Abstract
Post-fledging care constitutes a large proportion of the total costs of parental care in many bird species. Despite being recognized as of critical importance to the survival of the offspring and their recruitment into the breeding population, post-fledging care, including the relative contribution by male and female parents, is under-studied. In this study, we quantified food provisioning (prey deliveries) by male and female Tengmalm’s Owl (Aegolius funereus) parents to their offspring both in the nestling and the post-fledging stages, in years of differing natural prey abundance. Parents and at least one offspring in 26 families were fitted with radio-transmitters. Male parents exhibited higher delivery rates than did females throughout the late nestling and post-fledging stages, but the intersexual difference was smaller in broods that were not deserted by the female at any stage. The female deserted her mate and offspring at some stage in 63% of the broods. Overall, deserted males delivered more prey to their offspring than did non-deserted males. Delivery rates were generally higher post-fledging. Prey delivery rates differed among years, and were highest in low vole years (probably because of smaller prey items), intermediate in peak vole years, and lowest in years of vole increase. Prey delivery rates increased with increasing brood size for both sexes, but the response was stronger in females. We suggest that female Tengmalm’s Owls contribute less than males because they are in a position to decide on their level of provisioning effort first, and because of the potential for re-mating after deserting the first brood.
Zusammenfassung
Geschlechterrollen bei der Fürsorge für Flügglinge: Weibliche Raufußkäuze tragen wenig zur Futterversorgung bei
Die Fürsorge für Flügglinge macht bei vielen Vogelarten einen großen Anteil der Gesamtkosten der Brutpflege aus. Obwohl bekannt ist, dass die Fürsorge für Flügglinge von entscheidender Bedeutung für das Überleben der Nachkommen und ihre Rekrutierung in die Brutpopulation ist, ist dieser Aspekt der Brutpflege, einschließlich des relativen Beitrags von Männchen und Weibchen, nicht gut untersucht. In dieser Studie haben wir in Jahren mit unterschiedlicher natürlicher Beuteabundanz quantifiziert, wie männliche und weibliche Raufußkäuze (Aegolius funereus) ihre Nachkommen mit Futter versorgten, sowohl im Nestlingsstadium als auch nach dem Ausfliegen. Die Elternvögel und mindestens ein Jungvogel aus 26 Familien wurden mit Radiosendern ausgestattet. Männchen wiesen während des späten Nestlingsstadiums und nach dem Ausfliegen höhere Fütterraten auf als Weibchen, aber der Unterschied zwischen den Geschlechtern war geringer in Bruten, die nicht zu irgendeinem Zeitpunkt vom Weibchen verlassen wurden. Das Weibchen verließ irgendwann seinen Partner und seine Nachkommen in 63% der Bruten. Insgesamt brachten verlassene Männchen ihren Nachkommen mehr Beute als nicht verlassene. Die Fütterraten waren nach dem Ausfliegen generell höher. Sie unterschieden sich zwischen den Jahren und waren am höchsten in Jahren mit geringer Wühlmausabundanz (wahrscheinlich aufgrund kleinerer Beutestücke), mittel in Jahren mit höchster Wühlmausabundanz und niedrig in Jahren mit zunehmender Wühlmausabundanz. Die Fütterraten nahmen mit ansteigender Brutgröße für beide Geschlechter zu, doch die Antwort war bei Weibchen stärker ausgeprägt. Wir schlagen vor, dass weibliche Raufußkäuze einen geringeren Beitrag leisten als Männchen, da sie in der Lage sind, über das Ausmaß ihres Fütterungsaufwands zuerst zu entscheiden, und für sie die Möglichkeit besteht, sich nach Verlassen der ersten Brut wieder zu verpaaren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although both parents contribute to care in >80% of bird species, parental care is often not shared equally between mates (Clutton-Brock 1991; Cockburn 2006; Olson et al. 2008). According to parental investment theory, there is a conflict between males and females over how much care to provide for their joint offspring (Trivers 1972; Kokko and Jennions 2008). In bi-parental birds, each partner usually benefits from the effort invested by its mate and escapes some of the associated costs (e.g. Lessells and Parker 1999; Cockburn 2006). Parents should be expected to adjust their behaviour to their mate’s behaviour, environmental conditions and their brood’s need (e.g. Hinde 2006; Hinde and Kilner 2007; Kosztolányi et al. 2008; Eldegard and Sonerud 2010).
Caring for offspring after fledging constitutes a large proportion of the total costs of parental care in many altricial birds (Clutton-Brock 1991). The post-fledging dependency period is widely recognized as a critical, but under-studied, phase of avian life histories (e.g. Grüebler and Naef-Daenzer 2010). Most post-fledging studies have dealt with estimating survival or recruitment, while studies investigating factors potentially affecting survival in the post-fledging period, including parental behaviour, are scarce (e.g. Wells et al. 2007). We assume that post-fledging parental care is little studied because it is logistically challenging to quantify; fledgling birds are difficult to locate and follow because of their cryptic behaviour and plumage, and because they are not restricted to a fixed point like the nest, but rather move around within the parents’ home range.
Parental behaviour patterns, including the relative contribution of males and females in providing food for the offspring during the pre- and post-fledging periods, is of particular interest in owls and birds of prey (Strigiformes, Accipitriformes, Falconiformes). Most of these birds have strict sex roles pre-fledging; prior to nestlings being able to thermoregulate, the female performs most or all incubation, brooding and direct feeding of the nestlings, and the male provides most or all the food to the nestlings and the incubating female (Newton 1979, 1986; Cramp 1985; Eldegard et al. 2003). Thereafter, the female resumes hunting and may assist the male in providing for the young (e.g. Eldegard and Sonerud 2009, 2010). Offspring of predatory birds typically depend on their parents for food for a prolonged period post-fledging (e.g. Eldegard et al. 2003; Penteriani et al. 2005; Wiens et al. 2006; Sunde 2008; Vergara et al. 2010). Yet, quantitative data on the relative contribution of male and female parents to post-fledging food provisioning are scarce for most species.
