Abstract
Parental care may be costly to parents because it decreases resources allocated to self-maintenance and may thus reduce survival and future reproductive success. An inter-sexual conflict may exist in animals with obligatory bi-parental care, such as birds of prey, in which females incubate and brood, whereas males provision food for their families. We analysed 29 years of data (1981–2009) from a study population of Tengmalm’s owls Aegolius funereus in western Finland to examine the occurrence and timing of brood desertion and sequential polyandry, and recorded a total of 1,123 monogamous and 12 polyandrous females. These data were supplemented with the 29-year nationwide Finnish ringing data, which included 11,590 monogamous and 20 polyandrous females. The 12 polyandrous females started egg-laying in their two nests at intervals of 54–68 days (mean 60 days), thus deserting their first broods when the age of oldest young averaged 21 days. Thirty-two polyandrous females re-mated and raised a second brood at a median distance of 4.5 km (range 1–196 km). These females produced 79% more eggs, 93% more hatchlings and 73% more fledglings than did females that laid simultaneously but remained monogamous. Our results show that not only males, but also females of altricial species with bi-parental care can increase their fitness by deserting their first brood when it will be cared for by the males. Earlier studies have shown that male owls can increase their lifetime reproductive success by simultaneous polygyny, and we suggest that an inter-sexual “tug-of-war” over bi-parental care exists in Tengmalm’s owls.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parental care increases the survival and fitness of offspring (Clutton-Brock 1991) but may be costly to parents if it decreases the resources they allocate to self-maintenance and reduces their survival and future reproductive success (Roff 1992; Stearns 1992). Therefore, mating systems should not be considered as co-operative adventures in which females and males rear offspring in agreement, but rather as each individual attempts to maximise its own reproductive success even at the expense of its mate (Trivers 1972; reviews by Parker et al. 2002; Houston et al. 2005). Therefore, there is an inter-sexual conflict over parental care in animals (e.g., Hinde and Kilner 2007; Olson et al. 2008), in which both parents have important duties during the reproductive season. Thus, the costs and benefits of caring versus desertion need to be considered for each gender.
The main advantage proposed for monogamy is that males and females produce the most offspring if both parents help to raise a brood (Lack 1968). However, many individual-level population studies have revealed that regular social polygyny occurs in at least 10% of bird species from at least ten orders (review by Bennett and Owens 2002). In these cases, it is the male that partially or entirely deserts his offspring and re-mates in early or sometimes later phases of the breeding cycle. In addition, social polyandry has been documented in at least 11 different families including less than 5% of all bird species (Bennett and Owens 2002). Therefore, offspring desertion by females is far less common among birds, and in particular, the evolution of uni-parental male care has remained a puzzle (e.g., Clutton-Brock 1991). Uni-parental male care and “classical polyandry”, in which one female is paired with two or more males with separate nests in a season, is usually possible only in precocial birds, for example, in waders. Their chicks feed themselves and females can desert their broods after clutch completion to be cared by their mates (review by Oring 1983; Andersson 2005). Multi-nest sequential polyandry in birds with altricial chicks, where males are either unwilling or physiologically unable to perform the majority of incubation, is rare. It has been reported only in a handful of species (see Wiktander et al. 2000; Wiebe 2005 with references).
In species with bi-parental care, both sexes benefit from the reproductive effort of their partner and are suggested to somewhat decrease their own reproductive costs (Clutton-Brock 1991; Parker et al. 2002; but see Jones et al. 2002). When the benefits of desertion exceed the benefits of care, parents are expected to abandon their offspring (Houston et al. 2005). A main benefit of offspring desertion is that it allows faster re-mating for a new breeding attempt (Korpimäki 1989; Szekely et al. 1996; Roulin 2002). By shortening the period between two annual breeding events in seasonal environments, desertion could be beneficial in terms of fitness. Desertion may also allow individuals to re-allocate some resources from reproduction into body maintenance, moult or migration (e.g., Szekely et al. 1996; Currie et al. 2001). However, offspring desertion can be costly due to loss of a high-quality mate or territory (Roulin 2002) and a reduction of reproductive success at the first nest (Eldegard and Sonerud 2009). Offspring desertion may be constrained by re-mating opportunities (Emlen and Oring 1977) and reproductive success by the ability and decision of the partner to rear offspring on its own.
In birds of prey, the division of breeding duties between sexes is more marked than in most other birds and they thus have obligatory bi-parental care. The duties of females include production and incubation of eggs, brooding the young and distributing prey items delivered by males to the young until they are half-grown, whereas males specialise on hunting and providing their families with food from before the egg-laying until the independence of offspring (Newton 1979; Mikkola 1983). This division of duties has been mostly ignored in models of parental care that usually assume that a single estimate, such as food provisioning rate, is sufficient to characterise parental care behaviour (Houston et al. 2005). In birds of prey, prey deliveries by males are obligatory prior to egg-laying until the independence period of young, whereas maternal care probably is less crucial after the offspring are half-grown. Therefore, female birds of prey have been shown to desert their offspring more often than do males (Beissinger and Snyder 1987; Korpimäki 1989; Kelly and Kennedy 1993; Eldegard and Sonerud 2009; Zárybnická 2009). However, few studies have shown that deserting females re-mate (Korpimäki 1989; Roulin 2002), and no studies have shown fitness benefits of offspring desertion for female birds of prey.
