Abstract
Migratory birds face population declines attributed to habitat loss and modification in the wintering grounds, which may influence body condition, time of arrival to breeding grounds, and future reproductive opportunities. Despite this, very little is known of the wintering ecology of migratory birds. During three winter seasons, we assessed Wilson’s Warbler (Cardellina pusilla) density, territory size, and body condition at three cloud forest sites in southeast Mexico, with differing degrees of habitat disturbance and forest cover: preserved 125 ha of cloud forest actively protected for 40 years; moderately disturbed site of 67.5 ha of cloud forest under protection for 29 years; and highly disturbed unprotected site with 6.5 ha of cloud forest. We determined warbler density using 20 unlimited-radius point-counts at each site. We also captured and measured birds (n = 74) over three years, to obtain a body condition index, and re-sighted color-banded birds to determine individual territory size at each site. Bird densities were two times greater and territory size was smaller in the conserved forest site compared to disturbed sites with lower forest cover. However, there was no significant difference among sites in the body condition index of territorial birds. Furthermore, territory size and body condition were relatively constant among years for birds in conserved forest, but exhibited high inter-annual fluctuations at disturbed forest sites. Considering the higher bird density, smaller territory size, and inter-annually consistent body condition at the conserved cloud forest site, we propose that this represents higher quality wintering habitat for Wilson’s Warblers.
Zusammenfassung
Störungen im Winterquartier beeinflussen die Territorien-Größe und -Besetzungsdichte bei einem neotropischen Zugvogel ( Cardellina pusilla )
Verlust oder Veränderung ihrer Habitate in den Überwinterungsquartieren führen bei Zugvögeln zu einem Rückgang der Populationen und beeinflussen möglicherweise ihre gesamte physische Verfassung, ihre Ankunft in den Brutgebieten und die Fortpflanzungsmöglichkeiten. Dennoch weiß man nur sehr wenig über die Ökologie der Überwinterung von Zugvögeln. Über drei Winter hinweg bestimmten wir in Südost-Mexiko in drei Nebelwaldgebieten für den Mönchswaldsänger (Cardellina pusilla) die Größe und Besetzungsdichte der Territorien sowie ihre physische Verfassung. Diese Parameter wurden für drei Areale mit unterschiedlich starken Störungen und mit unterschiedlicher Bewaldung erfasst: (1) 125 ha große, über 40 Jahre aktiv geschützte Nebelwaldareale, (2) nur mäßig gestörte, über 29 Jahre geschützte, 67,5 ha große Areale, (3) stark gestörte, nicht geschützte Areale von 6,5 ha Größe. Anhand von 20 „unlimited-radius“-Punktzählungen bestimmten wir die Dichte/Häufigkeit der Vögel in jedem Areal. Ferner fingen wir über drei Jahre hinweg Vögel (n = 74) und nahmen ihre Maße, um einen Körperverfassungs-Index aufzustellen. Die Beobachtung von farbig markierten Vögeln gab uns außerdem Aufschluss über die individuellen Reviergrößen in den jeweiligen Nebelwald-Arealen. In den geschützten Arealen war die Individuendichte doppelt so groß, die Reviere jedoch kleiner als in den ungestörten Arealen mit geringerer Bewaldung. Beim Körperverfassungs-Index gab es jedoch keinen signifikanten Unterschied zwischen den einzelnen Arealen. Reviergröße und physische Verfassung erwiesen sich über die Jahre bei den Vögeln aus geschützten Waldarealen als ziemlich konstant, zeigten aber große Unterschiede zwischen den Jahren für die Vögel aus Arealen mit Störungen. Wegen der größeren Vogeldichte, der kleineren Reviere und der über die Jahre gleichbleibenden körperlichen Verfassung der Vögel in den geschützten Nebelwald-Arealen gehen wir davon aus, dass diese für Mönchswaldsänger ein qualitativ höherwertiges Winterquartier darstellen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last two decades, studies have documented population decline in numerous species of Neotropical migratory birds (Robbins et al. 1989; Askins et al. 1990; Ballard et al. 2003; Sauer et al. 2014), while resident bird species do not show a similar trend (Rappole and McDonald 1994). These population declines seem to be correlated with habitat loss on the wintering grounds (Robbins et al. 1989; Askins et al. 1990; Sutherland 1996), or at migration stopover sites (Weber et al. 1999; Iwamura et al. 2014), and are further indicated by a decrease in the number of individuals returning to breeding areas after migration (Rappole and McDonald 1994), leaving suitable nest sites unoccupied (McShea et al. 1995). One hypothesis to explain this population decline of Neotropical migratory birds is the negative influence of processes occurring in the wintering grounds (Rappole and McDonald 1994).
Wintering habitat quality may determine the body condition, survival, and reproductive fitness of migratory birds (Marra et al. 1998; Marra and Holmes 2001; Norris et al. 2004; Gunnarsson et al. 2005). In Jamaica, individual American Redstarts (Setophaga ruticilla) occupying higher quality mature mangrove forests in winter are in better condition, and are the first to depart their wintering territories, compared to individuals occupying suboptimal second-growth scrub, thereby increasing their chances of breeding success (Marra et al. 1998; Marra and Holmes 2001). However, there is a lack of knowledge on wintering habitat use of migratory birds in the tropics, which could vary greatly among species (Rappole and McDonald 1994; Marra et al. 1998; Brown and Long 2007).
In general, population size should be greater in higher quality habitats (Gilroy and Sutherland 2007), showing a positive correlation of bird density with resource abundance (Greenberg 1992; Lefebvre et al. 1994; Lefebvre and Poulin 1996) and body condition (Sherry and Holmes 1996). Furthermore, even when bird density is negatively correlated with body condition and food abundance (Marra and Holmes 2001; Hart et al. 2011), this may still provide an indication of habitat quality if complimented by data from other variables (van Horne 1983; Vickery et al. 1992). Migratory birds that maintain territoriality during the winter may be expected to follow the same pattern of higher densities and smaller territories in better quality habitats.
