Abstract
We studied avian dispersal of seeds from the hemiparasitic mistletoe Plicosepalus acaciae (Loranthaceae) to its tree hosts Acacia raddiana and A. tortilis in the Syrian–African Rift (Arava) valley, Israel. The Yellow-vented Bulbul (Pycnonotus xanthopygos) was the sole avian visitor observed feeding on mistletoe fruits. Bulbuls consumed mistletoe fruits whenever they were available, but the fruits only constituted a significant portion of the diet (71% of foraging attempts) when they were most abundant. These birds are potentially good dispersal vectors of P. acaciae because they swallowed the fruit whole and defecated viable seeds that were covered in a viscid pulp, which allowed the seeds to adhere to substrates when voided. In addition, bulbuls spent a large proportion (66–93%) of total observation time perched in Acacia trees, allowing for directed dispersal. Ephemeral river valleys (wadis) with high mistletoe infection were adjacent to those containing no infections, demonstrating that mistletoe dispersal is common within, but not among wadis. This is consistent with the flight behaviour in bulbuls, which do not typically move among wadis. We combined data on bulbul movements between Acacia trees with transit times of mistletoe seeds to create a hypothetical seed shadow as a function of distance from the parent mistletoe plant. Because they are directed dispersers, the movement patterns of bulbuls may explain the current distribution of P. acaciae in the Arava valley.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike the diffuse co-evolution found in most relationships between fruiting plants and vertebrate dispersal agents (Wheelwright and Orians 1982), bird–mistletoe interactions can be characterised by a high degree of specificity (Reid 1991; O’Donnell and Dilks 1994; Ladley and Kelly 1996). Stem-parasitic mistletoes have been cited as an example of directed dispersal, whereby dispersal agents non-randomly deposit seeds in sites that are suitable for establishment and growth (Howe and Smallwood 1982). Many mistletoe species rely on only one or a few species of frugivorous birds to deposit their seeds onto appropriate hosts (Reid 1989; López de Buen and Ornelas 1999). Under this scenario, a single dispersal agent can have a disproportionate effect on plant recruitment (Wenny 2001).
In the Syrian–African Rift (Arava) valley of Israel, the hemiparasitic mistletoe Plicosepalus acaciae (Loranthaceae) infects Acacia raddiana and A. tortilis trees. These species are native to the Middle East and Africa. In Israel, they co-occur in the main Arava valley and the adjoining ephemeral river valleys (called “wadis” in Arabic) (Halevy and Orshan 1972). As in many other mistletoe species, the fruits of P. acaciae are eaten by birds and later defecated. While the dispersal ecology of P. acaciae has not been studied in any part of its range, previous studies suggest that the Yellow-vented Bulbul (referred to by some sources as the White-spectacled Bulbul, Pycnonotus xanthopygos) is a frequent consumer of P. acaciae seeds in this region. This bird species is widely distributed in the Middle East (Paz 1987). In our study area, bulbuls were historically restricted to oases. However, an increase in agricultural settlements since the 1950s has provided conditions that have allowed the distribution of bulbuls to expand (Shirihai 1996).
Given the apparent low number of dispersers and the discrete distribution of host trees within wadis, this system is ideal for studying avian frugivory. The objective of this study was to identify the bird species that disperse P. acaciae and to evaluate whether the behaviour and physiology of the birds influence the distribution patterns of mistletoes. We first conducted observational studies, following bird movements over extended periods (up to 90 min per observation) to determine the diet composition and perch preferences of avian visitors to P. acaciae. These field surveys confirmed that the Yellow-vented Bulbul is the main dispersal vector of P. acaciae. We then used aviary studies to determine the retention time of P. acaciae seeds in the gut of bulbuls and ran germination trials to assess the viability of seeds that had been defecated. Finally, we developed a seed shadow estimate for P. acaciae by combining information on the transit time of seeds in the gut of bulbuls and data on the movement of these birds collected in field observations. These data were then used to evaluate the overall importance of dispersal afforded by the bulbul and to offer a possible explanation for the currently observed distribution of P. acaciae in the Arava valley.