In this study, we quantified provisioning to offspring by male and female Tengmalm’s Owls (Aegolius funereus) in the pre- and post-fledging periods. Tengmalm’s Owl is cavity-nesting, forest-dwelling and nocturnal with a Holarctic boreal distribution (Mikkola 1983). It exhibits pronounced reversed sexual dimorphism for linear body measurements (Hipkiss 2002). The female incubates and broods, and the male provides food for the female and nestlings until the end of the brooding period (Korpimäki 1981). The offspring hatch asynchronously and fledge 4–5 weeks after hatching (Korpimäki 1981), but depend on their parents for food for another 6–7 weeks post-fledging (own unpublished data). Body condition, breeding decisions and breeding success of Tengmalm’s Owls are affected by fluctuations in the abundance of their main prey, microtine rodents (voles) (Korpimäki 1987, 1988; Hörnfeldt et al. 1990; Korpimäki 1990; Eldegard and Sonerud 2009, 2010). In an experimental study carried out in a peak vole year, we found that female Tengmalm’s Owls contributed substantially less than males to food provisioning both at food-supplemented and control broods (Eldegard and Sonerud 2010). Furthermore, the frequency of female offspring desertion increased with natural and experimental increases in food supply (Eldegard and Sonerud 2009). Here, we report results from an observational study of food provisioning to 26 broods across a pulse of rodent abundance (a low, an increasing, and a peak vole year, and then another low vole year). For each brood, we quantified prey deliveries by male and female parents in the late nestling stage and in the early and late post-fledging stages.
We predicted that males would exhibit higher delivery rates than females throughout the offspring dependency period, but that the relative contribution by the sexes to food provisioning would be modulated by environmental conditions, with a higher relative contribution by females when the discrepancy between offspring need and amount of food received was large, i.e. when the natural food supply was low or when brood size was large.
Methods
Study area and natural food abundance
Our study took place at 60°45′–61°10′N and 10°55′–12°00′E at altitudes of 170–600 m in Hedmark County in southeast Norway during the years 1996–1999. The study area is situated in the boreal zone, and is dominated by coniferous forest, primarily of Scots Pine (Pinus sylvestris) and Norway Spruce (Picea abies), interspersed with bogs, and contains only negligible areas of farmland. The forest is heavily influenced by modern forestry based on clear-cutting of the old forest and replanting, and is a mosaic of stands of different ages. More detailed descriptions of the flora and fauna of this area are given by Sonerud (1986) and Steen et al. (1996).
Data on the relative abundance of the natural prey of Tengmalm’s Owls in our study area were obtained from a long-term monitoring study in which small mammals have been snap-trapped twice annually (spring and fall) since 1981 for several purposes (e.g. Sonerud 1986; Selås et al. 2001), following the method described by Sonerud (1988). There were two trapping sites about 30 km apart in our study area; a western site (≈60°56′N, 11°08′E) with ≈1,100 trap nights in each trapping session, and an eastern site (≈60°57′N, 11°42′E) with ≈600 trap nights in each trapping session. We defined a rodent (vole) index as the pooled indices (animals trapped per 100 trap nights) of Bank Vole (Myodes glareolus), Field Vole (Microtus agrestis), Root Vole (Microtus oeconomus) and Wood Lemming (Myopus schisticolor). Pooling these species is justified because of inter-specific population synchrony (Bjørnstad et al. 1999). Based on these trapping indices, we categorised 1998 as a peak vole year, 1996 and 1999 as low vole years, and 1997 as an increase vole year, i.e. a year with increasing vole population densities (see also Korpimäki and Lagerström 1988 for categorisation of vole years).
Nest box inspection, capturing and radio-tagging
Tengmalm’s Owls readily accept and breed in nest-boxes (e.g. Korpimäki 1981; Sonerud 1985). From mid-March, we checked available boxes at least every third week. After a nest box was found occupied, we checked it at least every week in order to determine the hatching date (±1 day) of the first egg in the clutch (hatching = day 0). Tengmalm’s Owl offspring hatch asynchronously, with a hatching interval of c. 1 day (Korpimäki 1981). Hatching date was determined by backdating from age of the oldest nestling (1–6 days). If all the eggs in a clutch were already hatched, the age of the oldest nestling was determined from body size (see Carlsson and Hörnfeldt 1994). Median clutch size for the 26 broods included in this study was 5.0 (range 4–7). Tengmalm’s Owls are sexually monochromatic, and therefore it is difficult to distinguish between the parents in the field. Also, as Tengmalm’s Owls are nocturnal and forest-dwelling, it is particularly challenging to locate the fledglings. Therefore, we fixed transmitters to both parents and offspring; the male and the female and at least one nestling in 26 nests; 3, 10, 10 and 3 nests in 1996, 1997, 1998 and 1999, respectively. Radio-transmitters (type TW-4; Biotrack, UK) were mounted as backpacks and attached with a tubular Teflon tape (Bally Ribbon Mills, PA, USA) (see Online Resource 1). Note that 10 food-supplemented nests from 1998 (Eldegard and Sonerud 2010) are excluded from the analyses here.
Adult females were caught by hand in the nest box. Males were captured at night in a swing-door trap mounted in front of the nest-box entrance, or in mist-nets in front of the nest tree. Adults and offspring were ringed for individual recognition. We radio-tagged the parents in the female brooding period (median day 17 after hatching, range 14–19, for females; median day 15, range 8–26, for males). In each brood, the nestlings that were assumed to fledge first, as judged from behaviour and wing length, were radio-tagged. Nestlings were tagged close to fledging [median 29.0 (range 26–31) days after hatching, n = 36 nestlings]. In this study, median fledging day for the first-fledged offspring was 33.0 (range 29–36) days after hatching of the first-hatched egg (n = 22 nests). In 1996–1997, we radio-tagged 1–2 offspring from each brood. However, we discovered that the brood mates keep together until about 60 days after hatching (own unpublished data), and that it was sufficient to tag only one offspring from each nest to keep track of the broods after fledging. Therefore, only one offspring from each brood was radio-tagged in 1998 and 1999. In total, we radio-tagged 26 adult males, 26 adult females, and 36 offspring for the purpose of this study. Prey deliveries by parents were not monitored until at least 3 days after the female had ceased brooding. Thus, parents were given minimum three nights for habituation to the tag before recording of prey deliveries started.
Field observations of prey deliveries and brood size
We recorded prey deliveries by both parents to each brood throughout three nights; in the late nestling stage, when the nestlings were no longer brooded by the female (median day 25, range 17–31) and in the early (median day 42, range 37–45) and late (median day 58, range 46–63) post-fledging stage. If the prey delivery of a parent ceased, the use of radio-telemetry enabled us to determine whether it had died or deserted (see Eldegard and Sonerud 2009 for detailed descriptions of the field methods). Disturbance was minimised by keeping a distance of c. 15–20 m between the observer and the nest or fledged brood, depending on vegetation and topography. Tengmalm’s Owls are not shy and habituate easily to the presence of a tranquil observer.
