Abstract
The main purpose of this study was to provide exhaustive and extensive data on the breeding biology of the Mediterranean Tawny Pipit Anthus campestris. Reproduction was studied within two populations nesting in shrubsteppes in central Spain. We compared breeding parameters (including hatching success, productivity, incubation, brood failures, predation rates, clutch and brood size) between study areas, and among years within each area. Our results suggest that Tawny Pipit reproduction in Spanish shrubsteppes is influenced by two main related factors: timing of breeding and nest predation. We detected seasonal declines in most breeding parameters, but a mid-season peak in productivity. Although large-sized broods were detected at the beginning of the breeding season, the recruitment of these juveniles was very low due to high predation rates on early broods. Finally, our results are compared with the scarce available data from previous breeding studies from other European Tawny Pipit populations.
Zusammenfassung
Brutleistung und Nestprädationsmuster bei steppegebundenen Vögeln des Mittelmeerraums: der Fall des Brachpiepers Anthus campestris
Der Hauptzweck dieser Studie war es, vollständige und umfassende Daten zur Brutbiologie mediterraner Brachpieper Anthus campestris bereitzustellen. Die Fortpflanzung wurde in zwei Populationen, die in Strauchsteppe in Zentralspanien nisten, untersucht. Wir haben Brutparameter (einschließlich Schlupferfolg, Produktivität, Bebrütung, Brutverluste, Prädationsraten, Gelege- und Brutgrößen) zwischen den Untersuchungsgebieten sowie innerhalb der Untersuchungsgebiete zwischen verschienenden Jahren verglichen. Unsere Ergebnisse deuten darauf hin, dass die Fortpflanzung von Brachpiepern in spanischen Strauchsteppen von zwei Hauptfaktoren beeinflusst wird: Zeitpunkt des Brütens und Nestprädation. Wir haben ermittelt, dass die meisten Brutparameter im Verlauf der Saison abnahmen, die Produktivität jedoch in der Mitte der Saison ihren Höhepunkt erreichte. Obwohl zu Beginn der Brutsaison große Bruten erfasst wurden, waren die Rekrutierungsraten für diese Jungvögel sehr niedrig, da frühe Bruten einem hohen Prädationsrisiko ausgesetzt waren. Schließlich vergleichen wir unsere Ergebnisse mit den wenigen verfügbaren Daten aus früheren Brutstudien in anderen europäischen Brachpieperpopulationen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds are one of the best-studied groups of the animal kingdom, yet there is still a lack of information on basic aspects of breeding biology in many avian species. This is particularly striking in ground-nesting passerines due to the difficulty in locating and monitoring their nests, which makes difficult to estimate breeding parameters (Martin and Geupel 1993; Sutherland et al. 2004a; Grzybek et al. 2008). The literature on avian life histories is clearly biased towards forest species or populations clustered in north-temperate latitudes (Bautista and Pantoja 2000; Moreno 2004). Clearly, there are major gaps in our knowledge about avian reproduction in steppe habitats within the Mediterranean basin, where there are differences in food seasonality (Blondel et al. 1991; Stamou et al. 2004) and predation rates (Yanes and Suárez 1995) with respect to populations located farther North (Moreno 2004).
The Tawny Pipit Anthus campestris is one of the Palaearctic passerines for which information concerning breeding ecology and life-history traits is mostly lacking. The species is a widespread summer visitor of well-preserved steppe-like habitats ranging from Europe and North Africa to Mongolia (Alström and Mild 2003). The species suffered a sharp decline between 1970 and 1990 (BirdLife International 2004), particularly marked in northern Europe (Van Turnhout 2005). Currently, Spain is considered the stronghold of the species in Europe (673.000–1,164.000 individuals during the reproductive period; Carrascal and Palomino 2008). Biological and ecological studies based on Spanish populations are thus mandatory to guarantee the long-term viability of the species (Sutherland et al. 2004b). Scientific knowledge on this species during the last two decades has been limited to studies on song structure (Osiejuk et al. 2007), changes in female body mass during the breeding season (Suárez et al. 2005a), nest features (Högstedt 1978; Suárez et al. 2005b), population densities (Van Turnhout 2005) and habitat selection (Brambilla and Rubolini 2005; Grzybek et al. 2008). Quantitative data on breeding biology can only be found in studies carried out in the 1970s and 1980s (Högstedt 1978; Bijlsma 1978; Krüger 1989), compiled in Del Hoyo et al. (2004) and Cramp (1998). These studies have provided pivotal information on the natural history of north-temperate Tawny Pipit populations. Nevertheless, much of this information is probably unhelpful for future conservation plans, because many of the identified key factors affecting populations have probably sharply changed during the last decade. This is the case of quantity and suitability of habitat, food resources, predation pressure or climate oscillations (Donald et al. 2001; Crick 2004; Gordo and Sanz 2005). Here, we provide the most extensive study on the breeding biology of the Tawny Pipit to date. We compare the breeding biology in two shrubsteppe populations of central Spain monitored during 3 and 6 years, with particular emphasis on the timing of breeding, reproductive parameters, and nest failures. We also assess the existence of seasonal trends in the breeding parameters in order to infer temporal constraints. Finally, the information collected is compared with the scarce available data of northern Tawny Pipit populations.