Methods
Study area and species
The Arava valley of Israel is a northern extension of the Syrian African Rift valley, located between the Dead Sea in the north and the Red Sea in the south. In this region, Acacia raddiana and A. tortilis trees are primarily restricted to wadis, where they are the dominant species in these ephemeral river valleys (Halevy and Orshan 1972). The mistletoe Plicosepalus acaciae parasitises at least five halophytic and 14 non-halophytic plant species in the Arava (Todt et al. 2000), but with the exception of A. raddiana, A. tortilis and Ziziphus spina-christi, most are incidental infections.
Field data were collected in seven different wadis in the Arava valley (Tamar/Zin: 31°1′12″N, 35°22′48″E; Saif: 35°0′9″N, 30°50′2″E; Shizaf: 35°10′4″N, 30°40′4″E; Nemiya: 35°10′1″N, 30°30′9.5″E; Katzra: 35°0′9″N, 30°30′2.5″E; Barak: 35°0′7.5″N, 30°20′5″E; Hai Bar: 35°0′3″N, 29°50′3″E) (Fig. 1). These sites lie between 50 and 400 m a.s.l. and receive between 25 and 40 mm of rainfall per annum. Previous surveys in the region have found that in five of these seven wadis, the percentage of Acacia trees infected by P. acaciae varied from 0 to 85% (T. Rödl, unpublished data; Fig. 1).
Map of the Negev desert and Arava valley in southern Israel showing the study sites located in wadis. The shaded lines represent political boundaries separating Israel from Egypt in the east and Jordan in the west, with Elat at the bottom of the map lying on the Red Sea. Elevations on the map are in feet. Numbers in parenthesis are percentages of Acacia raddiana and A. tortilis trees infected by the parasitic mistletoe Plicosepalus acaciae
Fruiting of P. acaciae occurs from June to April (Vaknin et al. 1996). The berry-like drupe fruits are 5–15 mm in diameter, with seeds that are approximately 3–5 mm in diameter. These fruits can easily be eaten by the Yellow-vented Bulbul, a medium-sized omnivorous passerine (mean adult body length 20 cm; adult mass 31–43 g).
Field observations
Over 100 h of point observations in the seven wadis were used to determine the potential dispersal vectors of P. acaciae seeds. Based on the fact that bulbuls were the only avian visitors observed on focal P. acaciae individual plants, we concentrated subsequent field observations on bulbuls. We followed focal bulbul individuals or pairs of bulbuls using 10 × 42 binoculars and recorded observations using a Psion data logger. Birds were observed for as long as possible (range 3 to 90 min). The duration of behaviours associated with food searching or food processing (flying between trees, perching in trees, hopping or flying within a tree, foraging attempts and defecation) were recorded, and we noted when birds were out of view. Data were collected from November 1998 to January 2000. Although no systematic study of P. acaciae fruiting phenology was carried out, fruits were most prevalent in October and November.
In a second field study focusing on the potential seed shadow of P. acaciae dispersed by bulbuls, all trees in three of the field sites were numbered and mapped on an aerial photograph (247 trees in Katzra, 182 trees in Nemiya and 110 trees in Barak). Every tree was also checked for mistletoe infection (84.6% of Acacia trees in Katzra, 36.8% in Nemiya and 61.0% in Barak). Using transparent graph paper, we converted tree locations to (x, y) coordinates. In the three wadis, observations were made on movements by bulbuls that consumed P. acaciae fruits. Each observation was carried out in a separate part of the wadi and, as the bulbuls are territorial, this limits the likelihood that the same birds were counted multiple times. Observations began when a bulbul consumed a fruit from what we defined as the hypothetical parent plant. Only observation sessions >15 min were used in the data analysis, giving a total of ten birds (two birds each in Katzra and Barak, six birds in Nemiya) followed over 6 h of observation time. Distance travelled was determined later using the coordinates of the mapped trees.