In Tengmalm’s Owl, in contrast to most other owls and birds of prey, the female does not leave the nest to retrieve prey from the male when he returns from hunting trips; the male delivers prey at the nest cavity entrance or even enters the cavity (Cramp 1985). This makes it possible to correctly assign each particular prey delivery to the parent that actually captured the prey. We assumed that all nest visits by males and females in the late nestling stage were prey deliveries (see Eldegard and Sonerud 2010). Nest visits were monitored either automatically, using a data-logging receiver (RX-900; Televilt, Sweden) and fixed two-element Yagi-antennas (Televilt), or by radio-tracking and visual observation, using portable receivers (Telonics, AZ, USA, and Televilt) and hand-held 2- or 4-element Yagi antennas (Televilt).
The data-logging receivers were calibrated by mounting a radio-transmitter on a long stick, and literally running through the forest simulating movements of adults on hunting trips, and parents arriving with food at the nest. The data-loggers were then tuned so that prey deliveries were displayed as clear peaks on scatter-plots of signal strength versus time. Tengmalm’s Owls rarely hunt near the nest and often >1 km away (own unpublished data), so it was not difficult to identify prey deliveries from signal strength, even though both the automatic and the manual methods pick up signals at a distance from the nest. The reliability of the automatic method was also tested by carrying out manual observations simultaneously with the automatic monitoring at five nests. The results from the manual and the automatic monitoring were always consistent with respect to number and timing of nest visits. Furthermore, during manual observation, none of the parents were observed entering the nest box without food in the late nestling period (own unpublished data). Also, video recording at six Tengmalm’s Owl nests for a total of 18 nights revealed no visits of males without prey and no post-brooding visits of females without prey (own unpublished data). Further evaluation of the reliability of the method is given in Eldegard and Sonerud (2010).
In the post-fledging period, prey deliveries were detected from signal strength of radio-transmitters, vocalisations of arriving parents, movements and vocalisations of offspring, and visual observations. The fledglings kept together as a group c. 4 weeks after fledging, so they were still together when we carried out the last of our three observation nights (the post-fledging dependency period is 6–7 weeks; own unpublished data). The fledglings gradually moved away from the nest, but the parents usually foraged far away, and the fledglings and parents did not travel together as a group (own unpublished data). Each brood was localised by homing in on the radio-tagged fledgling shortly before dusk. We followed the fledglings throughout the night (at distances of 5–30 m). Most of the time, the offspring could be seen (nights were light due to the high latitude; c. 61°N) or heard (begging calls). When a parent arrived with food, the fledglings called intensively, chased the parent and tried to grab the prey. Often the parent also vocalised upon arrival. Even if the fledglings had not been so vociferous upon arrival of the parent, it was generally not difficult to discern between directional movements of parents returning from hunting trips and weak (or none) transmitter signals detected inbetween prey deliveries. Our presence did not seem to disturb either the fledglings or their parents. We ended our night-time observations shortly after dawn.
We kept a count of brood size in the nestling stage on the basis of nest inspections, remains of dead nestlings and by X-raying prey remains from nests (post-fledging) in order to reveal rings of dead nestlings. Brood size in the post-fledging stage was determined from daytime observations of brood mates perching alongside or in the vicinity of the radio-tagged young, and from visual observations and begging calls of young at night. Sunde and Markussen (2005) found that using counts of begging young to estimate post-fledging survival in Tawny Owls (Strix aluco) reliably accounted for variation in brood size when used by a ‘naive’ observer.
Data analyses
Temporal distribution of prey deliveries throughout the night
In 1998, when prey deliveries in the late nestling stage were monitored automatically, we started the data-logging receivers well before sunset and stopped them well after sunrise at all nests. The results from this automatic monitoring showed that there was no difference in the temporal distribution of deliveries by male and female parents (Fig. 1). In the other study years, and in the post-fledging stage, we monitored prey deliveries by male and female parents from sunset to sunrise in most cases. However, sometimes the monitoring session was shorter; especially for late-hatched broods and in the post-fledging period, the night was short because our study area is situated at a high latitude. Therefore, monitoring by visual observations usually did not start until between 2100 and 2200 hours. In analyses of prey deliveries by male and female parents, we included all deliveries in the interval 2200–02:59 hours. Not all broods in all stages were monitored for as long as 5 h (mean ± SD observation time; late nestling stage: 4.7 ± 0.39 h, early post-fledging stage 4.4 ± 0.31 h, late post-fledging stage 4.3 ± 0.63 h). Therefore, we included ‘length of monitoring session’ (within this 5-h interval) as an offset variable in the analyses of prey deliveries. Although we found no statistically significant difference in temporal distribution between males and females, the males’ distribution tended to have more extended tails (Fig. 1). Thus, using data from only the middle 5 h slightly underestimates the relative contribution of male parents, and provides a conservative estimate of any intersexual difference in prey deliveries, because we predicted that males would exhibit higher delivery rates than females.
Sample size
Sample size was reduced from 26 broods in the late nestling stage to 18 in the late post-fledging stage because of predation of the whole brood of nestlings (n = 1), predation of the radio-tagged fledgling (n = 3), predation of the male parent (n = 1), loss of the radio-tag (n = 1 adult female), or because the brood mates were so widely dispersed in the late post-fledging stage that is was not possible to determine number of prey deliveries by the parents (n = 2).
Prey deliveries by one male parent and one breeding pair were monitored in two consecutive breeding seasons. Weighing the risk of pseudo-replication against reduction in sample size, we decided to include data from both years in both cases. Conditions (vole year, brood size) were not the same in the different years, and it is logistically demanding to obtain this kind of data on nocturnal, forest-dwelling birds.
Analysis of prey deliveries by males and females
The data on provisioning by parents were counts (number of prey deliveries). The data were not transformable to normality due to many zero values (many females did not deliver any food post-fledging). We used generalszed linear mixed models (GLMMs) for analyses of the prey delivery data, following procedures recommended by Bolker et al. (2008). First, we created a full (most complex) model including the fixed effects ‘sex’, ‘stage’, ‘vole year’, ‘brood size’, and the ‘sex × stage’, ‘sex × vole year’ and ‘sex × brood size’ interactions (“Appendix 1”). The three interaction terms were included because our purpose was to find out whether there was an intersexual difference in parental effort, and whether this intersexual difference changed when conditions (food, brood age, brood size) changed. ‘Brood size’ was the number of offspring alive at each individual monitoring session. ‘Parent identity’ nested within ‘brood identity’ was modelled as random effect because prey deliveries were measured repeatedly for the same parent individuals and for the same broods. In addition, ‘length of monitoring session’ (see “Temporal distribution of prey deliveries throughout the night”) was modelled as offset variable in order to control for variation in duration of monitoring sessions. We fitted a model with log link function, Poisson distribution, and Gauss-Hermite Quadrature [if one, or nested, random effect(s)] or Laplace (if more than one random effect) technique for GLMM parameter estimation (Bolker et al. 2008). We carried out graphical diagnostics and inspected the scaled Pearson statistic for the conditional distribution to check if the models fitted our data in each case. None of the models were over-dispersed, and there was only moderate under-dispersion in some cases (all χ 2/df values were in the range from 0.6 to <1.1).