Methods
Study areas
Fieldwork was carried out in two natural-steppe areas of 200 ha each located in central Spain (Fig. 1), and included in sites classified as Special Protection Areas (SPAs). Both areas maintain some traditional livestock and agricultural practices, such as extensive grazing (sheep, goats) and some cereal crops (Titricum, Hordeum). The first site, Layna (1,180 m a.s.l., Soria province, 41°05′N, 2°19′W) is a stony-flat terrain with low shrubsteppe vegetation dominated by Genista pumila, G. scorpius, Thymus spp., pastureland (Poa spp. and Stipa spp.) and scattered Juniperus phoenicea. The climate is continental Mediterranean (Peinado and Rivas-Martínez 1987), with an annual rainfall of 500 mm and a mean annual temperature of 10.2 °C (mean temperature in January: 0–2 °C; mean temperature in July: 18–20 °C). Tawny Pipit density is about 18–24 breeding pairs/km2 (personal observation). The second site, Valeria (1,090 m a.s.l., Cuenca province, 39°48′N, 2°10′W), is also flat and the vegetation dominated by calcicolous low shrub, Rosmarinus officinalis and Thymus spp. and the pastures dominated by Braquipodium phoenicoides. In this area, the climate is more similar to the typical Mediterranean conditions, where the annual rainfall reaches 750 mm and the mean annual temperature is 16 °C (mean temperatures in January and July, 4–6 °C and 24–26 °C, respectively). Tawny Pipit density in this area is about 11–15 breeding pairs/km2 (personal observation).
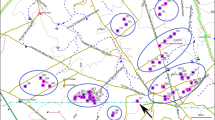
Breeding distribution of Tawny Pipits Anthus campestris according to BirdLife International and NatureServe (2011). The populations in which the length of the breeding period on the species is known are indicated, and a schedule of breeding period (from May to July) is shown for each population: Sweden (Sw), Germany (Ge), The Netherlands (Ne) and the two populations included in this study from Spain (Sp)
Nest monitoring
Data were collected during six breeding seasons in Layna (years 1991–1995, and 2007), and during three consecutive breeding seasons in Valeria (2008–2010). Fieldwork took place the last week of March each year, before the first birds arrived to the study areas, and lasted until the end of July, covering the complete breeding period of the species (Tyler 2004). We searched intensively for nests throughout the breeding season with a similar survey effort between study sites and years (mean ± SD; 93 ± 11 days/person/year). Nests (n = 235) were located by observing adult behaviour during nest building and incubation (n = 140 nests) or by following feeding adults (n = 95 nests). In order to monitor each nest during the breeding period, all nests were marked with a bamboo stick at a distance of 2 m from their exact position (to avoid disturbance), and GPS coordinates were recorded. Nests were visited regularly until fledging or nest failure, and nest status, reproductive parameters and chick growth were registered. Nestlings were individualised (colour-marking their claws) immediately after hatching, and colour-ringed when they were 7 days old. We measured tarsus length (tarsometatarsus; with a digital calliper to the nearest 0.01 mm), wing length (with a ruler to the nearest 0.5 mm; see Svensson 1992), and chick weight (with an electronic balance of 0.01 g resolution) during the nest visits. Nestlings were handled ~200 m apart from the nest in order to reduce, as much as possible, the potential risk of predation associated to our visits.
To assess if nest monitoring affected breeding failures, we recorded during three consecutive years (2008–2010) the following variables per nest: exposure days (i.e. the length of the period during which a nest was under observation (Mayfield 1961), number of visits (i.e. total number of visits during the breeding season), frequency of visits (number of visits/exposure days) and duration of visits (accumulated duration of all visits to each nest).
Breeding parameters and breeding failures
For each nest, the following breeding parameters were recorded: (1) laying date, as the date when the first egg was laid related to 1 April; (2) clutch size, as the number of laid eggs; (3) brood size, as the number of chicks that hatched in successful nest; (4) chick rearing time, as the time from hatching until chicks left the nest (only for nests with known hatching and fledging dates), (5) fledgling number, as the number of young that fledged in successful nests, and (6) productivity, as the number of chicks fledged per breeding attempt.