Tengmalm’s owl (Aegolius funereus) is a small nocturnal hole-nesting bird of prey that has obligatory bi-parental care until the offspring can thermo-regulate at the age of about 3 weeks (Kuhk 1969; Korpimäki 1981). These owls subsist mainly on voles of the genera Microtus and Myodes in northern Europe (Korpimäki 1981, 1988a). Populations of these voles show high-amplitude cycles with a period of 3 years in southern and central parts of North Europe (e.g., Korpimäki et al. 2004, 2005), which provides an excellent opportunity to study mating systems and parental care of these owls under highly fluctuating food conditions. Tengmalm’s owls are usually single-brooded with a typical clutch size of five to seven eggs in good vole years but only three to five eggs in poor vole years (Korpimäki and Hakkarainen 1991). Some 10–20% of male owls re-mate within the same season without deserting their first broods in years of high vole abundance (i.e., are simultaneously bigynous or even trigynous), whereas some 20–25% of males remain bachelors even if they possess a nest-hole and a territory (Korpimäki 1989, 1991; Carlsson 1991; Hakkarainen and Korpimäki 1998). A total of 14 polyandrous female Tengmalm’s owls were documented in Europe up to 1986. These females deserted their first broods in the late nestling period, re-mated and bred again (review by Korpimäki 1989). Recently, it has been found that some 70% of radio-tagged female owls deserted their first broods and that male owls continued to care the offspring alone (Eldegard and Sonerud 2009).
In this work, we analyse long-term data from a large population of Tengmalm’s owls in western Finland supplemented with corresponding nationwide ringing data to find out the timing of brood desertion, and occurrence and fitness consequences of sequential polyandry. Our first aim was to document whether females that deserted their broods re-mated and became polyandrous. If so, we determined inter-nest distances of polyandrous females. Third, we examined how the frequency of polyandry varied relative to fluctuating densities of prey. Our final and most important question was whether polyandrous females produced more offspring than did monogamous females that nested at the same time. To our knowledge, this last question has rarely been studied in altricial species, and never in birds of prey.
Material and methods
The study was conducted in the Kauhava region (62°55′–63°17′N, 22°55′–23°35′E) of western Finland in an area containing 395 (in 1981), 415 (1982), 450 (1983–1987), 500 (1989–1999) to 490 (in 2000–2009) nest-boxes and known natural cavities suitable for Tengmalm’s owls. The study area was gradually extended with the increasing number of nest-boxes so that the nest-site density remained stable (0.5–1 per km2). The study area covered 1,100 km2 during 1983–1987 and 1,300 km2 in 1988–2009. Different-aged managed spruce- and pine-dominated forests covered 61% of the area, farmlands 25%, clear-cut and sapling areas 6%, peat-land bogs 2%, lakes, ponds and rivers 2%, and other areas (settlements, roads, peat production, etc.) 3% of the study area. All the nest-boxes and known natural cavities were inspected twice per season and most nests were checked at least three times to determine final clutch size, laying date, number of hatchlings and the number of large (>3-week-old) nestlings when the chicks were also ringed. All the nests were inspected after the post-fledging period to find out the number of ringed nestlings succumbed on the bottom and in the vicinity of nest-boxes. The final number of chicks fledged (i.e., the number of fledglings) was estimated by subtracting the number of succumbed ringed nestlings from the number of nestlings at the stage of ringing (see Korpimäki 1987; Korpimäki and Hakkarainen 1991; Hakkarainen et al. 2003 for further details on the study area, dimensions of nest-boxes, the methods used for finding nests and determining breeding success).
We trapped (ringed or re-trapped) female Tengmalm’s owls at 1,135 nests and males at 934 nests during 1981–2009 early in the nestling period. During 1981–2001 and 2006–2009, most (80–100%) breeding females of the study population were ringed or re-trapped, and during 1981–1996, 1999–2000 and 2007–2009, 60–100% of breeding males of the study population were ringed or re-trapped. Females were caught in their nest-boxes by closing the entrance hole by hand or rod when they were incubating eggs or brooding young (see Korpimäki 1981, 1983 for methods for trapping males). Parent owls were weighed to an accuracy of 1 g and the maximum length of the flattened right wing was measured to the nearest 1 mm (Svensson 1992). We differentiated first-year breeders from older ones by checking the moult score of primaries, according to Glutz von Blotzheim and Bauer (1980). The reliability of this method was confirmed from 47 re-trapped owls of known age (Lagerström and Korpimäki 1988; see also Hörnfeldt et al. 1988).