Transit time
Ten bulbuls were captured using mist nets near Hazeva in the Arava valley, then transported to the Mitrani Department for Desert Ecology in Sede Boqer. Birds were individually housed in 63 × 44 × 56-cm cages under controlled conditions [12/12-h (light/dark) photoperiod (12L/12D); 25°C] and maintained on synthetic banana mash (Denslow et al. 1987). To assess possible variations in transit time associated with diet, transit time trials included three feeding treatments: P. acaciae fruits alone, with mealworms or with banana mash. All birds received trials with P. acaciae fruits alone, nine of the birds had trials involving mealworms and four of the birds were subjected to trials that included banana mash. For the trials that included banana mash, we fed birds both banana mash and mistletoe fruits ad libitum. In the mealworm trials, five mealworms were placed in the dish along with the mistletoe fruits. Water was provided ad libitum throughout all trials. Birds were observed constantly from the time when the fruits were offered until all ingested seeds were defecated (approximately 2 h after starting). Time of fruit ingestion and time of defecation of P. acaciae seeds were recorded to the nearest second. We assumed that seeds were defecated in approximately the same order in which they were ingested because when the birds ate more than one fruit at a time, a corresponding number of seeds was defecated together.
Germination of defecated seeds
We removed 66 defecated P. acaciae seeds from the cage floor immediately after the transit time trials and placed them in petri dishes. The dishes were placed indoors under a constant photoperiod (12L/12D) and at a temperature ranging from 15 to 22°C. While previous studies have demonstrated that petri dishes are a poor means for estimating germination of mistletoe seeds from intact fruits (Robertson et al. 2006), these conditions were successfully used in a similar study on germination of P. acaciae seeds removed by hand (Rödl and Ward 2002). As the seeds used in our study were all cleaned by passage through a bird and a reasonable germination percentage was achieved, we considered that the petri dishes most likely did not have a negative effect on germination. Seeds were monitored for 6 weeks, and successful germination was indicated by growth of the green radix.
Seed shadow
Field observations of bulbul movement (n = 10 individuals) were grouped into categories of time spent at different distances from the hypothetical parent plant. Observations for the three wadis were pooled to incorporate natural variation associated with different wadi widths and infection percentages. To develop a probability distribution for seed deposition, we organised movement data and transit time data into successive 5-min intervals. The distributions of movements during 5-min intervals were multiplied by the corresponding probability of defecating a mistletoe seed determined by the transit time distribution (Murray 1988). The results were summed over all time intervals to yield a probability distribution of seed deposition versus distance from the parent plant.
Results
Field observations
In more than 100 h of observation at Tamar/Zin, Saif, Shizaf, Nemiya, Katzra, Barak and Hai Bar, we never saw any birds other than bulbuls ingesting whole P. acaciae fruits and defecating intact seeds. Bulbuls appear to be opportunistic omnivores, as they had a varied diet that included P. acaciae nectar and fruits, Acacia flowers, insects and other fruits and plant parts. Bulbuls consumed P. acaciae fruits whenever they were available, but the fruits only constituted a major portion of the diet (71% of foraging attempts, n = 132 birds observed) during peak fruit availability in October and November.
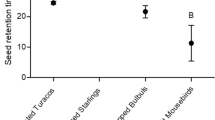
In observations of 103 individuals, bulbuls perched in Acacia trees for 76% of the total observation time in the summer when mistletoe nectar is abundant, 93% in autumn when mistletoe fruits are most abundant and 66% in winter when neither fruits nor nectar are abundant (Fig. 2). Seasonal differences in perch use in three wadis over ten separate days were statistically significant (one-way ANOVA with arcsine-square root transformation of the proportion of observation time: F 2,9 = 19.23, P = 0.001). These data indicate that P. acaciae seeds have a high probability of being defecated in a host Acacia tree during the times when the fruit is most abundant and that P. acaciae fruit represents the main component of the bulbul diet.