Wilson’s Warbler (Cardellina pusilla) is a long-distance migratory species that occupies forested habitats throughout its range, and exhibits territoriality both in breeding and wintering grounds (Eckhardt 1979; Ammon and Gilbert 1999; Rappole and Warner 1980; Hutto 1981). Wilson’s Warbler occurs in Canada, USA, and Mexico, and is undergoing a steep population decline (Berlanga et al. 2010), with a 2.21 % annual decline documented for the western population (Sauer et al. 2014; Ruiz-Sánchez et al. 2015) that is primarily related to intensive livestock grazing on their breeding grounds (Saab et al. 1995). During the breeding season, Wilson’s Warbler occupies both regenerated forests and clear-cut areas (Hejl et al. 1995; Desrochers et al. 2012), but uses a greater range of habitats in winter (Hutto 1981, 1994; Stiles et al. 1995) than in the summer (Eckhardt 1979; Finch 1989). Migratory birds often change their feeding habits between summer and winter, therefore habitat requirements are likely to vary between seasons (Long and Stouffer 2003; Pierce and McWilliams 2005; Martins et al. 2013). Furthermore, evidence suggests that forest habitats may be more favorable for Wilson’s Warbler during migration. Graham and Blake (2001) found that several migratory species, including Wilson’s Warbler, were more abundant in large areas of closed, mature forests during the winter. Yong et al. (1998) also found a higher proportion of adult Wilson’s Warblers in forest habitats during stopover in New Mexico, where they had positive rates of fat deposition, whereas birds in agricultural fields and edge habitats had the lowest rates of fat deposition and longer stopover times.
Studies on the wintering ecology and dynamics of migratory birds could give an insight into these species’ population declines, since there could be negative effects on populations due to habitat modification in the wintering areas. Carryover effects from wintering habitats on breeding success of migratory birds have been repeatedly proven (Marra et al. 1998; Norris et al. 2004; Reudink et al. 2009), although these have mainly been based on comparisons of birds wintering in different types of habitat (Sherry and Holmes 1996; Marra et al. 1998; Latta and Faaborg 2002; Saino et al. 2004). Little is known of the possible effects of changes in vegetation structure due to human disturbance on the wintering ecology and condition of migratory birds within the same habitat type. Therefore, our objective was to assess Wilson’s Warbler winter density, territory size, and body condition in three cloud forest sites with differing degrees of habitat disturbance and forest cover. We expected that Wilson’s Warblers in conserved cloud forest would present higher densities, smaller territory size, and improved body condition compared to birds wintering in disturbed cloud forest fragments.
Methods
Study area
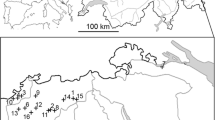
The study was conducted in the cloud forest of central Veracruz, Mexico, in an Important Bird Area (Arizmendi and Márquez Valdelamar 2000). We sampled three sites with varying degrees of forest modification that were separated by 5–11 km. The conserved forest site comprised 125 ha of continuous, mature cloud forest, surrounded by secondary growth cloud forest, 40 ha of which occurs in the Santuario de Bosque de Niebla Francisco Javier Clavijero (19.5ºN, 97.02ºW), that has been managed as a protected area by the Instituto de Ecología A.C. since 1976. The moderately disturbed site comprised 67.5 ha of mature cloud forest located in El Tejar Garnica, Xalapa (19.52ºN, 96.89ºW), which has been under protection since 1986, and is surrounded by secondary growth, grassland, and urban areas. Finally, the highly disturbed site was located in the 66-ha Rancho El Trébol, in Banderilla county (19.59ºN, 96.97ºW), and comprised several cloud forest fragments (totaling 6.5 ha of cloud forest), that were surrounded by disturbed and second growth forest, and immersed in an agricultural matrix. The region has a temperate-humid climate with mean temperature of 18 °C, and year-round rainfall of 1500–2000 mm annually (Williams-Linera et al. 2013). All sites were located within an altitudinal range of 1320–1690 m above sea level to reduce potential effects due to altitudinal variation.
Forest structure
To evaluate the influence of habitat modification on forest structure at the three sites, we measured five woody vegetation parameters: tree height, abundance and basal area, and shrub height and abundance. We established a 25-m-diameter circular plot in 80 marked Wilson’s Warbler territories (Blake and Hoppes 1986): 30 plots in the conserved forest site, 26 plots in the moderately disturbed site, and 24 plots in the highly disturbed site. Within each plot, we counted all trees and shrubs to quantify abundance, and calculated tree and shrub height using a clinometer (Suunto PM-5). We also estimated tree basal area from the center of the sample plot using an angle gauge (JIM-GEM Cruz-All) with an English basal area factor of five (Barret and Allen 1966).
Bird density
To determine the density of Wilson’s Warblers, we established 20 variable-radius point-counts within a ∼60-ha area at each site (Buckland et al. 1993), with a separation distance of 200 m between point-counts, which is sufficient to avoid pseudo-replication (Hurlbert 1984) and spatial correlation among sites (Rueda-Hernández et al. 2015). All point-counts were located in cloud forest vegetation, and at the highly disturbed site point-counts were distributed among various cloud forest patches maintaining a separation of 200 m among points. One observer (A. R. S.) conducted all point-counts during January in the winters of 2011–2012, 2012–2013, and 2013–2014. However, only eight point-counts were conducted at the highly disturbed site of Trébol during the first winter of 2011–2012. Each point-count was conducted for 5 min, during which we recorded all visual and audio detections of Wilson’s Warbler individuals (Ralph et al. 1996), and measured the distance from the observer to each detection with a range finder (Vortex Ranger 1000).