Trapping records in our study area showed that, after the first breeding attempt, male Tengmalm’s owls stay in the same area for their lifetime. They use one to five boxes in an area covering 2–5 km2, but the male rarely occupies the same nest-box in two or more successive years (only 28 cases out of 299 during 1979–2009; Korpimäki 1987, 1992, unpublished data). Moreover, the pair bond is annual since only in three cases (out of 923 during 1979–2009) did the pair bond last more than one breeding season (Korpimäki 1989, unpublished data).
Owl territories were graded into six categories according to the breeding frequency during the first 10 years when the nest-boxes on the territories were erected: 0 = not used for breeding, 1 = one nest, 2 = two nests, 3 = three nests, 4 = four nests, and 5 = 5–9 nests (Korpimäki 1988b). More voles (Microtus and Myodes spp.) were snap-trapped on frequently used territories than on seldom used territories, and the abundance of small birds (important alternative prey of Tengmalm’s owls; Korpimäki 1981, 1988a) was also higher on frequently used territories (Hakkarainen et al. 1997). This suggests that our territory-grading estimates were largely independent of the quality of territory occupants.
In our study area, the incubation period of the first egg averages 29.2 (±1.7 SD) days and the nestling period of the first-hatched young averages 32.6 (±2.3) days, while the brooding period of females (i.e., the period when the female warms the chicks and divides prey items delivered by males to chicks) lasts on average 21.1±2.5 days (range 15–23 days; Korpimäki 1981). Polyandrous females were defined as females that were trapped or re-trapped at two nests with a different male parent so that the first nest was successful until the young were at least 3–4 weeks old. All the other females were defined as monogamous, although some females of the late nests may have made their first breeding attempt outside our study area and might thus have been double-brooded. This proportion should be minor, however, because the effort of ringing female owls is also relatively high outside our large study area (see below). In fact, the first nesting attempts of all the polyandrous females were successful until the fledging phase, because all these females fledged ≥ 1 chick. Dispersal distances of these females were estimated as the linear distance between the nest-boxes that they used in successive breeding attempts. Females trapped for the first time at the nests that failed in the early stages of the breeding season were omitted, because these re-traps included re-nesting attempts, not real brood desertion and sequential polyandry cases. For comparing the reproductive success of polyandrous females and monogamous females laying simultaneously to primary and secondary nests of polyandrous females, a group of “control” females was selected among the monogamous females so that the laying dates of the first egg matched those of polyandrous females as much as possible.
Supplementary nationwide ringing data from Finland during 1981–2009 included a total of 11,610 females ringed or re-trapped at their nests (Table 1). Double-brooding and dispersal distances were defined using the same criteria noted above. In the nationwide data, annual numbers of Tengmalm’s owl nests in which the female parent was trapped or re-trapped varied from 92 to 1,203 (mean ± SD 400±259) during 1981–2009. The average (±SD) proportion of females ringed or re-trapped at nests in which chicks were ringed was 39.6% (±8.2%) with an annual range of 26% to 61% during 1981–2009 (Table 1). Therefore, the effort of ringing female parents was relatively high nationwide, which increased the probability of encountering double-brooded females, even at long dispersal distances.
The abundance of small mammals in our study area was estimated yearly by snap-trapping in May and in September during 1977–2009. Trap nights totalled 25,564 in the western part of the study area during 1977–1991, and 32,448 in the central part during 1973–1991. We pooled the results from 4-night trapping periods and standardized them to the number of animals captured per 100 trap nights. The final abundance index derived from the trapping results was the mean for the western and central parts of the study area (see Korpimäki 1981; Korpimäki and Wiehn 1998 for further details). Based on these data, the food abundance of the breeding season was classified into the following phases.
-
(1)
The increase years when vole abundance increased from a moderate level in early spring to the peak in the following autumn.
-
(2)
The decrease years when vole populations were moderate to high in early spring but declined to very low levels in the following autumn.
-
(3)
The low phase when the vole abundance was low in spring and summer but started to increase towards the next autumn.
Statistical analyses were made with SPSS 11.5. for Windows. When analysing body mass of monogamous and polyandrous females and their partners, structural size was controlled for by including wing length as the covariate in ANOVAs (Garcia-Berthou 2001).
Results
Of the 1,135 females trapped at their nests in our study area during 29 years, 12 (1.1%) were paired with two males in successive breeding attempts (Fig. 1) and were thus sequentially polyandrous. Ten of the 12 polyandrous females made both of their breeding attempts within our study area (maximum linear distance moved 35 km; Fig. 2). One female first bred south of our study area and made her second breeding attempt in our study area (linear distance moved 95 km in 1991; see Korpimäki 1993), whereas the other female first bred successfully in our study area and moved 95 km southwards to breed again in 2008. Of 11,610 females trapped at their nests elsewhere in Finland, 20 (0.2%) females were trapped at two nests and were thus defined as sequentially polyandrous (Table 1). Overall, polyandrous females searched for mates over relatively long distances (mean ± SD 22.9±48.2 km, median 4.5 km, range 1–196 km; Fig. 2).