After fitting the full model, selection among a set of candidate models was performed using the Akaike information criterion corrected for small sample size (AICc) as recommended by Anderson et al. (2001). In addition to the model with the lowest AICc value, models with ΔAICc < 2 were considered to be well supported. Model selection using information criteria like AICc will identify the best model in a set of candidate models, even if all models are poor. Therefore, we also provide Wald F tests of fixed effects, and likelihood ratio (LR) tests of random effects for the model best supported by the data (Table 1; “Appendix 1”) and competing model(s) (i.e. ΔAICc < 2; Online Resource 2; “Appendix 1”). Based on the results from this main analysis, we performed post hoc analyses of subsamples of the 26 broods. Data were analysed using SAS/STAT® 9.2 (SAS Institute, Cary, NC, USA), and Sigmastat 1.0 (Jandel Scientific, Ekrath, Germany) statistical software. All statistical tests are two-tailed.
Results
Prey deliveries by males and females
Among the candidate models explaining the number of prey deliveries to the offspring, two models had substantially higher AICc weight values than the others (“Appendix 1”). Both models included the main effects ‘sex’, ‘stage’, ‘vole year’ and ‘brood size’, and the ‘sex × brood size’ interaction. The model with the second highest AICc weight also included the ‘sex × stage’ interaction. Parameter estimates and Wald F tests of fixed effects for the model with the highest AICc weight (0.50) are given in Table 1 and Fig. 2. Parameter estimates and Wald F tests of fixed effects for the model with the second highest AICc weight (0.21) are given in Online Resource 2.
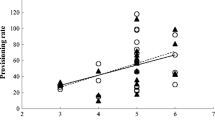
Prey deliveries by male and female Tengmalm’s Owl parents to nestlings and fledged offspring in years of a low, b increase and c peak vole abundance. ln late nestling stage, epf early post-fledging stage, lpf late post-fledging stage. Open symbols females, filled symbols males. Delivery rates are number of prey deliveries per brood and hour; log(observation time) was modelled as offset variable to correct for variation in duration of monitoring sessions. Sample size denotes number of broods. The symbols and error bars show estimated least squares means and 95% confidence limits (measurement scale), respectively, from the model with the highest AICc weight in “Appendix 1”
Males delivered more prey to the offspring than did females, both in the late nestling, early post-fledging and late post-fledging stage, and in all vole year categories (Table 1; Fig. 2; Online Resource 2). Number of prey deliveries differed among stages, with the highest number in the post-fledging stages, and the smallest in the late nestling stage (Table 1; Fig. 2; Online Resource 2). Number of deliveries was higher in low vole years and smaller in increase vole years, as compared to peak vole years (Table 1; Fig. 2; Online Resource 2). Number of deliveries increased with increasing brood size for both males and females, but females showed a stronger positive response than did males (Table 1; Online Resource 2; sex × brood size interaction). In addition, the parameter estimates for the ‘sex × stage’ interaction in the model with AICc weight 0.21 suggests that the intersexual difference was larger in the late post-fledging stage than in the late nestling and early post-fledging stages (Online Resource 2).
We also carried out an analysis of males separately, and found that prey deliveries by males were related to the behaviour of the mate (deserted or not deserted), vole year and stage (Table 2). Estimated mean delivery rate was more than twice as high for males that were deserted by the female at some stage than for males that were not deserted at any stage (Table 2). Delivery rates by males were c. 81% higher in low vole years, and c. 88% lower in increase vole years, as compared to peak vole years (Table 2). There was also a substantial difference in delivery rates by males between the late nestling stage and the post-fledging stages, with almost twice as high delivery rates in the early and late post-fledging stages compared to the late nestling stage (Table 2).
Prey deliveries to deserted versus non-deserted broods
By the end of the post-fledging stage, the female had deserted her mate and offspring in 63% of the broods (15 out of 24; for 2 broods we were unable to determine whether the female deserted or not). Males exhibited substantially higher delivery rates than did females even in nests receiving bi-parental care in the late nestling stage. Considering the late nestling stage only, and excluding all nests where the female had already deserted (8 of 26 nests), males delivered about 75% more prey to the offspring than did females (Fig. 3; Online Resource 3). In addition, the ‘brood size’ effect in the competing models indicates that both sexes increase their delivery rates as brood size increases, and the ‘sex × brood size’ interaction in one of these models suggests that females respond more strongly to increases in brood size (“Appendix 2”; Online Resource 3). This is in line with our findings for the whole offspring dependency period (Table 1; Online Resource 2). In the late nestling stage, number of prey deliveries was c. 36% higher for females that did not desert at any stage (X ± SE = 3.89 ± 2.32, n = 9) than for females that deserted at a later stage (2.86 ± 0.80, n = 9), but the 95% confidence interval for difference of means was wide (−1.38 to 3.44). Broods that were deserted by the females at some stage received more than twice as many prey from the male parent compared to broods that were not deserted at any stage (Table 2). Female offspring desertion in relation to natural and experimental changes in food abundance is analysed in Eldegard and Sonerud (2009).
Prey deliveries by male and female Tengmalm’s Owl parents to broods receiving bi-parental care in the late nestling stage (n = 18 nests). Nests already deserted by the female (n = 8 nests) were not included in the analysis. Delivery rates are number of prey deliveries per brood and hour; log(observation time) was modelled as offset variable to correct for variation in duration of monitoring sessions. The symbols and error bars show estimated least squares means and 95% confidence limits (measurement scale), respectively, from the model with the highest AICc weight in “Appendix 2”
Prey deliveries to broods not deserted by the female at any stage
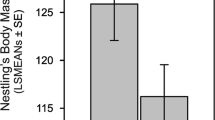
In order to analyse whether there was an intersexual difference in number of prey deliveries to broods that were not deserted by the female at any stage, we pooled the data from all years (n = 9 broods). Prey delivery rates by females were slightly lower than delivery rates by males both in the late nestling, early post-fledging and late post-fledging stages, but there was considerable overlap in the 95% confidence intervals of the estimated means for males and females (Fig. 4).