Body condition
We used up to four 12-m-long, 32-mm-mesh mist nets with playback (Johnson et al. 1981) to capture territorial Wilson’s Warbler individuals. Over three seasons we captured a total of 72 individuals with two recaptures, obtaining a total of 74 body condition measurements: 29 in the conserved forest site (2011–2012, n = 10; 2012–2013, n = 10; 2013–2014, n = 9); 25 captures in the moderate disturbance site (2011–2012, n = 6; 2012–2013, n = 9; 2013–2014, n = 10); and 20 captures in the highly disturbed site (2011–2012, n = 2; 2012–2013, n = 8; 2013–2014, n = 10). Captures were conducted in late December and January to exclude transitory migrating individuals and because birds were not responsive before mid-December, possibly because they had not yet established territories.
For each captured bird we determined age and sex following Pyle (1997) and Guallar et al. (2009), although most of the banded individuals were males (76 %) and adults (60 %), and this was consistent among the three sites and years. We also estimated a score (0–5) for fat accumulation in the furcular hollow or elsewhere on the body (North American Banding Council 2001). However, given that captures were undertaken in December shortly after birds established winter territories, 96 % of captured birds had no trace of body fat, and the rest had a fat score of 1 (three individuals); therefore, we pooled all data for body condition analysis. We measured wing chord (wing-ruler, 15-cm length, 0.5-mm precision), tarsus (digital caliper, 0.1-mm precision), and body mass (OHAUS Scout scale, 200-g capacity, 0.1-g precision) of each captured bird. We then calculated body condition following Strong and Sherry (2000, 2001) where body mass was first regressed against tarsus and wing length (r 2 = 0.308, F 2,76 = 16.9, P < 0.001). This provided the regression equation: predicted body mass = −1.49 + (0.13 × tarsus) + (0.11 × wing). We then calculated the body condition index by subtracting predicted body mass from actual body mass. Finally, we attached Darvic color bands for later visual identification of released birds (Ralph et al. 1996).
Territory size
To estimate territory size, we followed color-banded individuals within 4 h after sunrise, on two different occasions ~2 weeks apart during the winter (Marra and Holmes 2001), and only estimated territory size for individuals with at least five georeferenced location changes per visit. When a banded bird was re-sighted, we followed its movements for up to 2 h to record location changes and obtain a global positioning system coordinate for each new location. This provided a minimum of 10 min of location changes (excluding time when the bird was perched) per visit. When birds were lost from sight, movement recording was paused and only continued when the individual was re-sighted. Birds were detected and followed through mature and secondary growth cloud forest in the conserved and moderately disturbed sites as no individual at these sites was territorial in the surrounding disturbed matrix. However, in the highly disturbed site birds also used open areas with scattered trees that we confirmed were part of the territory by observations of agonistic behavior with conspecifics or response to playback.
We measured territory size for 63 individuals: 23 in the conserved forest site (2011–2012, n = 8; 2012–2013, n = 8; 2013–2014, n = 7 individuals); 20 territories in the moderate disturbance site (2011–2012, n = 5; 2012–2013, n = 7; 2013–2014, n = 8 individuals); and 20 territories in the highly disturbed site (2011–2012, n = 2, 2012–2013: n = 8, 2013–2014, n = 10 individuals). There were two territory estimates of the same individual in different winters: once in the moderately disturbed site, and once in the highly disturbed site. We calculated territory size using the minimum convex polygon function in Hawth’s tools (Beyer 2004) and the layer attributes function in ArcGIS 9.3 [Environmental Systems Research Institute (ESRI) 2008].
Statistical analysis
We evaluated normality using the Shapiro–Wilk test. Forest structure variables (mean tree height, mean shrub height, and tree basal area) were all normally distributed, therefore we performed one-way ANOVA tests to compare these structural characteristics among sites. Where a significant difference was found, we applied Tukey post hoc tests to determine which site was significantly different. However, we used Kruskal–Wallis ANOVA with Dunn post hoc test to compare tree and shrub density among sites since these data were not normally distributed.
We analyzed point-count bird survey data using the Distance program (Thomas et al. 2010) to obtain Wilson’s Warbler density estimates for each site, selecting the density model with the lowest Akaike value, which in this case was the half-normal model. To determine whether density estimates were significantly different among sites, we compared 84 % confidence intervals, assuming significant differences when confidence intervals did not overlap (Payton et al. 2003; MacGregor-Fors and Payton 2013). Density estimates based on 20 point-counts were compared among the three sites in the winters of 2012–2013 and 2013–2014. However, for the first winter of 2011–2012, we compared density estimates from just the conserved and moderately disturbed sites as only eight point-counts were conducted in the highly disturbed site during the first year, and these did not provide sufficient data for comparison.
We found no statistical differences in body condition by age (t 70 = 0.49, P = 0.61) or sex (t 70 = −0.46, P = 0.64) of individuals, therefore we combined data for further analyses. Body condition data were normally distributed only for the first winter of 2011–2012 (Shapiro–Wilk test) when comparing just the conserved and moderately disturbed sites as there was insufficient data to include the highly disturbed site in the analysis. Therefore, we applied a two-sample t-test to compare body condition of birds between the conserved and moderately disturbed sites in the first winter, and used Kruskal–Wallis ANOVA with a Dunn post hoc test to compare among the three sites in the second and third winters. When combining data from all three winters, we included data from recaptured birds only for the first winter they were captured, so as to preserve the assumption of independence for statistical tests.
Territory size also did not differ significantly by age (U 59 = −1.71, P = 0.09) or sex (U 59 = −0.78, P = 0.43), and data were combined for further analyses. Territory size data were only normally distributed for the first winter of 2011–2012; therefore, we performed a two-sample t-test to compare territory size of Wilson’s Warblers between the conserved and moderately disturbed sites in the first winter. We then performed Kruskal–Wallis ANOVA with a Dunn post hoc test to compare territory size among all three sites for the second and third winters, and for all three winters combined. For all statistical analyses we used α = 0.05 and descriptive statistics are presented as mean ± SD.