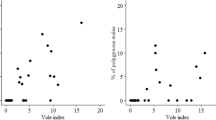
Number of monogamous (black bars) and polyandrous (grey bars) Tengmalm’s owl females in the three phases (low, increase and decrease) of the 3-year vole cycle in the Kauhava region, western Finland during 1981–2009. Note the different scale for monogamous and polyandrous females (left and right y-axis, respectively)
The 12 polyandrous females in our study area initiated egg-laying of their first annual breeding attempt on average 14 days earlier and produced on average 0.4 eggs more than monogamous females of the study population (mean ± SD: 15 March ±16 days, n = 12 vs. 29 March ± 18 days, n = 373 for laying date; 6.3±0.6, n = 12 vs. 5.9±1.2, n = 369 for clutch size; pooled data from 1985, 1988, 1991, 1992, 2008). These 12 polyandrous females started egg-laying in their two nests at intervals of 54–68 days (mean ± SD 59.7±4.0 days). If we assume conservatively that the time needed to search for and pair with a new mate, and putting on mass to lay a new clutch is at least 10 days, polyandrous females should have deserted their first broods when the oldest young were 21 days old on average. In one case, the oldest young was only 15 days old. This corresponds well with the brooding period observed in our study area and also the average brooding period of 22.2 days (+0.9 days) observed in a Czech study population in 2004, when most females deserted their broods (Zárybnická 2009).
Females were especially likely to desert their broods and pair with a new mate during the increase phase of the vole cycle (11 of 12 polyandry cases; Fig. 1), when the abundance of main prey rapidly increased in the course of the summer. The same tendency also appeared to be true for the nationwide ringing data from double-brooded females (11 of 20 polyandry cases), although this result is hampered by the fact that the classification of the different years outside our study area is not based on biannual trapping estimates of voles. This source of error should be minor, however, because the spatial synchrony of vole population cycles extends 80–100 km (Huitu et al. 2005, 2008), sometimes even up to 500–600 km in South and Central Finland (Sundell et al. 2004). There was a positive correlation between the pooled spring abundance index of Microtus voles and bank voles Myodes glareolus and the proportion of polyandrous females of all females trapped in our study population during 1981–2009 (Fig. 3).
Yearly percentage (%) of polyandrous females of all owl females plotted against the pooled abundance index of Microtus and bank voles (the number of voles captured per 100 trap nights) during 1981–2009 in the Kauhava region, western Finland (Spearman rank correlation, r s = 0.55, n = 29, two-tailed p = 0.002)
There was no apparent association between female age and polyandry in our study population, as the proportion of first-year breeders was 16.6% for polyandrous females and 41.7% for monogamous females that initiated egg-laying simultaneously (χ 2 test = 1.82, p = 0.18). The corresponding proportions were 29.4% for polyandrous females and 41.0% for monogamous females in the nationwide data (χ 2 test = 0.94, p = 0.33). Our results thus do not clearly suggest that older females are more successful in finding a new mate and in initiating a second clutch. All first-year breeders deserted their broods in the late nestling and fledging periods whereas the frequency of brood desertion was lower for older females (see Eldegard and Sonerud 2009).
There were no obvious differences in body mass, wing length, and in territory quality between simultaneously laying polyandrous and monogamous females (Table 2). Primary mates of polyandrous females tended to be heavier and thus in a better body condition than mates of monogamous females laying simultaneously (body mass: mean ± SD 112.7±5.7 vs. 107.6±8.9 g, respectively; two-way analysis of covariance [ANCOVA], body mass F 3,20 = 4.25, p = 0.05 for mating status, F 3,20 = 3.02, p = 0.10 for male age, wing length as the covariate F = 0.77, p = 0.39). Similar tendency was not found in the body mass and body condition between the secondary mates of polyandrous females and mates of monogamous females laying simultaneously (body mass: mean ± SD 106.9±6.0 vs. 109.5±7.3 g, respectively; two-way ANCOVA, body mass F 3,20 = 1.43, p = 0.25 for mating status, F 3,20 = 0.95, p = 0.34 for male age, wing length as the covariate F = 0.65, p = 0.43).
Polyandrous female owls produced 79% more eggs, 93% more hatched young, and 73% more fledglings within-season compared with monogamous females that laid at the same time (Table 2). In 11 of the 12 cases in our study area, the total number of fledglings produced by polyandrous females within a season was substantially higher than that of monogamous females that laid simultaneously with primary and secondary nests of polyandrous females (Fig. 4).