Laying date was recorded directly during nest visits (n = 20) or was back-calculated from hatching date (n = 65) assuming a laying interval of 1 day, that females start the incubation with the last egg (Cramp 1998) and considering an incubation period of 14 days (data obtained from this study, n = 7 nests). When hatching date was unknown (n = 104), both hatching and laying dates were calculated from chick measurements using nestling growth curves (authors, unpublished data), with an estimated error of ±1 day. When laying dates were unknown and the eggs failed to hatch because of predation or abandonment (n = 57), we assumed that the date the nest was found matched the midpoint of the incubation period.
To analyze trends in breeding failures (complete nest losses during the breeding season), the fate of each nest was coded as failure (1) or success (0). We discriminated in our analyses between incubation failures (complete nest losses during incubation) and brood failures (complete nest losses during the chick rearing period). Nests were assumed to be successful when chicks left the nest (most nestlings leave the nest when 9–11 days old) or if adults were subsequently observed feeding fledglings; otherwise, nests were coded as failed. We also assessed partial losses in successful nests as the proportion of unhatched eggs to the total clutch size, and the proportion of unfledged nestlings to the total number of hatched eggs per brood. Causes of nest failure were classified as predation, female desertion/starvation, or unknown. Nests found empty (or with evident signs of predation) before the expected hatching or fledging date were categorized as predated. Female desertion/starvation was assumed when females were absent from their nests during one or several days and, consequently, eggs never hatched or chicks were found dead in the nests.
For consistency with previous studies, daily survival rates (DSR) were estimated for incubation and chick rearing period period using the Mayfield method (Mayfield 1975) with variances calculated according to Johnson (1979). This method takes into account the fact that nests failing at an early stage have a low probability of being found, resulting in an underestimated nest failure rate (Mayfield 1975).
Data analyses
Between-population variation was analyzed using Student’s t tests for breeding parameters, and using Generalised Linear Models (GLZ) for breeding failures (failure = 1, success = 0) with a binomial error and logit link function. We included exposure days as continuous variable in GLZ models (Shaffer 2004).
Between-year variation in breeding parameters was analysed using one-way ANOVA tests for each population separately because fieldwork was done in different years in each of the study sites and, thus, the interaction population × year could not be evaluated directly. For analysing between-year variation in breeding failures, we fitted one GLZ test for each population and exposure days as continuous variable.
Seasonal changes in clutch size, productivity, the proportion of unhatched eggs and the proportion of unfledlged nestlings were assessed using General Lineal Models (GLM) with normal error distributions and identity link functions. For incubation and brood failures, we used GLZ with binomial error and logit link function, and exposure days included as an explanatory variable. The polynomial term of laying date was included as a explanatory variable to account for possible linear, quadratic or cubic patterns of variation in this parameter (Schielzeth 2010). Laying date was standardised separately for each year to account for between-year or between-population differences. We also included clutch size and brood size as explanatory variables in the analyses of incubation failures and brood failures, respectively, as both factors might influence parent activity and failures during reproduction (Martin et al. 2000).
To assess the possibility that our nest monitoring influenced nest failure, we used GLZ analysis (with binomial error and logit link function) including the frequency and duration of visits (as continuous predictors) and the number of visits (as a categorical predictor). We included the polynomial term of laying date, exposure days and clutch size as additional predictors. The relative importance of each explanatory variable was assessed by partitioning the total deviance of the model into different components.
All results are expressed as means ± standard deviations (SD). All the statistical analyses were carried out using STATISTICA 8.0 package (StatSoft 2007).
Results
Timing of breeding and nest construction
Tawny Pipits arrived to the breeding grounds during the first (Valeria population) or the second week of April (Layna population). Nest building lasted 3–4 days (n = 18 nests surveyed), and was carried out mainly by females (only 3 males contributed sporadically providing nest material). During this period, males spent most of the time in the vicinity of females, engaged in surveillance and mate-guarding activities. Mate guarding is very obvious during this phase, males being constantly involved in aggressive interactions against intruders until females begin the incubation (authors, unpublished data).
The onset of laying occurred earlier in Valeria (12 May ±3 days) than in Layna (20 May ±6 days), and the last clutches were also laid earlier in Valeria (25 June ±8 days) than in Layna (9 July ±11 days). We found significant differences in mean laying date between sites (Table 1), being earlier in Valeria than in Layna, and the laying period was overall shorter in the former (Fig. 1). Between-year variations in laying date were significant in Valeria but not in Layna (Table 1). The seasonal distribution of clutches in both populations showed no clear pattern (Fig. 2). Both populations showed a first laying peak during the second half of May and a much less visible peak in mid-June (Valeria) or early July (Layna) (Fig. 2).