Results
Habitat variation in forest structure
We found significant differences among forest sites in tree abundance (H 2,77 = 25.4, P < 0.001), tree height (F 2,77 = 3.73, P = 0.028), shrub abundance (H 2,77 = 40.1, P < 0.001), and shrub height (F 2,77 = 13.0, P < 0.001). Overall, plots at the conserved forest site had more trees and shrubs, and these were taller than in the disturbed forest sites (Fig. 1). In particular, tree and shrub abundance were significantly greater in the conserved cloud forest site compared to the moderately disturbed (trees, q = 2.53, P < 0.05; shrubs, q = 5.24, P < 0.05), and highly disturbed (trees, q = 5.02, P < 0.05; shrubs, q = 5.54, P < 0.05) sites. Moreover, the moderately disturbed site had significantly greater tree abundance than the highly disturbed site (q = 2.46, P < 0.05). Trees were also significantly taller in the conserved forest compared to the moderately disturbed forest (q = 3.79, P = 0.025), and shrubs were significantly taller in the conserved site compared to both disturbed sites (moderately disturbed, q = 6.95, P < 0.001; highly disturbed, q = 4.89, P < 0.003).
Variation in bird density
During each of the three winters, density of Wilson’s Warbler was highest in the conserved cloud forest site of Sanctuario Bosque Niebla (Fig. 2), with a mean 9.8 ± 1.6 individuals (ind)/ha, which was more than double the density of Wilson’s Warblers in the disturbed sites (moderately disturbed, 4.3 ± 0.26 ind/ha; highly disturbed, 4 ± 0.4 ind/ha). Furthermore, density estimates at each site were consistent among years (Fig. 2). Comparison of the 84 % confidence intervals demonstrated that in all three winters bird density was significantly higher in the conserved cloud forest site compared to disturbed sites (Fig. 2).
Density estimates [individuals (ind)/ha] with 84 % confidence intervals (CI) for Wilson’s Warblers at three cloud forest sites with different degrees of disturbance, in three consecutive winters a 2011–2012, b 2012–2013, and c 2013–2014, based on 20 point-counts per site. There is a significant difference among sites where CI do not overlap
Body condition
Wilson’s Warbler had an overall body condition index of −0.23 ± 0.29 (n = 74). Body condition indices did not differ significantly among sites in each winter season, or for all three winters combined (conserved forest site, −0.27 ± 0.26, n = 30; moderately disturbed, −0.16 ± 0.35, n = 23; highly disturbed, −0.26 ± 0.27, n = 21). Nevertheless, birds in conserved forest exhibited a relatively constant body condition index over all winters, whereas birds in disturbed forest sites showed greater inter-annual fluctuations in body condition indices (Fig. 3).
Variation in territory size
Overall, mean winter territory size of Wilson’s Warbler in cloud forest was 766.2 ± 858.3 m2 (n = 61 territories). Taking all 3 years together, birds in the conserved cloud forest site had smaller territories of 361.7 ± 228.2 m2 (n = 23 birds), compared to a territory size of 1092.6 ± 1226.4 m2 for 18 birds in the moderately disturbed site, and 890.2 ± 743.6 m2 for 20 birds in the highly disturbed site. Furthermore, in each of the three winter seasons territory size was smaller in the conserved forest site compared to the disturbed forest sites (Fig. 4). We found a significant difference in territory size among sites for the third winter season (H 2,24 = 7.8, P = 0.021), and for all three seasons combined (H 2,58 = 8.41, P = 0.015). Dunn post hoc analysis showed that in both cases birds in the conserved forest site had significantly smaller territories compared to birds in the highly disturbed forest site (2013–2014 winter, q = 2.79, P < 0.05; combined winters, q = 2.77, P < 0.05). Moreover, in the conserved forest territory sizes were small in each of the three winters, but birds in disturbed forest sites showed higher inter-annual variation in territory size (Fig. 4).
Discussion
We found that Wilson’s Warblers in the conserved cloud forest site had greater densities, smaller territory sizes, and more consistent inter-annual variation in body condition, suggesting that the conserved forest site represents higher quality winter habitat for the species. The conserved forest site also had greater abundance of taller trees and shrubs than the disturbed forest sites. Therefore, mature, conserved forests may have greater structural complexity able to hold a larger number of birds, with territorial individuals able to meet their resource requirements within a smaller defended area than birds in disturbed forests. A high-quality habitat is considered to have sufficient resources to support a higher population size than a low-quality habitat (Gilroy and Sutherland 2007). Nevertheless, density estimation alone may not be a good indicator of habitat quality, and needs to be accompanied by the evaluation of other variables (van Horne 1983; Vickery et al. 1992; Marra and Holmes 2001). Thus, the fact that Wilson’s Warblers also have smaller territories in the conserved forest, and that all three variables of density, territory size, and body condition are consistent among winter seasons in the conserved forest site, strengthens the conclusion that this represents higher quality habitat for migrating Wilson’s Warblers.
Wilson’s Warbler appears able to breed in both disturbed and undisturbed habitats (Hejl et al. 1995; Desrochers et al. 2012). During migration, however, forest habitats may be more suitable stopover sites for the species, as forest sites with tall trees and a mix of shrubs enabled birds to gain body mass at a higher rate and spend less time in stopovers compared to agricultural fields and edge habitats (Yong et al. 1998). This is supported by our findings for the winter season, where conserved cloud forest, with more abundant and taller shrubs and trees, may provide homogeneous and consistent habitat conditions among years, enabling migrating birds to maintain similar behavior and condition through time, as indicated by the relative constancy of bird density, territory size, and body condition among years at the conserved forest site.