Pooled number of fledglings produced by each of the 12 double-brooded polyandrous females (black bars) in their primary and secondary nests compared with the mean (SD) number of fledglings produced by two monogamous females (grey bars) laying simultaneously to primary and secondary nests of each polyandrous females (total number of monogamous females = 24) during 1985, 1988, 1991, 1992 and 2008 in the Kauhava region, western Finland
The inter-nest distance of polyandrous females correlated negatively with the laying date and the number of fledglings produced in the first breeding attempt within a season (Spearman rank correlation, r s=−0.70, n = 12, two-tailed p = 0.012 for laying date and r s=−0.59, n = 12, p = 0.049 for no. of fledglings). There was, however, no obvious relationship between the inter-nest distance and the laying date of the second breeding attempt (r s=−0.35, n = 12, two-tailed p = 0.27), the number of fledglings produced in the second breeding attempt (r s=−0.21, n = 12, p = 0.52), or the body mass of polyandrous females (r s=−0.41, n = 12, p = 0.18).
Discussion
Our most important findings were: (1) a total of 32 cases in which female Tengmalm’s owls deserted their first broods, re-mated and successfully raised a second brood with a new mate. While Eldegard and Sonerud (2009) showed that some 70% of radio-tagged female owls deserted their first broods and that male owls continued to care the offspring alone, they found only one case in which the deserting females re-mated and bred again. (2) Most polyandrous females were recorded in the increase phase of the 3-year vole cycle when the densities of voles are intermediate in the early spring but rapidly increase in the course of the summer. The frequency of polyandry was also higher in years of high vole density than in years of low vole density. (3) Polyandrous females had nearly twice the within-season egg and offspring production as monogamous females that nested at the same time.
By using nationwide long-term ringing and re-trapping data from Finland, we overcame the methodological challenge of relocating deserting females that appeared to be unsuccessful in an earlier radio-tracking study (Eldegard and Sonerud 2009). But, because the mean inter-nest distance of polyandrous females was 23 km (median 4.5 km), the frequency of double-brooding (1.1%) documented here was probably an underestimate owing to our restricted study area and lower ringing effort of females outside our main study area. In a total of 14 polyandry cases recorded in Finland, Norway and Germany up to 1986, the mean inter-nest distance was 5 km (review by Korpimäki 1989). Another confounding factor may be the large variation in dispersal distances: in four of the 32 polyandry cases reported here, inter-nest distances of double-brooded females were up to 95–196 km. An inter-nest distance of 194 km for a double-brooded female Tengmalm’s owl has also been documented in Switzerland (Ravussin et al. 1993). However, the majority (87%) of polyandrous females in our pooled data re-mated and produced a second brood within <35 km of their first nest, which suggests that our data are not heavily biased towards shorter inter-nest distances.
There appeared to be a cost for females in searching for mates over long distances, because there was a negative relationship between inter-nest distance of polyandrous females and the number of fledglings they produced in their first breeding attempt within a season. The courtship, egg-laying, incubation and nestling periods of Tengmalm’s owls take 10–12 weeks, and thereafter fledglings are fed by parents for 3–7 weeks (März 1968; Eldegard and Sonerud 2009). If a female initiates her first brood as early as mid-March, she will be free from brooding duties in late May, allowing her to quickly find a new mate for a second attempt. In light of the need to re-mate quickly, the longest inter-nest distances recorded in this study appeared to be unexpectedly long, because females likely move only during the 4 to 6 h of darkness that occurs in mid-summer at northern latitudes. In addition, some 20–25% of male Tengmalm’s owls remain bachelors even if they possess a territory and suitable nest-box (Carlsson 1991; Korpimäki 1991), and even when these bachelors are able to feed their mates and successfully raise a brood if experimentally provided with a partner (Hakkarainen and Korpimäki 1998). We surmise that brood-deserting females are choosy in their search for a new mate and may move over long distances because they attempt to reduce the risk of becoming a secondary female of polygynous males. This interpretation is also supported by the result that early laying polyandrous females moved over longer distances between successive breeding attempts than later laying polyandrous females. Therefore, those polyandrous females that were able to initiate their first nesting attempt early had probably more time to search for a second mate and thus to be more choosy in mate choice. Secondary females have a substantially reduced reproductive success in terms of both quantity and quality of offspring (Korpimäki 1991).