Clutch size, brood size and incubation failures
Females lay eggs at a rate of one egg every 24 h (data calculated for 15 eggs from 6 different nests), and the incubation begins with the last but one egg. The incubation period ranged 13–15 days (mean = 14 ± 0.48 days, modal value = 14 days; n = 7 nests), and the average clutch size was 4.19 ± 0.74 eggs (range = 1–5 eggs, modal value = 4 eggs, n = 235 nests). Nests with less than three eggs were occasional (2.5 % of nests). There were no significant differences in clutch size between populations (Table 1). Between-year variation in clutch size was significant in Layna but not in Valeria (Table 1), and post hoc analyses revealed that the differences in Layna arose from the years 1992 and 1994 (Tukey test: P = 0.03), all other pairwise comparisons being non-significant (P > 0.1). Clutch size was largest during the first part of the breeding period in both populations (Fig. 3), as revealed by the negative relationship with the quadratic term of laying date (Table 2). No such relationship was found for either the linear term or the cubic term (Table 2).
Of all clutches (n = 140), 279 out of 569 eggs did not hatch (46.9 %). Most (87 %) failures to hatch referred to complete losses (87 %) whereas only 13 % of unhatched eggs apply to partial losses. Incubation failures were detected in 42 % of clutches. The main causes of failure during this period were predation (85 % of cases), female desertion (13 %) and other causes (2 %). Partial predation during incubation was detected only in two clutches, with subsequent female desertion. Minimum and maximum Mayfield probability of failure during incubation were 48.3 and 67.3 %, respectively (DSRincubation = 0.938 ± 0.008). Incubation failure was similar in both populations (W = 0.66, P = 0.42; Fig. 4), no significant between-year differences were found in any of them (Layna: W = 1.51, P = 0.91; Valeria: W = 2.21, P = 0.33; Fig. 4), and nor did we find any seasonal pattern in incubation failures (Table 2). There was no significant relationship between incubation failure and clutch size (Table 2), although smaller clutches tended to have higher probability of failure than larger ones.
Failure probability in Tawny Pipits (mean ± SE) during incubation (white circles) and chick rearing (black circles) in two studied areas of Central Spain. Sample sizes in Table 1
Partial losses were detected in 33.3 % of successful clutches (n = 81). The number of unhatched eggs per nest was one (71.6 % of the cases) or two (28.6 % of the cases), representing an overall proportion of unhatched eggs per clutch of 0.10 ± 0.15. As a result, brood size was only slightly smaller than clutch size (Table 1; Fig. 3). There was a weak indication of between-population differences in the proportion of unhatched eggs, being higher in Layna than in Valeria. On the other hand, we found no significant annual differences in the proportion of unhatched eggs in either of the two populations (Table 1). Finally, the proportion of unhatched eggs was not related to clutch size or to laying date (Table 2).
Productivity and brood failures
Chick rearing period was 10.8 ± 1.30 days (range 7–14 days, modal value = 11; n = 113 nests). Chick feeding during rearing period was carried out almost exclusively by females; males cooperated only occasionally (behaviour observed only in 3 out of 112 nests surveyed during this period).
During the 9 years of the study, 40 % (275 out of 688) of the nestlings died. Complete brood failures accounted for 90 % of the losses during this period, whereas partial failures accounted for 10 % of the losses. Furthermore, brood failures affected 56 % of the nests and were caused mainly by predation (89 %), starvation (6 %) and unknown causes (5 %). The daily survival rate was 0.939 ± 0.008, and the minimum and maximum Mayfield probability of brooding failures were 37.4 and 54.6 %, respectively. Probability of brood failure did not differ significantly between sites (W = 0.30, P = 0.58; Fig. 4). Between-year differences in brood failure were found in Layna (W = 20.09, P < 0.01) but not in Valeria (W = 1.79, P = 0.41; Fig. 4). Post hoc analyses revealed that the highest number of brood failures occurred in 1995 and 2007, contrasting with the lowest values observed in 1994 (Fig. 4). Brood failures were not significantly affected by brood size (Table 2), but showed a significant quadratic relationship with laying date (Table 2).
Partial failures in this period occurred in 17.4 % of the fledged nests (n = 113) and were mainly due to the death of one of the nestlings (75 % of cases). The proportion of unfledged nestlings was 0.06 ± 0.15 (n = 113), being significantly higher in Valeria than in Layna (Table 1). There was no significant between-year variation in partial failures in any of the two populations (Table 1), and we did not find any indication that the proportion of unfledged nestlings varied with the laying date nor the brood size (Table 2; Fig. 3).
Tawny Pipit productivity was 1.75 ± 1.95 (range = 0–5, modal value = 0 chicks, n = 234) and fledgling number was 3.64 ± 0.99 (range = 1–5, modal value = 4 chicks, n = 113). Productivity changed significantly over the breeding season (Table 2), being higher in the middle than earlier or later in the season (Fig. 3). After accounting for exposure days, neither brood size nor clutch size significantly affected nest productivity (Table 2). Productivity was similar for both populations (Table 1), and a significant between-year variation was observed in Layna but not in Valeria (Table 1). These differences were mainly due to the high productivity recorded in 2007 as compared to 1992 and 1994 (Tukey test P < 0.01) and 1994–1995 (P = 0.04).