Number of prey deliveries per brood to Tengmalm’s Owl broods not deserted by the female at any stage (n = 9 broods). Open symbols females, filled symbols males. ln late nestling stage, epf early post-fledging stage, lpf late post-fledging stage. The symbols and error bars show parameter estimates and 95% lower and upper confidence limits (total number of prey deliveries delivered during a monitoring session; i. e. one night), respectively. Fixed effects were only main effects ‘sex’ and ‘stage’; ‘sex’ × ‘stage’ interaction (P = 0.89) not included. Generalised mixed model with log link function, Poisson distribution, and Laplace approximation to the likelihood. Model fit: Pearson χ 2/df = 0.70. Random effects were ‘broods size’ and ‘brood identity’. Note that because sample size was small, we had to restrict the number of parameters in the model, so that we did not include ‘duration of monitoring session’ as offset variable
Discussion
Provisioning by male and female parents
Tengmalm’s Owl males delivered substantially more prey to the offspring than did females throughout the nestling and post-fledging stages, both in low, increase and peak vole years. This large contribution by male parents corroborates our previous findings from the peak vole year (i.e. 1998; see Eldegard and Sonerud 2010). Nearly two-thirds of the females deserted at some stage. Males that were deserted had more than twice as high delivery rates as males that were not deserted, and this indicates that deserted males at least partially compensated for low female contribution. The intersexual difference in food provisioning was not merely a consequence of desertion of the offspring by the parent female; in the late nestling stage, there was a pronounced intersexual difference in delivery rates to broods that still received bi-parental care. At this stage, delivery rates appeared to be lower for females that deserted at a later stage (“intentional deserters”) than for females that did not desert at any stage. The intersexual difference in delivery rates was smaller among broods not deserted by the female at any stage (females’ proportion c. 0.43 for the whole offspring dependency period) than among broods that were deserted by the female at some stage.
Parental care, such as food provisioning, is assumed to have life-history costs (Clutton-Brock 1991; Ydenberg 2007), and parents are expected to terminate care when the net benefits of continued care are exceeded by the benefits of deserting (Stearns 1992; Székely et al. 1996). Birds are unusual among animals in that most species (>80%) exhibit bi-parental care at least during parts of the offspring dependency period (Cockburn 2006). However, males are generally assumed to be less involved, and if one parent deserts, it is usually the male (Clutton-Brock 1991; Székely et al. 1996). For Tengmalm’s Owls, we have found the opposite pattern (Eldegard and Sonerud 2009, 2010; this study), and the question is why females are less involved in provisioning and frequently desert the offspring. Eldegard and Sonerud (2009) found that there is a cost of deserting in terms of reduced offspring survival, and argued that the most important benefit of deserting may be re-mating. Korpimäki et al. (2011) found that sequential polyandry by brood desertion increased female fitness. Whereas female Tengmalm’s Owls must desert to re-mate (sequential polyandry), males may re-mate without deserting their first brood (i.e. sequential or simultaneous polygyny) (Eldegard and Sonerud 2009; Korpimäki et al. 2011). Furthermore, by deserting or contributing little to provisioning, females may prevent the male from attempting to attract a secondary female and become bigynous (Eldegard and Sonerud 2009). Also, after having devoted almost all available time to brooding and processing food for the nestlings for about 3 weeks, the female is likely to be better informed about offspring need, and thus in a position to decide on her level of effort first (see also Johnstone and Hinde 2006). Observed patterns of parental care decisions may be viewed as the outcome of negotiations within the family (Hinde and Kilner 2007), and the observed pattern of care in Tengmalm’s Owls may at least partly be a consequence of females being in a better negotiating position than their partners. Females that choose not to desert may make the best of the situation by contributing more to provisioning and thereby increase the survival prospects of the current brood. Yet, even though the intersexual difference in provisioning was smaller in non-deserted broods, we conclude that, in Tengmalm’s Owls, males are generally more heavily involved than females in pre- and post-fledging provisioning.
Our finding that males contributed substantially more than females to food provisioning in the late nestling stage is in accordance with the results from previous studies in Barn Owls (Tyto alba) (Roulin et al. 1999; Roulin and Bersier 2007) and Tawny Owls (Sunde et al. 2003). We are not aware of any studies (except Eldegard and Sonerud 2010) that have quantified provisioning rates by male and female owls in the post-fledging period. However, Sunde et al. (2003) found that males attended the offspring more often than did females in Tawny Owls pairs monitored during the post-fledging period, and that the relative intersexual difference in estimated minimum displacement distances (an indirect measure of parental effort) was highest in the post-fledging period. Among diurnal birds of prey, it appears to be a common pattern that male parents feed offspring more frequently than do females both in the nestling and the post-fledging stages (e.g. Collopy 1984; Masman et al. 1988; Vekasy et al. 1996; Wiehn and Korpimäki 1997; Eldegard et al. 2003 with references; Vergara and Fargallo 2008), but some studies report that females contribute equally or more than males (e.g. Bustamante 1994; Real et al. 1998; Dawson and Bortolotti 2002).
For non-raptorial birds, there are substantial among-species differences with respect to male and female contributions to post-fledging care. For Little Auks (Alle alle) and other alcids (Alcidae), the usual pattern appears to be a transition from bi-parental to uni-parental male care during late chick-rearing, with males accompanying their chick(s) to sea and subsequently caring for their fledgling(s) alone (see Harding et al. 2004 with references). In contrast, post-fledging feeding rates of male Barn Swallows (Hirundo rustica) were considerably lower and dropped earlier than those of females (Naef-Daenzer et al. 2011). In American Dippers (Cinclus mexicanus), parents contributed equally to provisioning in the first week of post-fledging care, but males took longer time to return with food than females in the second week (Middleton et al. 2007). For Great Tits (Parus major), Verhulst and Hut (1996) found that females participated less than males in post-fledging care for their first-brood offspring, both for females without a second clutch and for double-brooded females that had their clutch experimentally removed. In some species, each parent feeds a different subset of offspring post-fledging (brood division), and males and females provision offspring at the same rate (e.g. Eastern Whipbird Psophodes olivaceus, Rogers and Mulder 2004; Toc–toc Foudia sechellarum, Vega et al. 2007). Given the large among-species differences in phylogeny and ecology, substantial variation in the patterns of post-fledging care is not surprising. Yet, we conclude that large male contributions to post-fledging care appears to be widespread among birds.