Amount of time spent by 103 Yellow-vented Bulbuls (Pycnonotus xanthopygos) perching on A. raddiana and A. tortilis, sitting on the ground, perching in various shrub species or in transit between perching sites. Data are based on number of seconds bulbuls spent on each of the behaviours during 2-day periods at each of three study sites during three seasons (16 h of observation in total)
Seeds were often defecated while birds were perched in Acacia trees or P. acaciae plants (36 of 40). Although bulbuls defecated seeds surrounded by a sticky viscin layer, the birds were not observed to wipe the seeds from their cloacas onto a branch. Rather, they waited until the seed fell from the cloaca, occasionally bobbing the rump repeatedly to shake off the seeds. Observations of defecated seeds in the field indicate that a high percentage germinated within several days of being defecated. Not all seeds defecated when bulbuls were perched in Acacia trees necessarily landed on a safe site. Of 36 defecated seeds for which we knew the location of deposit, 58% landed on a small Acacia branch, 17% landed on the ground and 25% landed on a mistletoe plant. Only the Acacia branches can be considered reliable hosts for this species of mistletoe, although hyperparasitism has been recorded in a number of Loranthaceae.
Only movements between Acacia trees (considered to be safe sites for the dispersed seeds) were included in the movement distribution. Movement patterns of bulbuls were generally limited to within a small area, with a maximum distance of 267 m from the location at the start of the observation. Movements on a larger scale probably occur but were not observed and are presumed to be rare.
Transit time
Of 148 P. acaciae fruits ingested by bulbuls in the transit time trials, 97.3% were defecated and 2.7% were regurgitated. All regurgitated seeds were brought up within a few seconds of ingestion, and these retention times were included in subsequent calculations of transit times. The overall mean transit time (± 1 SD) of P. acaciae seeds in the bulbul was 19.3 ± 4.1 min (Fig. 3). There were no significant differences among transit times when birds were fed diets of mistletoe seeds alone, mistletoe seeds with banana mash and mistletoe seeds with meal worms (one-way ANOVA: F 2,20 = 0.64, P = 0.538). Further analysis using only those birds that were fed all three diet types also resulted in no significant difference (repeated measures ANOVA: F 2,6 = 0.02, P = 0.980). The transit time when bulbuls consumed only one seed (20.0 ± 12.8 min; n = 8 bulbuls) was not significantly different from that for bulbuls that consumed more than one seed at a time (19.1 ± 3.3 min; n = 9 bulbuls) (one-way ANOVA: F 1,15 = 0.04, P = 0.830).
Germination of defecated seeds
Of the seeds defecated by bulbuls during the transit time studies (n = 66 seeds from ten birds), 51.5% germinated. A study by Rödl and Ward (2002) found that hand-cleaned fruits of P. acaciae only had a 35% germination rate in petri dishes. We therefore conclude that passage through the bulbul gut increases the germination rate in P. acaciae.
Seed shadow
The transit time data combined with movement patterns in Katzra, Nemiya and Barak indicate that 73.3% of P. acaciae seeds will be deposited within 100 m of a parent plant (Fig. 4). At the same time, the probability that a P. acaciae seed is defecated while a bulbul perches on a host Acacia tree infected by the parent mistletoe plant is low (3.7%). Although there is a high likelihood that a P. acaciae seed is dispersed away from the parent plant, bulbuls perch in infected trees a substantial amount of the time (45–96% of observed perches).
Discussion
The results of our study implicates the Yellow-vented Bulbul as the primary dispersal vector of P. acaciae in the Arava valley of Israel. Bulbuls appear to provide directed dispersal (Howe and Smallwood 1982) of P. acaciae, based on the fact that they swallow fruits whole, defecate the seeds in germinable condition and non-randomly deposit the seeds onto appropriate Acacia tree hosts. Consequently, bulbuls are likely to have a disproportionate effect on P. acaciae recruitment in Israel. This could, in turn, have an impact on native tree populations. While Acacia species infected with P. acaciae do not show negative effects (Bowie and Ward 2004), Z. spina-christi suffers high mortality when infected by these mistletoes (Ward et al. 2006).