Effects of nest monitoring on breeding failures
The mean number of visits per nest each year was 4.04 ± 2.74, the frequency of the visits was 0.74 ± 0.71 per day, and the total duration of visits was 63.28 ± 36.69 min. Researcher disturbance explained 8.43 % of the deviance in the probability of nest predation, representing 67.1 % of the variation explained by the whole model. The number of visits to each nest and the frequency of visits did not significantly influence nest predation (W 1,61 = 0.01, P = 0.92; W 1,61 = 0.60, P = 0.44, respectively). The only variable that significantly influenced the probability of nest predation was the accumulated duration of the nest visits (W 1,61 = 5.57, P = 0.02).
Discussion
This study represents the most exhaustive report to date on the reproduction of the Tawny Pipit in the Mediterranean region, which holds most of its European population (BirdLife International 2010). Overall, our results suggest that breeding performance and reproductive output of Tawny Pipits in Spanish shrubsteppes are controlled by two main related factors: timing of breeding/seasonality of resources, and nest predation.
The environmental conditions experienced by Mediterranean birds fall somewhere between the temperate north and the tropical environmental conditions, with warmer springs and, overall, less marked seasons than farther north. This fact allows, according with the traditional hypothesis of latitudinal variation in life-history traits (Lack 1950; Ashmole 1961; Slagsvold 1982), longer breeding periods in the Mediterranean regions (due to earlier arrivals to breeding grounds and shorter migratory routes) and consequently an increase in the number of broods that can be raised in a given season. However, in the Mediterranean region, there seems to exist some unclear breeding constraint that may be induced by environmental conditions such as photoperiod, ambient temperature or food availability, all of them determinant variables for breeding performance (review in Dunn 2004). This means that the observed seasonal and latitudinal trends in breeding patterns could present notable variations from the expected (examples in Moreno 2004). According to our data, this seems to be the case of Spanish Tawny Pipit populations since they did not show an earlier timing of breeding nor a longer reproductive period than populations studied farther north (Fig. 1). Moreover, according to theory, clutch size should be larger in northern and more seasonal areas than in Mediterranean populations (e.g. Lack 1947; Sanz 1997). Interestingly, this pattern was not evident in our results since clutch size was similar and within the range of those previously reported for north European populations (Table 3). Specific features of the Mediterranean shrubsteppes may play an important role in explaining why the expected latitudinal pattern is truncated there. Our results for Spanish Tawny Pipits are in fully agreement with studies describing seasonal variation in clutch size in species that are sometimes multi-brooded (Crick et al. 1993). Single-brooded species usually begin the laying period when the optimal clutch size is greatest, in contrast to multi-brooded birds which start laying before the optimum, so they can raise more broods in a season (Crick et al. 1993). Accordingly, the reproductive output of single-brooded birds normally declines as the season advances, whereas multi-brooded species usually have a mid-season peak (Crick et al. 1993; Perrins 1970; Svensson 1995). In our case, the initial slope of the quadratic relationship between laying date and clutch size was positive (whereas it should be 0 or negative in single-brooded species), but the clutch size reached its maximum value early in the season (Fig. 3). Taking into account the time needed to complete one breeding attempt (about 32 days; Cramp 1998; present study), and the length of the breeding season for the two studied populations (Fig. 1), there is theoretically enough time to have more than one successful breeding attempt per season. However, second clutches in Mediterranean populations of this species are anecdotic (Calero-Riestra et al. 2010), probably in relation to the decline of food abundance towards the end of the breeding season (Martin 1987) and the high predation rates. Whilst the larger clutches observed in this study during the first nesting attempts suggest that food resources are not limited at the start of the breeding season, the smaller clutch sizes of late broods might be interpreted as a decline in food abundance and an indication of the ending limit of the breeding season. The decrease in mass and dimensions of Tawny Pipit nests and in the female condition as the season advances (Suárez et al. 2005a, b) corroborate the existence of severe constraints towards the end of the season. This issue, together with the high nest loss rate detected in our study which force most females to lay replacement clutches (Suárez et al. 2005a), might prevent the existence of double-brooding in Mediterranean populations of this species.
Individual variation in reproductive output in this species was highly related to predation rates. Nest predation influences the number of young fledged in other open-land ground-nesting passerines (Suárez and Manrique 1992; Martin 1993; Yanes and Suárez 1995), and is considered to be a strong selective factor in the evolution of nest-site selection and nesting strategies in birds (Magnhagen 1991; Skutch 1949; Lack 1968; Ricklefs 1969). As far as we know, the nest predation rates reported in our study areas are the largest for the species, being responsible for 85 % of failures during incubation and 89 % during chick rearing. Predation rates were higher in central Spain than in northern populations, especially during incubation, probably because of the larger predator communities in those lower latitudes (Suárez and Manrique 1992; Yanes and Suárez 1995, 1996). We are aware that our results can be biased by disturbance by researchers (repeated visits to the nests) (Götmark 1992). However, our analyses support that neither the number or frequency of visits nor the handling time reduced significantly nest success, in agreement with previous studies on closely related birds (e.g. Weidinger 2008; Jacobson et al. 2011; Lloyd et al. 2000; but see Major 1990; Tryjanowski and Kuźniak 1999). It should also be noted that failures occurring after fledging are not contemplated in this study. According to Bijlsma (1978), approximately 64 % of fledged nestlings do not survive until adulthood. We can thus infer that the total number of recruits produced in a given season should be very low in our studied populations.