Provisioning in relation to natural food abundance
For both sexes, provisioning rates differed among years of different prey abundance, and were higher in low vole years and smaller in increase vole years, as compared to peak vole years. Similarly, Hakkarainen and Korpimäki (1995) found that male Tengmalm’s Owls delivered more prey items to the nest in low than in increase and peak vole years. It may appear counterintuitive to find the highest provisioning rates in years of low natural prey abundance. However, this is probably due to the fact that Tengmalm’s Owls capture relatively more shrews (Sorex spp.) when voles are scarce, and the fact that shrews are smaller than voles, so that the owls have to compensate by increasing the number of hunting trips (Korpimäki 1981, 1988; Hakkarainen and Korpimäki 1995). Thus, even though provisioning rates were high in low vole years, the total abundance of prey was quite low (own unpublished data). Based on our previous findings that the probability of female offspring desertion increased with increasing food supply, and that, in response to experimentally increased food supply, females showed a stronger reduction provisioning rates than did males (Eldegard and Sonerud 2009, 2010), we predicted that females would contribute relatively more to food provisioning in low vole years. However, we did not find larger among-year differences in delivery rates for females than for males.
Provisioning in relation to offspring age
We found that delivery rates were substantially higher post-fledging than in the late nestling stage. In addition, one of the competing models indicated that the intersexual difference in delivery rates was at its largest in the late post-fledging stage, which is consistent with the finding that a large proportion of the females had deserted by this stage. Our results are consistent with those of Sunde et al. (2003), who found that foraging male Tawny Owls moved longer distances per time unit in the post-fledging than in the nestling stage, and interpreted this as evidence of increased male parental effort post-fledging. Most studies have found prey biomass or delivery rates by the parents combined to be higher in the post-fledging than in the late nestling stage (Montagu’s Harriers Circus pygargus, Arroyo et al. 2002; Red-footed Booby Sula sula, Guo et al. 2010; Western Slaty-antshrike Thamnophilus atrinucha, Tarwater and Brawn 2010). In contrast, Bustamante and Negro (1994) found that the combined delivery rates of Lesser Kestrel (Falco naumanni) parents were higher in the late nestling than in the post-fledging stage, but the duration of the post-fledging dependence period in this species is unusually short (5 days). As the Tengmalm’s Owl fledglings improved their flight skills, they gradually moved towards the parents and thereby prey transportation distances were reduced (own unpublished data). In addition, prey availability and vulnerability probably varied throughout the offspring dependency period, and this may also have affected the parent’s energy expenditure. Thus, because we did not measure the parents’ energy expenditure directly, we could not demonstrate unequivocally that parental effort was higher in the post-fledging stage. However, post-fledging care undoubtedly constitutes a substantial part of the total parental effort in Tengmalm’s Owl, especially for male parents.
Provisioning in relation to brood size
We found that delivery rates increased with brood size for both sexes, but this relationship was stronger for females than for males. Similarly, Vergara and Fargallo (2008) found that the rate of prey delivery by Eurasian Kestrel (Falco tinnunculus) parents increased with the number of fledglings remaining in the surroundings of the nest. We have previously shown that broods receiving male-only care following female desertion suffered higher offspring mortality than broods receiving bi-parental care throughout the offspring dependency period (Eldegard and Sonerud 2009). When brood size increases, the brood’s need also increases, and this may explain the increased female involvement in large broods.
Provisioning in relation to sexual size dimorphism
As Tengmalm’s Owls exhibit pronounced reversed sexual size dimorphism (RSD) (Hipkiss 2002), one may ask if the larger female could potentially provide the same amount of food as the smaller male parent by delivering fewer but larger prey. We did not measure prey size in this study (which would have been impossible post-fledging), but in 2002, a total of 63 prey deliveries by males and females at four Tengmalm’s Owl nests at which both parents provided prey in the late nestling stage were filmed (G.A. Sonerud, unpublished data). Only 11 (17.5%) of the recorded deliveries were performed by the female. There was no indication of females delivering larger prey than males; estimated mean prey body mass (95% confidence limits), females: 13.0 g (7.0–24.4 g), males: 13.6 g (10.2–18.2 g) (nest identity modelled as random effect; G.A. Sonerud, unpublished data). We are not aware of any published data on prey size delivered by male and female Tengmalm’s Owls. However, in order for females to match the amount of food provided by the male, they would have had to catch substantially larger prey (e.g. 75% larger in the late nestling stage). We assert that that is highly unlikely, because the size range of potential prey species in our study area is limited, and male Tengmalm’s Owls regularly capture the largest prey species available (Microtus spp.). Even if the females did capture fewer but larger prey, we question whether the females’ provisioning effort (energy expenditure) would equal that of the males, as they would still exhibit fewer hunting trips.
Given the extensive post-fledging paternal care found in raptorial birds studied so far, a female should benefit from having an efficient hunter to provide for her offspring. The distinct role division between male and female parents, including the male’s role as forager for the whole family, has provided the grounds for several hypotheses proposing that natural selection may explain the evolution of RSD in predatory birds: (1) selection for males to be small during the breeding season, in order to be effective hunters and minimise flight costs (Andersson and Norberg 1981; Temeles 1985; Massemin et al. 2000; Sunde et al. 2003; Krüger 2005; Slagsvold and Sonerud 2007), and (2) selection for both males and females to be large in the non-breeding season, in order to maximise survival (Ydenberg and Forbes 1991; Slagsvold and Sonerud 2007). Sunde et al. (2003) suggested that female Tawny Owls move less than males and contribute less to post-fledging food provisioning because they are larger and therefore less energy-efficient. As a consequence, Sunde et al. (2003) argued that females are “predetermined to take the job of guarding the young”. The degree of RSD is even larger in Tengmalm’s Owl than in Tawny Owl (Cramp 1985). In many owl species, female parents can provide care for the offspring by staying close to the nest or fledged offspring in order to guard them against predators (e.g. Hawk Owl Surnia ulula, Tawny Owl, Ural Owl Strix uralensis, Great Grey Owl Strix nebulosa; Mikkola 1983; Cramp 1985). However, in a radio-tracking study of fledgling behaviour and spatial associations between fledglings and parents, we found no indication of female Tengmalm’s Owls being more closely associated (guarding) with the offspring post-fledging than were the males; in fact, neither sex appeared to guard the fledglings at all (own unpublished data).