The frequency of polyandry was highest in the increase phase of the 3-year vole cycle (Fig. 1) and also in years of high spring vole density than in years of low vole density (Fig. 3). This latter result was also consistent with earlier findings that the frequency of brood desertion of Tengmalm’s owls was positively correlated with the abundance of voles in spring and that this frequency can be increased with supplementary feeding (Eldegard and Sonerud 2009). We suggest that it is not only the abundance of main foods in early spring that is crucial for the adaptive advantages of brood desertion, but also that the increase in vole abundance toward autumn in the increase phase of the vole cycle is essential for the success of polyandry. Also, late owl nests have high fledgling production in the increase phase of the vole cycle (Korpimäki and Hakkarainen 1991), and the first-year survival rate of independent offspring during improving food conditions is at least twice as high as in the other phases of the vole cycle (Korpimäki and Lagerström 1988). Therefore, larger fitness benefits of brood desertion by females can be obtained during improving food conditions because double-brooding is more successful. Temporal changes in food supply that allow females to breed again have been predicted to increase polyandry (Graul 1977). For example, the successive polyandry of black-shouldered kites Elanus caeruleus seems to occur in good food conditions (Mendelsohn 1983). Moreover, one of the snail kite Rostrhamus sociabilis parents appeared to desert their first broods during favourable food conditions (Beissinger and Snyder 1987). Also the skewed operational sex ratio in favour of males may contribute to re-mating opportunities of brood deserting female owls (Emlen and Oring 1977; Pilastro et al. 2001). However, in our study this appears to be less important than improving food conditions, because bachelor males are known to exist in both increasing and decreasing years of vole abundance (Carlsson 1991; Korpimäki 1991; Hakkarainen and Korpimäki 1998).
Our most important novel result was that polyandrous female owls were able to produce 73% more fledglings than monogamous females. Because we compared offspring production of polyandrous females with simultaneously laying monogamous females, the well-known seasonal decline in clutch size with later laying date (see Korpimäki and Hakkarainen 1991) cannot explain our results (Table 2). Single-handed male owls, even if they increased their provisioning rates (Eldegard and Sonerud 2010), appeared not to be able to fully compensate, as their fledglings had an estimated 30% lower survival until independence than fledglings of non-deserted females (Eldegard and Sonerud 2009). Even if we conservatively suppose that this lowered survival rate also applies to our case, the larger offspring production of polyandrous than monogamous females indicates that polyandry confers substantial fitness benefits to females. Earlier it was shown that the number of fledglings produced by Tengmalm’s owls in any one breeding attempt is closely correlated with the lifetime number of recruits to the breeding population (Korpimäki 1992). This in turn has been considered as one of the most accurate estimates of fitness in wild bird populations (Newton 1985, 1989).
To our knowledge, fitness benefit for females owing to sequential polyandry have only rarely been demonstrated for altricial bird species with bi-parental care and also not in birds of prey. In lesser spotted woodpeckers (Dendrocopos minor), male contribution to parental care (incubation and food provision duties) is equal to or larger than that of females, and polyandrous females more than double their number of fledglings produced within a season compared with monogamous females (Wiktander et al. 2000). However, because Wiktander et al. (2000) compared the number of fledglings produced between all polyandrous and monogamous females, it is unclear whether the differences were partially due to an earlier start of egg-laying in the primary nests of polyandrous females. The number of fledglings produced by deserting female rock sparrows Petronia petronia was also higher than that of both single-brooded and non-deserting double brooded females (Pilastro et al. 2001). Double-brooded female barn owls Tyto alba deserting their first broods initiated their second broods 2 weeks earlier and produced significantly more eggs than non-deserting double-brooded females but their reproductive success did not obviously differ (Roulin 2002).
Our novel results show that not only males but also females of altricial species with bi-parental care can increase their fitness by deserting their first broods, provided that these broods will be cared for by the males. Earlier studies have shown that male Tengmalm’s owls can increase their annual offspring production and also their lifetime reproductive success by simultaneous polygyny (Korpimäki 1992). Polygynous males provision chicks in primary (earlier) nests preferentially over secondary (later) nests which leads to reduced fledgling production of secondary females of bigynous males (Korpimäki 1989, 1991; Carlsson 1991). Polyandrous females desert their first broods when the chicks are 3–4 weeks old, but the deserted males substantially increase their offspring feeding rate during the 6- to 7-week post-fledging period (Eldegard and Sonerud 2010). Because primary mates of polyandrous females tended to be in a better body condition than mates of monogamous females laying simultaneously, single-handed males were at least partly able to compensate for the suddenly reduced parental care by their deserting partner. High fitness costs of complete nest failures after female brood desertion probably force the single-handed males to partially compensate for the decreased care of their partners. This notion appears to be in agreement with many older models of parental care in which incomplete compensation has been suggested to be an evolutionarily stable strategy (see Houston et al. 2005; Wiebe 2010 with references). Both polyandrous female owls and polygynous male owls thus appear to leave their mates in a “cruel bind” (sensu Jones et al. 2002), which suggests that there is an inter-sexual “tug-of-war” over bi-parental care in Tengmalm’s owls.