Our study also shows that bulbuls disperse P. acaciae seeds away from parent plants. However, these birds frequently forage and perch in Acacia trees infected with P. acaciae, making it likely that these hosts will receive more seeds. This finding is consistent with other studies demonstrating that re-infection of host trees by mistletoes is common. Bird dispersers are often attracted to fruiting mistletoe plants in trees that are already infected, and they preferentially visit the tallest trees in an area (Martínez del Rio et al. 1996; Aukema and Martínez del Rio 2002). In addition, it has been demonstrated that some host trees are intrinsically more susceptible to infection (Reid and Stafford-Smith 2000), although that finding has been contradicted by other studies (Overton 1994). In the case of this study system, foraging bulbuls may be attracted to Acacia trees for reasons other than mistletoe plants. Bulbuls also consume Acacia flowers and insects found in Acacia canopies, and this mixed diet could facilitate the infection of uninfected Acacia host trees.
We never observed bulbuls to leave the wadi and fly over the open, unvegetated desert that characterises the areas between wadis, an observation supported by Paz (1987). While wadis are only 1–2 km apart in most places and bulbuls do occasionally fly from one wadi to another via the main Arava valley, we suggest that the majority of movements by bulbuls are within the same wadi, with movements between wadis occurring only rarely. Because P. acaciae dispersal is closely linked to the flight behaviour of bulbuls, this could lead to local adaptation of P. acaciae populations restricted to individual wadis, as has been demonstrated in A. raddiana populations that are dispersed by large mammals and winter floods within but not between wadis (Shrestha et al. 2002).
Even when the dispersal of P. acaciae between wadis does occur, it is possible that P. acaciae seedlings will not survive on Acacias in these new sites. Rödl and Ward (2002) cross-infected A. raddiana trees in two different wadis with seeds from the same P. acaciae plant and found the foreign seeds to have significantly a lower germination and holdfast formation than local seeds. This strong genotype-by-environment interaction for P. acaciae suggests strong selection against dispersal between wadis. These results combined with the propensity of bulbuls to move primarily within wadis, perch on Acacia trees and defecate P. acaciae seeds within a short time of ingestion indicate that the dispersal of P. acaciae to a new wadi and subsequent germination are likely to be limited. Such a mechanism may explain how wadis with high P. acaciae infection can exist directly adjacent to those with no infection.
Foraging observations indicate that P. acaciae accounts for a substantial percentage of the bulbul diet. In fact, mistletoes are considered to be keystone species in many systems because their flowers and fruits are such an important food source for birds (Watson 2001). Beyond providing food, large mistletoe plants may also protect bulbuls and their nests from environmental factors and predation. If a dispersal vector of a plant species relies on that same plant species for food and microhabitat, then the mutually beneficial association can result in a positive feedback loop. Martínez del Rio et al. (1996) found that increased abundance of the Chilean mockingbird (Mimus thenca) increased seed transmission of a mistletoe (Tristerix aphyllus) among columnar cacti hosts (Eulychnia acida and Echinopsis skottsbergii). A similar positive feedback loop is possible between the bulbul and P. acaciae in Israel. In this case, an increasing population of bulbuls resulting from the expansion of agriculture in the Arava valley (Shirihai 1996) may lead to increases in the abundance and range of P. acaciae. This, in turn, could allow bulbul populations to be established in the desert, independent of oases and agricultural settlements.
Regardless of whether such a positive feedback loop exists, there is a high degree of specificity between P. acaciae and the Yellow-vented Bulbul in the Arava valley of Israel. The fruits of P. acaciae are an important food source for the bulbul in this region and, in turn, the bulbuls disperse viable seeds to host Acacia trees. This suggests that the system is one of directed dispersal. The dispersal behaviour of bulbuls is consistent with the observed variability in P. acaciae infection percentage among wadis, but further research is necessary to establish the details of this system. The simplicity of the bulbul–mistletoe–Acacia interaction makes this system ideal for future study.