Potential correlates of RSD in raptors were investigated by Krüger (2005), but none of the predictor variables included in his analyses contained information on the relative contribution of the sexes in parental duties. We assume that this reflects the fact that quantifying the relative contribution of the sexes is logistically demanding; many species are secretive, cryptic and even nocturnal, and the fledged young perform extensive spatial movements before they become independent of their parents for food. As quantitative evidence from more species accumulate in the future, we believe that it will be worthwhile to investigate the potential relationship between the degree of reversed sexual size dimorphism and the relative contribution by male and female parents to food provisioning throughout the whole offspring dependency period.
References
Anderson DR, Link WA, Johnson DH, Burnham KP (2001) Suggestions for presenting the results of data analyses. J Wildl Manag 65:373–378
Andersson M, Norberg RÅ (1981) Evolution of reversed sexual size dimorphism and role partitioning among predatory birds, with a size scaling of flight performance. Biol J Linn Soc 15:105–130
Arroyo BE, DeCornulier Th, Bretagnolle V (2002) Parental investment and parent-offspring conflicts during the postfledging period in Montagu’s harriers. Anim Behav 63:235–244
Bjørnstad ON, Ims RA, Lambin X (1999) Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol Evol 14:427–432
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2008) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bustamante J (1994) Behavior of colonial Common Kestrels (Falco tinnunculus) during the post-fledging dependence period in southwestern Spain. J Raptor Res 28:79–83
Bustamante J, Negro JJ (1994) The post-fledging dependence period of the lesser kestrel (Falco naumanni) in southwestern Spain. J Raptor Res 28:158–163
Carlsson B-G, Hörnfeldt B (1994) Determination of nestling age and laying date in Tengmalm’s Owl: use of wing length and body mass. Condor 96:555–559
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc Lond B 273:1375–1383
Collopy MW (1984) Parental care and feeding ecology of Golden Eagle nestlings. Auk 101:753–760
Cramp S (1985) The birds of the western palearctic, vol IV. Oxford University Press, Oxford
Dawson RD, Bortolotti GR (2002) Experimental evidence for food limitation and sex-specific strategies of American kestrels (Falco sparverius) provisioning offspring. Behav Ecol Sociobiol 52:43–52
Eldegard K, Sonerud GA (2009) Female offspring desertion and male-only care increase with natural and experimental increase in food abundance. Proc R Soc Lond B 276:1713–1721
Eldegard K, Sonerud GA (2010) Experimental increase in food supply influences the outcome of within-family conflicts in Tengmalm’s owl. Behav Ecol 64:815–826
Eldegard K, Selås V, Sonerud GA, Steel C, Rafoss T (2003) The effect of parent sex on prey deliveries to fledgling Eurasian sparrowhawks Accipiter nisus. Ibis 145:667–672
Grüebler MU, Naef-Daenzer B (2010) Survival benefits of post-fledging care: experimental approach to a critical part of avian reproductive strategies. J Anim Ecol 79:334–341
Guo H, Cao L, Peng L, Zhao G, Tang S (2010) Parental care, development of foraging skills, and transition to independence in the red-footed booby. Condor 112:38–47
Hakkarainen H, Korpimäki E (1995) Contrasting phenotypic correlations in food provision of male Tengmalm’s owls (Aegolius funereus) in a temporally heterogenous environment. Evol Ecol 9:30–37
Harding AMA, van Pelt TI, Lifjeld JT, Mehlum F (2004) Sex differences in little auk Alle alle parental care: transition from biparental to paternal-only care. Ibis 146:642–651
Hinde CA (2006) Negotiation over offspring care?—a positive response to partner-provisioning rate in great tits. Behav Ecol 17:6–12
Hinde CA, Kilner RM (2007) Negotiations within the family over the supply of parental care. Proc R Soc Lond B 274:53–60
Hipkiss T (2002) Sexual size dimorphism in Tengmalm’s owl (Aegolius funereus) on autumn migration. J Zool 257:281–285
Hörnfeldt B, Carlsson B-G, Löfgren O, Eklund U (1990) Effects of cyclic food supply on breeding performance in Tengmalm’s owl (Aegolius funereus). Can J Zool 68:522–530
Johnstone RA, Hinde CA (2006) Negotiation over offspring care–how should parents respond to each other’s efforts? Behav Ecol 17:818–827
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Korpimäki E (1981) On the ecology and biology of Tengmalm’s owl (Aegolius funereus) in Southern Ostrobothnia and Suomenselkä, western Finland. Acta Univ Oulu Ser A Bio 13:1–84
Korpimäki E (1987) Clutch size, breeding success and brood size experiments in Tengmalm’s owl Aegolius funereus: a test of hypotheses. Ornis Scand 18:277–284
Korpimäki E (1988) Diet of breeding Tengmalm’s owls Aegolius funereus: long term changes and year-to-year variation under cyclic food conditions. Ornis Fenn 65:21–30
Korpimäki E (1990) Body mass of breeding Tengmalm’s owls Aegolius funereus: seasonal, between-year, site and age-related variation. Ornis Scand 21:169–178
Korpimäki E, Lagerström M (1988) Survival and natal dispersal of fledglings of Tengmalm’s owl in relation to fluctuating food conditions and hatching date. J Anim Ecol 57:433–441
Korpimäki E, Salo P, Valkama J (2011) Sequential polyandry and brood desertion increases female fitness in a bird with obligatory bi-parental care. Behav Ecol Sociobiol 65:1093–1102
Kosztolányi A, Cuthill IC, Székely T (2008) Negotiation between parents over care: reversible compensation during incubation. Behav Ecol 20:446–452
Krüger O (2005) The evolution of reversed sexual size dimorphism in hawks, falcons and owls: a comparative study. Evol Ecol 19:467–486
Lessells CM, Parker GA (1999) Parent-offspring conflict: the full-sib half-sib fallacy. Proc R Soc Lond B 266:1637–1643
Masman D, Daan S, Dijkstra C (1988) Time allocation in the kestrel (Falco tinnunculus), and the principle of energy minimization. J Anim Ecol 57:411–432
Massemin S, Korpimäki E, Wiehn J (2000) Reversed sexual size dimorphism in raptors: evaluation of the hypotheses in kestrels breeding in a temporally changing environment. Oecologia 124:26–32
Middleton HA, Green DJ, Krebs EA (2007) Fledgling begging and parental responsiveness in American dippers (Cinclus mexicanus). Behaviour 144:485–501
Mikkola H (1983) Owls of Europe. Poyser, Calton
