav 82:3–19
Sapolsky RM (1982) The endocrine-stress response and social status in the wild baboon. Horm Behav 16:279–287
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory and preparative actions. Endocrin Rev 21:55–89
Sarpong K, Madliger CL, Harris CM, Love OP, Doucet SM, Bitton PP (2019) Baseline corticosterone does not reflect iridescent plumage traits in female tree swallows. Gen Comp Endocrinol 270:123–130. https://doi.org/10.1016/j.ygcen.2018.10.015
Senar JC, Polo V, Uribe F, Camerino M (2000) Status signalling, metabolic rate and body mass in the siskin: the cost of being a subordinate. Anim Behav 59(1):103–110
Sepp T, Desaivre S, Lendvai AZ, Németh Jm McGraw KJ, Giraudeau M (2018) Feather corticosterone levels are not correlated with health or plumage coloration in juvenile house finches. Biol J Linne Soc 20:1–8
Sirkiä PM, Adamík P, Artemyev AV, Belskii E, Both C, Bureš S, Burgess M, Bushuev AV, Forsman JT, Grinkov V, Hoffmann D, Järvinen A, Král M, Krams I, Lampe HM, Moreno J, Mägi M, Nord A, Potti J, Ravussin PA, Sokolov L, Laaksonen T (2015) Fecundity selection does not vary along a large geographical cline of trait means in a passerine bird. Biol J Linn Soc 114:808–827
Spencer KA, Evans NP, Monaghan P (2009) Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinol 150:1931–1934
Svensson L (1984) Identification guide to European passerines. Stockholm
West-Eberhard MJ (1979) Sexual selection, social competition, and evolution. Proc Am Phil Soc 123:222–234
Will A, Wynne-Edwards K, Zhou RK, Kitaysky A (2019) Of 11 candidate steroids, corticosterone concentration standardized for mass is the most reliable steroid biomarker of nutritional stress across different feather types. Ecol and Evol 9:11930–11943. https://doi.org/10.1002/ece3.5701
Windsor RL, Fox GA, Bowman R (2019) Consistency of structural color across molts: the effects of environmental conditions and stress on feather ultraviolet reflectance. Auk 136:1–17
Wingfield JC (2013) Ecological processes and the ecology of stress: the impacts of abiotic environmental factors. Funct Ecol 27:37–44
Wingfield JC, Smith JP, Farner DS (1982) Endocrine responses of white-crowned sparrows to environmental stress. Condor 84:399–409
Wingfield JC, Romero, LM (2001) Adrenocortical responses to stress and their modulation in free-living vertebrates. In: McEwen BS, Goodman HM (eds) Handbook of Physiology, Section 7, vol IV, pp. 211–234
Woodcock EA, Rathburn MK, Ratcliffe LM (2005) Achromatic plumage reflectance, social dominance and female mate preference in black-capped chickadees (Poecile atricapillus). Ethology 111:891–900
Zanollo V, Griggio M, Robertson J, Kleindorfer S (2012) The number and coloration of white flank spots predict the strength of a cutaneous immune response in female Diamond Firetails, Stagonopleura guttata. J Ornithol 153:1233–1244
Acknowledgements
The study was supported by project CGL2017-83843-C2-1 from Ministerio de Economía, Industria y Competitividad (MINECO, Spanish Government). Two reviewers offered valuable criticism. Permits—Permissions for capturing, ringing and handling birds were provided by ‘Consejería de Medio Ambiente, Comunidad de Madrid’ (regional government). The study was approved by the Ethical Committee of CSIC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Fusani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreno, J., López-Arrabé, J. The extent of white plumage patches in female Pied Flycatchers Ficedula hypoleuca is negatively associated with corticosterone concentration in partly unpigmented feathers. J Ornithol 162, 511–520 (2021). https://doi.org/10.1007/s10336-020-01851-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-020-01851-z