Previous studies have also reported high failure rates in ground-nesting birds due to predation (Wright et al. 2009; Martin 1993; Suárez et al. 1993), and most of them showed a constant or monotonic increase in the probability of nest predation over the season (e.g. Skutch 1949; Slagsvold 1982; Grant et al. 2005; Evans et al. 2005). Unexpectedly, we found that incubation failures were more or less constant through the season, whereas brood failures showed a negative relationship with laying date, suggesting a seasonal decrease in predation rates. Predation during incubation may be related to certain predator species, such as lizards, snakes or rodents, which may maintain a constant predator pressure through time (Weatherhead et al. 2003). These are typical predators on bird’s eggs in the Mediterranean areas (Yanes and Suárez 1996), where they reach higher abundances than at higher latitudes (Blondel et al. 2010).
The mismatch between the number of young hatched and the number of young fledged resulted in nesting attempts from mid-season being more productive than earlier or later ones (Fig. 3). Life-history theory and empirical studies predict that breeding early confers higher reproductive success (Perrins 1970) since it allows more nesting attempts per season or the possibility to relay after predation. In addition, nest survival rates should decrease significantly as the season progresses. In our study populations, the percentage of predated nestlings stabilises just prior to the mid-season, coinciding with the time when Tawny Pipit productivity is the highest (Fig. 5). Therefore, the observed seasonal decrease in the probability of nest failure probably relates to an increased availability of nestlings for predators, not only of Tawny Pipit but also of other passerines breeding in the same area, such as Skylarks Alauda arvensis or Short-Toed Larks Calandrella brachydactyla, which have the same phenology (Suárez et al. 2005c). Whether nest predation is sufficient to constraint reproduction at a global scale of open-landscape ground-nesting birds in the Mediterranean remains an open question. Nest predation reduces the benefits of early clutch initiation in Tawny Pipits, a pattern already detected in other passerine species (Mallord et al. 2008), so there is a clear reason to suspect that nest predation is a potentially strong selective force on Tawny Pipit reproduction in Mediterranean habitats.
In summary, the breeding biology of Tawny Pipits at Mediterranean latitudes seems to be strongly influenced by nest predation, which imposes a severe limit to population productivity. This, together with the infrequency of double-brooding in this species at low latitudes (Davis 2009; Calero-Riestra et al. 2010), entails considerable scepticism about the long-term viability of the Mediterranean populations of Tawny Pipit. The comparison of breeding parameters from Mediterranean and northern populations highlights the idiosyncrasy of the former, and the necessity for integrating their study with those at higher latitudes in order to give a full picture of avian life histories of this species. Future research on this and closely related species should be focused on connectivity among populations (source––sink population dynamics), and specific responses against predation to really understand the future viability of open-landscape breeding species.
References
Alström P, Mild K (2003) Pipits and Wagtails of Europe, Asia and North America: identification and systematics. Christopher Helm, London
Ashmole N (1961) The biology of certain terms. PhD dissertation. Oxford University, Oxford
Bautista LM, Pantoja JC (2000) A bibliometric review of the recent literature in ornithology. Ardeola 47:109–121
Bijlsma RG (1978) Het voorkomen van de Duinpieper Anthus campestris op de Zuidwest-Veluwe, Nederland, deel 1: broedtijd. Limosa 51:107–121
BirdLife International and NatureServe (2011) Bird species distribution maps of the world. BirdLife International/NatureServe, Cambridge/Arlington
BirdLife-International (2004) Species factsheet: Anthus campestris. http://www.birdlife.org
BirdLife-International (2010) Species factsheet: Anthus campestris. http://www.birdlife.org
Blondel J, Dervieux A, Maistre M, Perret P (1991) Feeding ecology and life history variation of the blue tit in Mediterranean deciduous and sclerophyllous habitats. Oecologia 88:9–14
Blondel J, Aronson J, Bodiou JY, Boeuf G (2010) The Mediterranean region: biological diversity in space and time. Oxford University Press, Oxford