Wilson’s Warbler territory size in cloud forest of central Veracruz was smaller than previous territory estimates for the species. Our overall mean territory size of 737 m2 was smaller than the territory sizes reported for Wilson’s Warbler at summer breeding grounds in North America, which range from 2000 to 13,000 m2 (Stewart 1973; Stewart et al. 1977). This pattern of smaller winter territories compared to breeding territories is shared by other insectivorous warblers such as the Hooded Warbler, Setophaga citrina (Rappole and Warner 1980; Howlett and Stutchbury 1997), and American Redstart, Setophaga ruticilla (Sturm 1945; Ficken 1962; Sherry and Holmes 1989, 1997). However, territory sizes obtained in our study were also smaller than the 3000-m2 home range reported for Wilson’s Warbler during winter in the rainforest of Veracruz (Rappole and Warner 1980), although home range may include exploratory movements and may comprise area not actively defended by individuals. Furthermore, Rappole and Warner (1980) estimated home range by dividing the survey area with the number of individuals, and may include some dead space in the estimate of home range. Therefore, we recognize that there may be limitations in comparison of territory size estimates among studies as not all studies use the same method for determining territory size.
Nevertheless, territories as small as those maintained by Wilson’s Warblers in the cloud forest of Veracruz have also been reported for the Yellow Warbler, Setophaga petechia (520 m2) in Chiapas, Mexico, where individuals defend the richest arthropod habitat (several trees) within a pasture matrix (Greenberg and Salgado-Ortiz 1994). Therefore, the overall small territory size recorded for Wilson’s Warblers in our study suggests that cloud forest may be a resource-rich wintering habitat. Cloud forest may present benign microclimatic conditions for Wilson’s Warblers since humid habitats with increased rainfall have greater arthropod abundance, and are better habitats for primarily insectivorous migratory birds (Latta and Faaborg 2002; Studds and Marra 2005, 2007; Brown and Sherry 2006; Smith et al. 2010). Cloud forest has high levels of precipitation, similar to other wet-forest habitats, although even rainforests have been reported to have lower arthropod abundance than cloud forest (Townsend et al. 2012). Moreover, humid habitats have been linked to improved body condition of another Neotropical migratory warbler, the American Redstart (Marra et al. 1998; Marra and Holmes 2001).
Although territory size of Wilson’s Warbler was larger in the disturbed forest sites, there was no difference in body condition among degrees of forest disturbance. This suggests that birds modify their behavioral strategies to compensate for resource differences among habitats. Birds can modify their diet through foraging plasticity (Martins et al. 2013), store more fat in habitat with few or less constant resources (Strong and Sherry 2000), and defend a larger territory under low food availability. It is possible that, even when resources may be unevenly distributed in cloud forest fragments of differing sizes and degrees of disturbance, varying behavioral strategies may enable individuals to maintain similar body condition by defending larger territories when needed. Furthermore, the fact that Wilson’s Warblers maintain territories in disturbed cloud forest shows that such disturbed habitats may still be beneficial, since territorial defense implies trade-offs by making the individual more conspicuous to predators (Campos et al. 2009), and leading to aggressive behavior with conspecifics that has high energy costs by restricting foraging time (Cresswell 2008)—risks that birds would not take unless there was a worthwhile benefit. Coincidently, larger winter territories have been reported for migratory birds in disturbed habitats (pastures and hedgerows), added to which a high proportion of birds are non-territorial (Rappole and Warner 1980; Rappole and Morton 1985). Furthermore, territorial individuals of the Ovenbird (Seiurus aurocapillus), another migratory insectivorous warbler, were found to have higher body mass with lower foraging rates when compared to floaters (Kresnik and Stutchbury 2014), therefore territoriality may have benefits in enabling exclusive access to resources.
Low variation in body condition and territory size among Wilson’s Warbler individuals in the conserved forest suggests that this is the most homogeneous habitat of the three sites, and that forest size and structure could be affecting wintering ecology of migratory birds. The greater area of continuous forest cover in the conserved forest may lead to greater food resource availability, since higher insect abundance has been found in continuous forest compared to fragmented forests (Ruiz-Guerra et al. 2012). Accordingly, in central Veracruz there is higher abundance of forest specialist birds, such as understory insectivores, in large fragments of mature cloud forest compared to smaller fragments in disturbed areas (Rueda-Hernández et al. 2015). Furthermore, the inter-annually consistent territory size and body condition of birds in the conserved forest indicate resource stability, similar to that found in other evergreen forests when compared to drier habitats (Brown and Sherry 2006; Smith et al. 2010). This inter-annual resource stability could be an additional benefit for migratory birds and make conserved forest conditions more predictable from year to year compared to disturbed forests; this predictability could ultimately be reflected in individual overwintering survivorship. In support of this, Wolfe et al. (2015) found that the negative effects of climatic variability, such as El Niño droughts, on the White-collared Manakin (Manacus candei) were reduced in large mature forests compared to early successional forests.
The high inter-annual variability in territory size and body condition of Wilson’s Warblers in disturbed forests suggests that forest habitats subject to human disturbance are less stable over time, which may represent a drawback when selecting winter territories. Greater resource stability in mature, conserved cloud forest would make this a more reliable habitat over time for wintering Wilson’s Warblers, increasing their chances of survival, and the likelihood that they will maintain good body condition, essential for an early return to breeding grounds and increased fitness (Marra et al. 1998). By comparison, variable conditions in disturbed and smaller forest fragments could work as an ecological trap, preventing birds from seeking better territories when conditions appear to be good, which in subsequent years may be radically different (Ekroos et al. 2012).
Our results are of greater relevance considering that we found differences in Wilson’s Warbler wintering ecology within the same forest type, but under differing levels of disturbance. Variations in winter ecology have generally been determined between distinct and more contrasting habitat types (Sherry and Holmes 1996; Marra et al. 1998; Latta and Faaborg 2002; Saino et al. 2004), where differences are more likely to occur. However, our results demonstrate that even changes in area and structure of the same habitat type could significantly affect wintering performance of migratory birds, and there may be distinct carryover effects for individuals wintering in different sites within the same habitat.