References
Andersson M (2005) Evolution of classical polyandry: three steps to female emancipation. Ethology 111:1–23
Beissinger SR, Snyder NFR (1987) Mate desertion in the snail kite. Anim Behav 35:477–487
Bennett PM, Owens IPF (2002) Evolutionary ecology of birds life histories, mating systems and extinction, 1st edn. Oxford University Press, Oxford
Carlsson B-G (1991) Recruitment of mates and deceptive behavior by male Tengmalm’s owls. Behav Ecol Sociobiol 28:321–328
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Currie D, Valkama J, Berg Å, Boschert M, Norrdahl K, Hänninen M, Korpimäki E, Pöyri V, Hemminki O (2001) Sex roles, parental effort and offspring desertion in the monogamous Eurasian curlew Numenius arquata. Ibis 143:642–650
Eldegard K, Sonerud GA (2009) Female offspring desertion and male-only care increase with natural and experimental increase in food abundance. Proc R Soc B 276:1713–1721
Eldegard K, Sonerud GA (2010) Experimental increase in food supply influences the outcome of within-family conflicts in Tengmalm’s owl. Behav Ecol Sociobiol 64:815–826
Emlen ST, Oring LW (1977) Ecology, sexual selection and the evolution of mating systems. Science 197:215–223
Garcia-Berthou E (2001) On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J Anim Ecol 70:708–711
Glutz von Blotzheim UN, Bauer KM (1980) Handbuch der Vögel Mitteleuropas, vol 9. Akademische Verlagsgesellschaft, Wiesbaden
Graul WD (1977) The evolution of avian polyandry. Am Nat 111:812–816
Hakkarainen H, Korpimäki E (1998) Why do territorial male Tengmalm’s owls fail to obtain a mate? Oecologia 114:578–582
Hakkarainen H, Koivunen V, Korpimäki E (1997) Reproductive success and parental effort of Tengmalm’s owls: effects of spatial and temporal variation in habitat quality. Ecoscience 4:35–42
Hakkarainen H, Mykrä S, Kurki S, Korpimäki E, Nikula A, Koivunen V (2003) Habitat composition as a determinant of reproductive success of Tengmalm’s owls under fluctuating food conditions. Oikos 100:162–171
Hinde CA, Kilner RM (2007) Negotiations within the family over supply of parental care. Proc R Soc B 274:53–60
Hörnfeldt B, Carlsson B-G, Nordström LL (1988) Molt of primaries and age determination in Tengmalm’s owl (Aegolius funereus). Auk 105:783–789
Houston AI, Szekely T, McNamara JM (2005) Conflict between parents over care. Trends Ecol Evol 20:33–38
Huitu O, Laaksonen J, Norrdahl K, Korpimäki E (2005) Spatial synchrony in vole population fluctuations—a field experiment. Oikos 109:583–593
Huitu O, Laaksonen J, Klemola T, Korpimäki E (2008) Spatial dynamics of Microtus voles in continuous and fragmented agricultural landscapes. Oecologia 155:53–61
Jones KM, Ruxton GD, Monaghan P (2002) Model parents: is full compensation for reduced partner nest attendance compatible with stable biparental care? Behav Ecol 13:838–843
Kelly EJ, Kennedy PL (1993) A dynamic state variable model of mate desertion in Cooper’s hawks. Ecology 74:351–366
Korpimäki E (1981) On the ecology and biology of Tengmalm’s Owl (Aegolius funereus) in Southern Ostrobothnia and Suomenselkä western Finland. Acta Univ Ouluensis, A Sci Rerum Nat 118:1–84
Korpimäki E (1983) Polygamy in Tengmalm’s owl Aegolius funereus. Ornis Fenn 60:86–87
Korpimäki E (1987) Selection for nest-hole shift and tactics of breeding dispersal in Tengmalm’s owl Aegolius funereus. J Anim Ecol 56:185–196
Korpimäki E (1988a) Diet of breeding Tengmalm’s Owls Aegolius funereus: long-term changes and year-to-year variation under cyclic food conditions. Ornis Fenn 65:21–30
Korpimäki E (1988b) Effects of territory quality on occupancy, breeding performance and breeding dispersal in Tengmalm’s owl. J Anim Ecol 57:97–108
Korpimäki E (1989) Mating system and mate choice of Tengmalm’s Owls Aegolius funereus. Ibis 131:41–50
Korpimäki E (1991) Poor reproductive success of polygynously mated female Tengmalm’s owls: are better options available? Anim Behav 41:37–47
Korpimäki E (1992) Fluctuating food abundance determines the lifetime reproductive success of male Tengmalm’s owls. J Anim Ecol 61:103–111
Korpimäki E (1993) Helmipöllönaaraan pitkä puolisonetsintämatka. Suomenselän Linnut 28:130–131
Korpimäki E, Hakkarainen H (1991) Fluctuating food supply affects the clutch size of Tengmalm’s Owl independent of laying date. Oecologia 85:543–552
Korpimäki E, Lagerström M (1988) Survival and natal dispersal of fledglings of Tengmalm’s owl in relation to fluctuating food conditions and hatching date. J Anim Ecol 57:433–441