Naef-Daenzer L, Grüebler MU, Naef-Daenzer B (2011) Parental care trade-offs in the inter-brood phase in barn swallows Hirundo rustica. Ibis 153:27–36
Newton I (1979) Population ecology of raptors. Poyser, Berkhamsted
Newton I (1986) The sparrowhawk. Poyser, Calton
Olson VA, Liker A, Freckleton RP, Székely T (2008) Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proc R Soc Lond B 275:301–307
Penteriani V, Delgado MM, Maggio C, Aradis A, Sergio F (2005) Development of chicks and predispersal behavior of young in the eagle owl Bubo bubo. Ibis 147:155–168
Real J, Mañosa S, Codina J (1998) Post-nestling dependence period in the Bonelli’s eagle Hieraaetus fasciatus. Ornis Fenn 75:129–137
Rogers AC, Mulder RA (2004) Breeding ecology and social behaviour of an antiphonal duetter, the eastern whipbird (Psophodes olivaceus). Aust J Zool 52:417–435
Roulin A, Bersier L-F (2007) Nestling barn owls beg more intensely in the presence of their mother than in the presence of their father. Anim Behav 74:1099–1106
Roulin A, Ducrest A-L, Dijkstra C (1999) Effect of brood size manipulations on parents and offspring in the barn owl Tyto alba. Ardea 87:91–100
Selås V, Sonerud GA, Histøl T, Hjeljord O (2001) Synchrony in short-term fluctuations of body mass of moose calves and population density of bank voles supports the mast depression hypothesis. Oikos 92:271–278
Slagsvold T, Sonerud GA (2007) Prey size and ingestion rate in raptors: importance for sex roles and reversed sexual size dimorphism. J Avian Biol 38:650–661
Sonerud GA (1985) Nest hole shift in Tengmalm’s owl Aegolius funereus as defence against nest predation involving log-term memory in the predator. J Anim Ecol 54:179–192
Sonerud GA (1986) Effect of snow cover on seasonal changes in diet, habitat, and regional distribution of raptors that prey on small mammals in boreal zones of Fennoscandia. Holarctic Ecol 9:33–47
Sonerud GA (1988) What causes extended lows in microtine cycles? Analysis of fluctuations in sympatric shrew and microtine populations in Fennoscandia. Oecologia 76:37–42
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Steen H, Ims RA, Sonerud GA (1996) Spatial and temporal patterns of small-rodent population dynamics at a regional scale. Ecology 77:2365–2372
Sunde P (2008) Parent-offspring conflict over duration of parental care and its consequences in tawny owls Strix aluco. J Avian Biol 39:242–246
Sunde P, Markussen BEN (2005) Using counts of begging young to estimate post-fledging survival in tawny owls Strix aluco. Bird Study 52:343–345
Sunde P, Bølstad MS, Møller JD (2003) Reversed sexual dimorphism in tawny owls, Strix aluco, correlates with duty division in breeding effort. Oikos 101:265–278
Székely T, Webb JN, Houston AI, McNamara JM (1996) An evolutionary approach to offspring desertion in birds. In: Nolan V, Ketterson ED (eds) Current ornithology. Plenum, New York, vol 13, pp 271–330
Tarwater CE, Brawn JD (2010) The post-fledging period in a tropical bird: patterns of parental care and survival. J Avian Biol 41:479–487
Temeles EJ (1985) Sexual size dimorphism of bird-eating hawks: the effect of prey vulnerability. Am Nat 125:485–499
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–1971. Aldine Atherton, Chicago, pp 136–179
Vega LB, Holloway GJ, Millett JE, Richardson DS (2007) Extreme gender-based post-fledging brood division in the toc–toc. Behav Ecol 18:730–735
Vekasy MS, Marzluff JM, Kochert MN, Lehman RN, Steenhof K (1996) Influence of radio transmitters on prairie falcons. J Field Ornithol 67:680–690
Vergara P, Fargallo JA (2008) Sex, melanic coloration, and sibling competition during the postfledging dependence period. Behav Ecol 19:847–853
Vergara P, Fargallo J, Martínez-Padilla J (2010) Reaching independence: food supply, parent quality, and offspring phenotypic characters in kestrels. Behav Ecol 21:507–512
Verhulst S, Hut RA (1996) Post-fledging care, multiple breeding and the costs of reproduction in the great tit. Anim Behav 51:957–966
Wells KMS, Harvey HT, White JD et al (2007) A review of the avian post-fledging literature: major themes and future recommendations. COS 4-2 contributed oral abstract. In: COS4—conservation ecology and ecosystem management: planning and policy. Ecological Society of America/Society for Ecological Restoration International, Joint Meeting August 2007
Wiehn J, Korpimäki E (1997) Food limitation on brood size: experimental evidence in the Eurasian kestrel. Ecology 78:2043–2050
Wiens JD, Noon BR, Reynolds RT (2006) Post-fledging survival of northern goshawks: the importance of prey abundance, weather, and dispersal. Ecol Appl 16:406–418
Ydenberg RC (2007) Provisioning. In: Stephens DW, Brown JS, Ydenberg RC (eds) Foraging: behaviour and ecology. University of Chicago Press, Chicago, pp 273–303
Ydenberg RC, Forbes LS (1991) The survival-reproduction selection equilibrium and reversed size dimorphism in raptors. Oikos 60:115–120
Acknowledgments
We thank O. Heie, E.J. Hildrum, I. Løvdal, and H. Vognild for assistance in the field; R. Bjørnstad, G. Nyhus, the late F. Rønning, K. Skjærvik, O. Skjærvik, T. Wernberg, and E. Østby for finding some of the owl nests; and H. Antonsen, G. Fry, V. Selås, P. Sunde, J. O. Vik and three anonymous reviewers for comments on previous drafts of the manuscript. The Directorate for Nature Management and the National Animal Research Authority in Norway granted permission to trap and radio-tag the owls, and the Directorate for Nature Management granted permission to trap small mammals. The Research Council of Norway (grant no. 123604/410) and the Nansen Endowment (grants no. 75/96 and 81/97) provided financial support for the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study complies with Norwegian law; trapping, handling, radio-tagging and follow-up of radio-tagged individuals were done with permission from, and in accordance with, the ethical standards provided by, the Directorate for Nature Management and the National Animal Research Authority of Norway. An ethical note is given in Online Resource 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Friedl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1
See Table 3.
Appendix 2
See Table 4.
Rights and permissions
About this article
Cite this article
Eldegard, K., Sonerud, G.A. Sex roles during post-fledging care in birds: female Tengmalm’s Owls contribute little to food provisioning. J Ornithol 153, 385–398 (2012). https://doi.org/10.1007/s10336-011-0753-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-011-0753-7