Taken together our findings suggest that mature, conserved cloud forest represents a high-quality wintering habitat for Wilson’s Warbler, and this habitat condition could also benefit other migratory birds that have similar ecological requirements. Our population-level analysis of bird density demonstrated that conserved forest was able to hold a greater number of territorial and non-territorial birds. On the other hand, evaluation of individual territory size and body condition suggests that territorial birds inhabiting disturbed forest maintain body condition by expanding territory size, since territoriality reduces intra-specific competition (Odum and Kuenzler 1955), and provides exclusive access to food resources (Parrish and Sherry 1994; Sogge et al. 2007). However, the high variability in territory size and body condition of birds in disturbed forests among years suggests that the effectiveness of adjusting territory size may vary from year to year.
To properly direct conservation efforts it is important to understand the effects of wintering habitat on the behavior and population traits of migratory birds, particularly since wintering habitat has important carryover effects on breeding success (Marra et al. 1998; Norris et al. 2004; Reudink et al. 2009). Knowledge of habitat use by Neotropical migratory warblers during the winter helps to reveal features of the habitat that could be driving population declines. We stress the importance of actively protecting remnants of mature cloud forest, as well as second-growth forest that can be restored, which bird density, territory size and body condition all indicate are better quality habitats for Wilson’s Warblers. Future studies addressing Wilson’s Warbler wintering ecology in different habitats would help us to understand the importance of each habitat in the species’ wintering dynamics and its entire life cycle. The results of our study confirm that even when birds are able to offset resource limitations through physiological and behavioral plasticity (Weber and Hedenström 2001; Pierce and McWilliams 2005), disturbed habitats are not ideal for migratory birds, and we still do not know the implications for trade-offs when balancing resource shortages. Migratory birds undergo seasonal changes in needs and behavioral traits, and only by understanding the way in which they utilize available habitats will we be able to propose the most appropriate strategies to preserve, and as a more ambitious goal, possibly to improve the status of wild populations.
References
Ammon EM, Gilbert WM (1999) Wilson’s Warbler (Wilsonia pusilla). In: Poole A, Gill F (eds) The birds of North America 478. The Birds of North America Inc, Philadelphia, p 28
Arizmendi MC, Márquez Valdelamar L (2000) Áreas de importancia para la conservación de las aves de México. Sociedad para el Estudio y Conservación de las Aves en México AC. Mexico
Askins RA, Lynch JF, Greenberg R (1990) Population declines in migratory birds in eastern North America. In: Johnston R (ed) Current Ornithology, vol 7. Plenum, New York, pp 1–57
Ballard G, Geupel GR, Nur N, Gardali T (2003) Long-term declines and decadal patterns in population trends of songbirds in western North America, 1979–1999. Condor 105:737–755
Barret JP, Allen PH (1966) Angle-guage sampling a small hardwood tract. For Sci 12:83–89
Berlanga H, Kennedy JA, Rich TD, Arizmendi MC, Beardmore CJ, Blancher PJ, Butcher GS, Couturier AR, Dayer AA, Demarest DW, Easton WE, Gustafson M, Iñigo-Elías E, Krebs EA, Panjabi AO, Rodríguez Contreras V, Rosenberg KV, Ruth JM, Santana Castellón E, Vidal RM, Will T (2010) Saving our shared birds: partners in flight tri-national vision for landbird conservation. Cornell Lab of Ornithology, Ithaca
Beyer HL (2004) Hawth’s analysis tools for ArcGIS. http://www.spatialecology.com/htools. Accessed 11 May 2015
Blake JG, Hoppes WG (1986) Influence of resource abundance on use of tree-fall gaps by birds in an isolated woodlot. Auk 103:328–340
Brown DR, Long JA (2007) What is a winter floater? Causes, consequences, and implications for habitat selection. Condor 109:548–565
Brown DR, Sherry TW (2006) Food supply controls the body condition of a migrant bird wintering in the tropics. Oecologia 149:22–32
Buckland ST, Anderson DR, Burnham KP, Laake JL (1993) Distance sampling: estimating abundance of biological populations. Chapman & Hall, London
Campos DP, Bander LA, Raksi A, Blumstein DT (2009) Perch exposure and predation risk: a comparative study in passerines. Acta Ethol 12:93–98
Cresswell W (2008) Non-lethal effects of predation in birds. Ibis 150:3–17
Desrochers A, Tardif J, Mazerolle MJ (2012) Use of large clear-cuts by Wilson’s Warbler in an eastern Canadian boreal forest. Avian Conserv Ecol 7:1. doi:10.5751/ACE-00521-070201
Eckhardt RC (1979) The adaptive syndromes of two guilds of insectivorous birds in the Colorado Rocky Mountains. Ecol Monogr 49:129–149
Ekroos J, Fox AD, Christensen TK, Petersen IK, Kilpi M, Jónsson JE, Green M, Laursen K, Cervencl A, de Boer P, Nilsson L, Meissner W, Garthe S, Öst M (2012) Declines amongst breeding Eider Somateria mollissima numbers in the Baltic/Wadden Sea flyway. Ornis Fenn 89:81–90
ESRI (2008) ArcGIS desktop: release 10. Environmental Systems Research Institute, Redlands
Ficken MS (1962) Agonistic behavior and territory in the American Redstart. Auk 79:607–632
Finch DM (1989) Habitat use and habitat overlap of riparian birds in three elevational zones. Ecology 70:866–880
Gilroy JJ, Sutherland WJ (2007) Beyond ecological traps: perceptual errors and undervalued resources. Trends Ecol Evol 22:351–356
Graham CH, Blake JG (2001) Influence of patch- and landscape-level factors on birds assemblages in a fragmented tropical landscape. Ecol Appl 11:1709–1721