Brambilla M, Rubolini D (2005) Caratteristiche macroambientali dell’habitat riproduttivo del calandro Anthus campestris. Avoceta 29:105
Calero-Riestra M, García J, Suárez F (2010) ¿Dobles puestas o reposiciones? El caso del bisbita campestre Anthus campestris. Paper presented at the XX Congreso Español de Ornitología, Tremp
Carrascal LM, Palomino D (2008) Las aves comunes reproductoras en España. Población en 2004–2006. SEO/Birdlife, Madrid
Cramp S (ed) (1998) The Birds of the Western Palearctic, vol 5. Oxford University Press, Oxford
Crick HQP (2004) The impact of climate change on birds. Ibis 146:48–56
Crick H, Gibbons D, Magrath R (1993) Seasonal changes in clutch size in British birds. J Anim Ecol 62:263–273
Davis SK (2009) Renesting intervals and duration of the incubation and nestling periods of Sprague’s Pipits. J Field Ornithol 80:265–269
Del Hoyo J, Elliott A, Christie D (eds) (2004) Handbook of the birds of the world (volume 9): Cotingas to Pipits and Wagtails. Del Hoyo J, Elliott A, Sargatal J (eds) 1992. Handbook of the birds of the world, vol 1. Lynx, Barcelona
Donald PF, Green RE, Heath MF (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc R Soc Lond B 268:25–29
Dunn P (2004) Breeding dates and reproductive performance. Adv Ecol Rs 35:69–87
Evans DM, Redpath SM, Evans SA (2005) Seasonal patterns in the productivity of Meadow Pipits in the uplands of Scotland. J Field Ornithol 76:245–251
Gordo O, Sanz J (2005) Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia 146:484–495
Götmark F (1992) The effects of investigator disturbance on nesting birds. Curr Ornithol 9:63–104
Grant TA, Shaffer TL, Madden EM, Pietz PJ, Johnson D (2005) Time-specific variation in passerine nest survival: new insights into old questions. Auk 122:661–672
Grzybek J, Michalak I, Osiejuk TS, Tryjanowski P (2008) Densities and habitats of the Tawny Pipit Anthus campestris in the Wielkopolska region (W Poland). Acta Ornithol 43:221–225
Högstedt G (1978) Orientation of the entrance in Tawny Pipit Anthus campestris nests. Ornis Scand 9:193–196
Jacobson MD, Tsakiris ET, Long AM, Jensen WE (2011) No evidence for observer effects on Lark Sparrow nest survival. J Field Ornithol 82:184–192
Johnson D (1979) Estimating nest success: the Mayfield method and an alternative. Auk 96:651–661
Krüger S (1989) Der Brachierper Anthus campestris., vol 598. Ziemsen, Wittenberg Lutherstadt
Lack D (1947) The significance of clutch-size. Ibis 89:302–352
Lack D (1950) The breeding seasons of European birds. Ibis 92:288–316
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lloyd P, Little RM, Crowe TM (2000) Investigator effects on the nesting success of arid-zone birds. J Field Ornithol 71:227–235
Magnhagen C (1991) Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–186
Major RE (1990) The effect of human observers on the intensity of nest predation. Ibis 132:608–612
Mallord J, Dolman P, Brown A, Sutherland W (2008) Early nesting does not result in greater productivity in the multi-brooded Woodlark Lullula arborea. Bird Study 55:145–151
Martin T (1987) Food as a limit on breeding birds: a life-history perspective. Annu Rev Ecol Syst 18:453–487
Martin TE (1993) Nest predation among vegetation layers and habitat types: revising the dogmas. Am Nat 141:897–913
Martin TE, Geupel GR (1993) Nest-monitoring plots: methods for locating nests and monitoring success. J Field Ornithol 64:507–519
Martin TE, Scott J, Menge C (2000) Nest predation increases with parental activity: separating nest site and parental activity effects. Proc R Soc Lond B 267:2287–2293
Mayfield H (1961) Nesting success calculated from exposure. Wilson Bull 73:255–261
Mayfield H (1975) Suggestions for calculating nest success. Wilson Bull 87:456–466
Moreno J (2004) Avian reproduction in a Mediterranean context: contributions of ornithological research in Spain. Ardeola 51:51–70
Osiejuk TS, Grzybek J, Tryjanowski P (2007) Song structure and repertoire sharing in the Tawny Pipit Anthus campestris in Poland. Acta Ornithol 42:157–165
Peinado M, Rivas-Martínez S (1987) La vegetación de España., vol 3. Colección Aula Abierta. Servicio de Publicaciones de la Universidad de Alcalá, Alcalá de Henares
Perrins C (1970) The timing of birds’ breeding seasons. Ibis 112:242–255
Ricklefs RE (1969) An analysis of nesting mortality in birds, vol 9. Smithsonian contributions to zoology. Smithsonian Institution Press, Washington
Sanz JJ (1997) Geographic variation in breeding parameters of the Pied Flycatcher Ficedula hypoleuca. Ibis 139:107–114