Zusammenfassung
Gerichtete Verbreitung von Misteln Plicosepalus acaciae durch Gelbsteißbülbüls Pycnonotus xanthopygos
Wir haben die Vogelverbreitung von Samen der halbparasitischen Mistel Plicosepalus acaciae (Loranthaceae) auf ihre Wirtsbäume Acacia raddiana und A. tortilis im Syrisch-Afrikanischen Rift-Valley (Arava-Tal), Israel untersucht. Der Gelbsteißbülbül Pycnonotus xanthopygos war die einzige Vogelart, die beim Fressen von Mistelbeeren beobachtet wurde. Bülbüls fraßen Mistelbeeren, wann immer sie verfügbar waren, aber die Beeren machten nur dann einen maßgeblichen Anteil an der Nahrung aus (71% der Futtersuch-Ereignisse), wenn sie am häufigsten waren. Die Vögel sind potentiell gute Verbreitungsvektoren von P. acaciae, weil sie die Früchte komplett schlucken und die keimfähigen Samen mit einer zähflüssigen Masse umgeben ausscheiden, die es den Samen ermöglicht, am Substrat haften zu bleiben, wenn sie abgegeben werden. Außerdem hielten sich Bülbüls eine großen Teil (66–93%) der gesamten Beobachtungszeit in den Akazien auf, was eine direkte Verbreitung gestattete. Täler temporärer Flüsse (Wadis) mit einer hohen Mistelinfektionsrate lagen benachbart zu solchen ohne Infektionen, was zeigt, dass die Mistelverbreitung innerhalb der Wadis häufig ist, aber nicht zwischen den Wadis. Das deckt sich mit dem Flugverhalten der Bülbüls, die normalerweise nicht zwischen den Wadis umherwechseln. Um eine hypothetische Samenverbreitungsfläche als eine Funktion der Distanz zur Mistel-Mutterpflanze zu erzeugen, verschnitten wir Daten der Bülbül-Bewegungen zwischen Akazienbäumen mit der Verweildauer der Mistelsamen im Magen-Darm-Trakt. Weil die Bülbüls direkte Samenverbreiter sind, könnten ihre Bewegungsmuster die gegenwärtige Verteilung von P. acaciae im Arava-Tal erklären.
References
Aukema JE, Martínez del Rio C (2002) Where does a fruit-eating bird deposit mistletoe seeds? Seed deposition patterns and an experiment. Ecology 83:3489–3496
Bowie M, Ward D (2004) Water and nutrient status of the mistletoe Plicosepalus acaciae parasitic on isolated Negev Desert populations of Acacia raddiana differing in level of mortality. J Arid Environ 56:487–508. doi:10.1016/S0140-1963(03)00067-3
Denslow JS, Levey DJ, Moermond TC, Wentworth BC (1987) A synthetic diet for fruit-eating birds. Wilson Bull 99:131–134
Halevy G, Orshan G (1972) Ecological studies on Acacia species in the Negev and Sinai: I. Distribution of Acacia raddiana, A. tortilis and A. gerrardii ssp. negevensis as related to environmental factors. Isr J Bot 21:197–208
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228. doi:10.1146/annurev.es.13.110182.001221
Ladley JJ, Kelly D (1996) Dispersal, germination and survival of New Zealand mistletoes (Loranthaceae): dependence on birds. NZ J Ecol 20:69–79
López de Buen L, Ornelas JF (1999) Frugivorous birds, host selection, and the mistletoe Psittacanthus schiedeanus, in central Veracruz, Mexico. J Trop Ecol 15:329–340. doi:10.1017/S0266467499000851
Martínez del Rio C, Silva A, Medel R, Hourdequin M (1996) Seed dispersers as disease vectors: bird transmission of mistletoe seeds to plant hosts. Ecology 77:912–921. doi:10.2307/2265511
Murray GK (1988) Avian seed dispersal of three neotropical gap-dependent plants. Ecol Monogr 58:271–298. doi:10.2307/1942541