Greenberg R (1992) Forest migrants in non-forest habitats on the Yucatan Peninsula. In: Hagan JM, Johnston DW (eds) Ecology and conservation of Neotropical migrant landbirds. Smithsonian Institution Press, Washington, pp 273–286
Greenberg R, Salgado-Ortiz J (1994) Interspecific defense of pasture trees by wintering Yellow Warblers. Auk 111:672–682
Guallar S, Santana E, Contreras S, Verdugo H, Gallés A (2009) Passeriformes del occidente de México: biometría, datación y sexado. Museu de Ciències Naturals de Barcelona, Barcelona
Gunnarsson TG, Gill JA, Newton J, Potts PM, Sutherland WJ (2005) Seasonal matching of habitat quality and fitness in a migratory bird. Proc R Soc B 272:2319–2323. doi:10.1098/rspb.2005.3214
Hart PJ, Woodworth BL, Camp RJ, Turner K, McClure K, Goodall K, Henneman C, Spiegel C, Lebrun J, Tweed E, Samuel M (2011) Temporal variation in bird and resource abundance across an elevational gradient in Hawaii. Auk 128:113–126
Hejl SJ, Hutto RL, Preston CR, Finch DM (1995) Effects of silvicultural treatments in the Rocky Mountains. In: Martin TE, Finch DM (eds) Ecology and management of Neotropical migratory birds. Oxford University Press, New York
Howlett JS, Stutchbury BJM (1997) Within-season dispersal, nest-site modification, and predation in renesting Hooded Warblers. Wilson Bull 109:643–649
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Hutto RL (1981) Seasonal variation in the foraging behavior of some migratory western Wood Warblers. Auk 98:765–777
Hutto RL (1994) The composition and social organization of the mixed-species flocks in a tropical deciduous forest in western Mexico. Condor 96:105–118
Iwamura T, Fuller RA, Possingham HP (2014) Optimal management of a multispecies shorebird flyway under sea-level rise. Conserv Biol 28:1710–1720. doi:10.1111/cobi.12319
Johnson RR, Brown BT, Haight LT, Simpson JM (1981) Playback recording as a special avian censusing technique. In: Ralph CJ, Scott JM (eds) Estimating the numbers of terrestrial birds. Studies in avian biology, vol 6, pp 68–75
Kresnik RJ, Stutchbury BJM (2014) Space-use strategies of wintering Ovenbirds in Belize: causes and consequences. J Field Ornithol 85:274–288
Latta SC, Faaborg J (2002) Demographic and population responses of Cape May Warblers in multiple habitats. Ecology 83:2502–2515
Lefebvre G, Poulin B (1996) Seasonal abundance of migrant birds and food resources in Panamanian mangrove forests. Wilson Bull 108:748–759
Lefebvre G, Poulin B, McNeil R (1994) Temporal dynamics of mangrove bird communities in Venezuela with special reference to migrant warblers. Auk 111:405–415
Long JA, Stouffer PC (2003) Diet and preparation for spring migration in captive Hermit thrushes (Catharus gutattus). Auk 120:323–330
MacGregor-Fors I, Payton ME (2013) Contrasting diversity values: statistical inferences based on overlapping confidence intervals. PLoS One 8:e56794. doi:10.1371/journal.pone.0056794
Marra PP, Holmes RT (2001) Consequences of dominance-mediated habitat segregation in American Redstarts during the nonbreeding season. Auk 118:92–104
Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282:1884–1886
Martins RC, Catry T, Santos CD, Palmeirim JM, Granadeiro JP (2013) Seasonal variations in the diet and foraging behaviour of Dunlins Calidris alpina in a south European estuary: improved feeding conditions for northward migrants. PLoS One 8:e81174. doi:10.1371/journal.pone.0081174
McShea WJ, McDonald MV, Morton ES, Meier R, Rappole JH (1995) Long-term trends in habitat selection by Kentucky Warblers. Auk 112:375–381
Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliffe LM (2004) Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc R Soc B 271:59–64. doi:10.1098/rspb.2003.2569
North American Banding Council (2001) The North American bander’s study guide. Point Reyes Station, CA
Odum EP, Kuenzler EJ (1955) Measurement of territory and home range size in birds. Auk 72:128–136
Parrish JD, Sherry TW (1994) Sexual habitat segregation by American Redstarts wintering in Jamaica: importance of resource seasonality. Auk 111:38–49
Payton ME, Greenstone MH, Schenkerk N (2003) Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci 3:1–6
Pierce BJ, McWilliams SR (2005) Seasonal changes in composition of lipid stores in migratory birds: causes and consequences. Condor 107:269–279
Pyle P (1997) Identification guide to North American birds. Part 1. Slate Creek Press, Bolinas
Ralph CJ, Geupel R, Pyle P, Martin TE, DeSante DF, Milá B (1996) Handbook of field methods for monitoring landbirds. General technical report PSW-GTR-159. US Department of Agriculture, Albany
Rappole JH, McDonald MV (1994) Cause and effect in population declines of migratory birds. Auk 111:652–660
Rappole JH, Morton ES (1985) Effects of habitat alteration on a tropical avian forest community. In: Buckley PA, Morton ES, Ridgely R, Buckley FG (eds) Neotropical ornithology, ornithological monographs 36, pp 1013–1021
Rappole JH, Warner DW (1980) Ecological aspects of migrant bird behavior in Veracruz, Mexico. In: Keast A, Morton ES (eds) Migrant birds in the Neotropics: ecology, behavior, distribution and conservation. Smithsonian Institution Press, Washington, pp 353–393
Reudink MW, Marra PP, Kyser TK, Boag PT, Langin KM, Ratcliffe LM (2009) Non-breeding season events influence sexual selection in a long-distance migratory bird. Proc R Soc B 276:1619–1626
Robbins CS, Sauer JR, Greenberg RS, Droege S (1989) Population declines in North American birds that migrate to the Neotropics. Proc Natl Acad Sci USA 86:7658–7662
Rueda-Hernández R, MacGregor-Fors I, Renton K (2015) Shifts in resident bird communities associated with cloud forest patch size in Central Veracruz, Mexico. Avian Conserv Ecol 10:2. doi:10.5751/ACE-00751-100202