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Method Ecol Evol 1:103–113
Shaffer T (2004) A unified approach to analyzing nest success. Auk 121:526–540
Skutch AF (1949) Do tropical birds rear as many young as they can nourish. Ibis 91:430
Slagsvold T (1982) Clutch size variation in passerine birds: the nest predation hypothesis. Oecologia 54:159–169
Stamou GP, Stamou GV, Papatheodorou EM, Argyropoulou MD, Tzafestas SG (2004) Population dynamics and life history tactics of arthropods from Mediterranean-type ecosystems. Oikos 104:98–108
StatSoft (2007) STATISTICA (data analysis software system), version 8.0. Statsoft, Tulsa
Suárez F, Manrique J (1992) Low breeding success in Mediterranean shrubsteppe passerines: thekla lark Galerida theklae, lesser short-toed lark Calandrella rufescens, and black-eared wheatear Oenanthe hispanica. Ornis Scand 23:24–28
Suárez F, Yanes M, Herranz J, Manrique J (1993) Nature reserves and the conservation of Iberian shrubsteppe passerines: the paradox of nest predation. Biol Conserv 64:77–81
Suárez F, Morales MB, Minguez I, Herranz J (2005a) Seasonal variation in nest mass and dimensions in an open-cup ground-nesting shrub-steppe passerine: the Tawny Pipit Anthus campestris. Ardeola 52:43–51
Suárez F, Traba J, Herranz J (2005b) Body mass changes in female Tawny Pipits Anthus campestris during the nesting stage. J Ornithol 146:372–376
Suárez F, Herranz J, Yanes M, Sánchez AM, García JT, Manrique J (2005c) Variación estacional e interanual en la reproducción de cuatro alondras en Espana mediterránea: fecha y tamano de puesta, tamano de los huevos y asincronía de puesta. Ardeola 52:103–117
Sutherland WJ, Newton I, Green R (eds) (2004a) Bird ecology and conservation: a handbook of techniques. Oxford University Press, Oxford
Sutherland WJ, Pullin AS, Dolman PM, Knight TM (2004b) The need for evidence-based conservation. Trends Ecol Evol 19:305–308
Svensson L (ed) (1992) Identification guide to European Passerines, 4th edn. British Trust for Ornithology, Stockholm
Svensson E (1995) Avian reproductive timing: when should parents be prudent? Anim Behav 49:1569–1575
Tryjanowski P, Kuźniak S (1999) Effect of research activity on the success of Red-backed Shrike Lanius collurio nests. Ornis Fenn 76:41–43
Tyler SJ (2004) Family Motacillidae (pipits and wagtails). In: Del Hoyo J, Elliott A, Christie D (eds) Handbook of the birds of the world (volume 9): Cotingas to Pipits and Wagtails. Lynx, Barcelona, pp 686–786
Van Turnhout C (2005) The disappearance of the Tawny Pipit Anthus campestris as a breeding bird from The Netherlands and Northwest-Europe. Limosa 78:1–14
Weatherhead PJ, Blouin-Demers G, Cavey KM (2003) Seasonal and prey-size dietary patterns of black ratsnakes (Elaphe obsoleta obsoleta). Am Midl Nat 150:275–281
Weidinger K (2008) Nest monitoring does not increase nest predation in open-nesting songbirds: inference from continuous nest-survival data. Auk 125:859–868
Wright L, Hoblyn R, Green R, Bowden C, Mallord J, Sutherland W, Dolman P (2009) Importance of climatic and environmental change in the demography of a multi-brooded passerine, the woodlark Lullula arborea. J Anim Ecol 78:1191–1202
Yanes M, Suárez F (1995) Nest predation patterns in ground-nesting passerines on the Iberian Peninsula. Ecography 18:423–428
Yanes M, Suárez F (1996) Incidental nest predation and lark conservation in an Iberian semiarid shrubsteppe. Conserv Biol 10:881–887
Acknowledgments
This study was funded by JCCM projects (PAC06-0137 and PII1C09-0128-4724) and CSIC-MICINN project (PIE 201030I019). M.C.-R. was supported by a predoctoral grant (I3P) from the Consejo Superior de Investigaciones Científicas (CSIC). We are grateful to the Junta de Comunidades de Castilla-La Mancha for capture licenses, the landowners of Layna and Valeria, I. Hervás and J. Hernández-Justribó for their help during the fieldwork. A. Benitez, B. Frasure and Sally Bach checked the language in different versions of the manuscript. This paper is dedicated to the memory of our beloved colleague F. Suárez (“Quico”).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. H. Becker.
Rights and permissions
About this article
Cite this article
Calero-Riestra, M., García, J.T., Herranz, J. et al. Breeding output and nest predation patterns in steppe-associated Mediterranean birds: the case of the Tawny Pipit Anthus campestris . J Ornithol 154, 289–298 (2013). https://doi.org/10.1007/s10336-012-0893-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0893-4