Korpimäki E, Wiehn J (1998) Clutch size of kestrels: seasonal decline and experimental evidence for food limitation under fluctuating food conditions. Oikos 83:259–272
Korpimäki E, Brown PR, Jacob J, Pech RP (2004) The puzzles of population cycles and outbreaks of small mammals solved? BioSci 54:1071–1079
Korpimäki E, Oksanen L, Oksanen T, Klemola T, Norrdahl K, Banks PB (2005) Vole cycles and predation in temperate and boreal zones of Europe. J Anim Ecol 74:1150–1159
Kuhk R (1969) Schlüpfen und Entwicklung der Nestjungen beim Rauhfusskauzes (Aegolius funereus). Bonn zool Beitr 20:141–150
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lagerström M, Korpimäki E (1988) Helmipöllö. In: Pietiäinen H, Ahola K, Forsman D, Haapala J, Korpimäki E, Lagerström M, Niiranen S (eds) Pöllöjen iän määrittäminen. Helsingin yliopiston eläinmuseo, Helsinki
März R (1968) Der Rauhfusskauz. Die neue Brehm-Bücherei, Wittenberg
Mendelsohn JM (1983) Social behaviour and dispersion of the black-shouldered kite. Ostrich 54:1–18
Mikkola H (1983) Owls of Europe. Poyser, Calton
Newton I (1979) Population ecology of raptors. Poyser, Berkhamsted
Newton I (1985) Lifetime reproductive output of female Sparrowhawks. J Anim Ecol 54:241–253
Newton I (ed) (1989) Lifetime reproduction in birds. Academic, London
Olson VA, Liker A, Freckleton RP, Szekely T (2008) Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proc R Soc B 275:301–307
Oring LW (1983) Avian polyandry. Curr Ornithol 3:309–351
Parker GA, Royle NJ, Hartley IR (2002) Intrafamiliar conflict and parental investment: a synthesis. Philos Trans R Soc Lond B 357:295–307
Pilastro A, Biddau L, Marin G, Mingozzi T (2001) Female brood desertion increases with number of available mates in the Rock Sparrow. J Avian Biol 32:68–72
Ravussin P-A, Trolliet D, Willenegger L, Béguin D (1993) Observations sur les fluctuations d’une population de Chouette de Tengmalm (Aegolius funereus) dans le Jura vaudois (Suisse). Nos Oiseaux 42:127–142
Roff DA (1992) The evolution of life histories; theory and analysis, 1st edn. Chapman & Hall, New York
Roulin A (2002) Offspring desertion by double-brooded female barn owls (Tyto alba). Auk 119:515–519
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Sundell J, Huitu O, Henttonen H, Kaikusalo A, Korpimäki E, Pietiäinen H, Saurola P, Hanski I (2004) Large-scale spatial dynamics of vole populations in Finland revealed by the breeding success of vole-eating avian predators. J Anim Ecol 73:167–178
Svensson L (1992) Identification guide to European passerines. Svensson, Stockholm
Szekely T, Webb JN, Houston AI, McNamara JN (1996) An evolutionary approach to offspring desertion in birds. In: Nolan V, Ketterson ED (eds) Current ornithology, vol 13. Plenum, New York
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Heinemann, Chicago
Wiebe KL (2005) Asymmetric costs favor female desertion in the facultatively polyandrous northern flicker (Colaptes auratus): a removal experiment. Behav Ecol Sociobiol 57:429–437
Wiebe KL (2010) Negotiation of parental care when the stakes are high: experimental handicapping of one partner during incubation leads to short-term generosity. J Anim Ecol 79:63–70
Wiktander U, Olsson O, Nilsson SG (2000) Parental care and social mating system in the Lesser Spotted Woodpecker Dendrocopos minor. J Avian Biol 31:447–456
Zárybnická M (2009) Parental investment of female Tengmalm’s Owls Aegolius funereus: correlation with varying food abundance and reproductive success. Acta Ornithol 44:81–88
Acknowledgements
The valuable comments by Tapio Eeva, Jeffrey S. Marks and two anonymous referees substantially improved this paper. The study was supported financially by the Emil Aaltonen Foundation, the South Ostrobothnia Foundation of the Finnish Cultural Foundation, the Otro Seppä Memorial Foundation of the Finnish Cultural Foundation, and the Academy of Finland. Harri Hakkarainen, Mikko Hast, Timo Hyrsky, Mikko Hänninen, Jorma Nurmi and Rauno Varjonen assisted in the fieldwork.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Leonard
Rights and permissions
About this article
Cite this article
Korpimäki, E., Salo, P. & Valkama, J. Sequential polyandry by brood desertion increases female fitness in a bird with obligatory bi-parental care. Behav Ecol Sociobiol 65, 1093–1102 (2011). https://doi.org/10.1007/s00265-010-1118-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1118-6