Ruiz-Guerra B, Renton K, Dirzo R (2012) Consequences of fragmentation of tropical moist forest for birds and their role in predation of herbivorous insects. Biotropica 44:228–236
Ruiz-Sánchez A, Renton K, Landgrave-Ramírez R, Mora-Aguilar EF, Rojas-Soto O (2015) Ecological niche variation in the Wilson’s Warbler Cardellina pusilla complex. J Avian Biol 46:516–527
Saab VA, Bock CE, Rich TD, Dobkin DS (1995) Livestock grazing effects in western North America. In: Martin TE, Finch DM (eds) Ecology and management of Neotropical migratory birds. Oxford University Press, New York, pp 311–353
Saino N, Szép T, Ambrosini R, Romano M, Møller AP (2004) Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proc R Soc B 271:681–686
Sauer JR, Hines JE, Fallon JE, Pardieck KL, Ziolkowski Jr DJ, Link WA (2014) The North American breeding bird survey, results and analysis 1966–2013. Version 01.30.2015 USGS Patuxent Wildlife Research Center, Laurel
Sherry TW, Holmes RT (1989) Age-specific social dominance affects habitat use by breeding American Redstarts (Setophaga ruticilla): a removal experiment. Behav Ecol Sociobiol 25:327–333
Sherry TW, Holmes RT (1996) Winter habitat quality, population limitation, and conservation of Neotropical-Nearctic migrant birds. Ecology 77:36–48
Sherry TW, Holmes RT (1997) American Redstart (Setophaga ruticilla). In: Poole A, Gill FB (eds) The birds of North America 277. Academy of Natural Science, Philadelphia and American Ornithological Union, Washington, p 32
Smith JAM, Reitsma LR, Marra PP (2010) Moisture as a determinant of habitat quality for a nonbreeding Neotropical migratory songbird. Ecology 91:2874–2882
Sogge MK, Koronkieweicz TJ, VanRiper C III, Durst SL (2007) Willow flycatcher nonbreeding territory defense behavior in Costa Rica. Condor 109:475–480
Stewart RM (1973) Breeding behavior and life history of the Wilson’s Warbler. Wilson Bull 85:21–30
Stewart RM, Henderson RP, Darling K (1977) Breeding ecology of the Wilson’s Warbler in the high Sierra Nevada, California. Living Bird 16:83–102
Stiles FG, Skutch AF, Gardner D (1995) Birds of Costa Rica. Cornell University Press, Ithaca
Strong AM, Sherry TW (2000) Habitat-specific effects of food abundance on the condition of ovenbirds wintering in Jamaica. J Anim Ecol 69:883–895
Strong AM, Sherry TW (2001) Body condition of Swainson’s Warblers wintering in Jamaica and the conservation value of Caribbean dry forest. Wilson Bull 113:410–418
Studds CE, Marra PP (2005) Nonbreeding habitat occupancy and population processes an upgrade experiment with a migratory bird. Ecology 86:2380–2385
Studds CE, Marra PP (2007) Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird, Setophaga ruticilla. Clim Res 35:115–122
Sturm L (1945) A study of the nesting activities of the American Redstart. Auk 62:189–206
Sutherland WJ (1996) Predicting the consequences of habitat loss for migratory populations. Proc R Soc B 263:1325–1327
Thomas L, Buckland ST, Rexstad EA, Laake JL, Strindberg S, Hedley SL, Bishop JRB, Marques TA, Burnham KP (2010) Distance software: design and analysis of distance sampling surveys for estimating population size. J Appl Ecol 47:5–14
Townsend JM, Rimmer CC, McFarl KP, Goetz JE (2012) Site-specific variation in food resources, sex ratios, and body condition of an overwintering migrant songbird. Auk 129:683–690
van Horne B (1983) Density as a misleading indicator of habitat quality. J Wildl Manage 47:893–901
Vickery PD, Malcolm ML Jr, Wells JV (1992) Is density an indicator of breeding success? Auk 109:706–710
Weber TP, Hedenström A (2001) Long-distance migrants as a model system of structural and physiological plasticity. Evol Ecol Res 3:255–271
Weber TP, Houston AI, Ens BJ (1999) Consequences of habitat loss at migratory stopover sites: a theoretical investigation. J Avian Biol 30:416–426
Williams-Linera G, Toledo-Garibaldi M, Gallardo Hernández C (2013) How heterogeneous are the cloud forest communities in the mountains of central Veracruz, Mexico? Plant Ecol 214:685–701
Wolfe JD, Ralph CJ, Elizondo P (2015) Changes in the apparent survival of a tropical bird in response to the El Niño oscillation in mature and young forest in Costa Rica. Oecologia 178:315–721
Yong W, Finch DM, Moore FR, Kelly JF (1998) Stopover ecology and habitat use of migratory Wilson’s Warblers. Auk 115:829–842
Acknowledgments
The study was conducted in partial fulfillment of a Ph.D. in Biological Sciences at the Universidad Nacional Autónoma de México (UNAM). Funding for the research was provided by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (UNAM-DGAPA-PAPIIT grant IN203012), and the Consejo Nacional de Ciencias y Tecnología (CONACyT 179877) to K. R. CONACyT also provided a Doctoral scholarship to A. R. S. (197592) and R. R. H. (171086). The Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT), Mexico, granted permits for the research, which complies with Mexican law. We thank Ángel F. Rueda Hernández for his technical assistance while carrying out the fieldwork. We are also grateful to Marduck Obrador for allowing us to study birds on his property Rancho el Trébol, and to the Instituto de Ecología A.C. for allowing access to Santuario de Niebla, Francisco Javier Clavijero. We appreciate the constructive comments of the anonymous reviewers that enriched the quality of our paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
Permits for the research were granted by SEMARNAT in compliance with Mexican law.
Additional information
Communicated by C. G. Guglielmo.
Rights and permissions
About this article
Cite this article
Ruiz-Sánchez, A., Renton, K. & Rueda-Hernández, R. Winter habitat disturbance influences density and territory size of a Neotropical migratory warbler. J Ornithol 158, 63–73 (2017). https://doi.org/10.1007/s10336-016-1368-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1368-9