O’Donnell CFJ, Dilks PJ (1994) Foods and foraging of forest birds in temperate rainforest, South Westland, New Zealand. NZ J Ecol 18:87–107
Overton JM (1994) Dispersal and infection in mistletoe metapopulations. J Ecol 82:711–723. doi:10.2307/2261437
Paz U (1987) The birds of Israel. Ministry of Defense, Tel-Aviv
Reid N (1989) Dispersal of mistletoes by honeyeaters and flowerpeckers: components of seed dispersal quality. Ecology 70:137–145. doi:10.2307/1938420
Reid N (1991) Coevolution of mistletoes and frugivorous birds. Aust J Ecol 16:457–469. doi:10.1111/j.1442-9993.1991.tb01075.x
Reid N, Stafford-Smith M (2000) Population dynamics of an arid zone mistletoe (Amyema preissii, Loranthaceae) and its host Acacia victoriae (Mimosaceae). Aust J Bot 48:45–58. doi:10.1071/BT97076
Robertson AW, Trass A, Ladley JJ, Kelly D (2006) Assessing the benefits of frugivory for seed germination: the importance of the deinhibition effect. Funct Ecol 20:58–66. doi:10.1111/j.1365-2435.2005.01057.x
Rödl T, Ward D (2002) Host recognition in a desert mistletoe: early stages of development are influenced by substrate and host origin. Funct Ecol 16:128–134. doi:10.1046/j.0269-8463.2001.00592.x
Shirihai H (1996) The birds of Israel. Academic, London
Shrestha MK, Golan-Goldhirsh A, Ward D (2002) Population genetic structure and the conservation of isolated populations of Acacia raddiana in the Negev desert. Biol Conserv 108:119–127. doi:10.1016/S0006-3207(02)00100-3
Todt H, Breckle S-W, Veste M (2000) The mistletoe Loranthus acaciae (Loranthaceae) on halophytic and non-halophytic hosts in the southern Arava-Valley (Israel). In: Breckle S-W (ed) I Schimper symposium—Ergebnisse weltweiter Forschung. Verlag Gunter Heimbach, Stuttgart, pp 475–480
Vaknin Y, Yom Tov Y, Eisikowitch D (1996) Flowering seasonality and flower characteristics of Loranthus acaciae Zucc. (Loranthaceae): implications for advertisement and bird-pollination. Sex Plant Reprod 9:279–285. doi:10.1007/BF02152703
Ward D, Shrestha MK, Musli I (2006) Are invasive mistletoes killing Ziziphus spina-christi? Isr J Plant Sci 54:113–117. doi:10.1560/IJPS_54_2_113
Watson DM (2001) Mistletoe—a keystone resource in forests and woodlands worldwide. Annu Rev Ecol Syst 32:219–249. doi:10.1146/annurev.ecolsys.32.081501.114024
Wenny DG (2001) Advantages of seed dispersal: a re-evaluation of directed dispersal. Evol Ecol Res 3:51–74
Wheelwright NT, Orians GH (1982) Seed dispersal by animals: contrasts with pollen dispersal, problems of terminology, and constraints on coevolution. Am Nat 119:402–413. doi:10.1086/283918
Acknowledgements
We thank Thomas Rödl and Iris Musli for assistance with many aspects of this research and Berry Pinshow for the use of his laboratory. Financial assistance came from Keren Keyemet L’Israel and DISUM grant 00046A from the German Ministry of Environmental Affairs and the Israeli Ministry of Science to D. Ward, Sigma Xi and NSF pre-doctoral fellowships to A.K. Green, and a Claude Leon Foundation postdoctoral fellowship to M.E. Griffiths.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Friedl.
Rights and permissions
About this article
Cite this article
Green, A.K., Ward, D. & Griffiths, M.E. Directed dispersal of mistletoe (Plicosepalus acaciae) by Yellow-vented Bulbuls (Pycnonotus xanthopygos). J Ornithol 150, 167–173 (2009). https://doi.org/10.1007/s10336-008-0331-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-008-0331-9