Abstract
Cormorants and shags are foot propelled pursuit divers that forage on benthic and pelagic fish. Previous studies suggested that diving in cormorants is extremely costly, and this is usually attributed to their partially wettable plumage and inefficient mode of propulsion. We investigated the energetic requirements of three Phalacrocorax species during diving. Our results indicate that, when the differences in experimental conditions and calculation methods are accounted for, energy expenditure during shallow horizontal diving in these species is similar and not considerably different from other avian divers. In the absence of direct measurements, thermodynamic modelling has been used to assess the impact of dive depth on the energetic costs of diving. Based on this, the energetic costs of Great Cormorants (Phalacrocorax carbo) diving during the winter in Greenland were estimated to be as high as 64 W kg−1 (∼21 × RMR). A recent study measured the effect of depth on the diving energetics of Double-crested Cormorants (P. auritus) to be much less drastic than suggested by this model. Extrapolating from the latter study to conditions encountered by birds wintering in Greenland shows that the thermodynamic model might greatly overestimate the energetic demand of Great Cormorants during diving. Using an improved model, the daily food intake of Great Cormorants wintering in Greenland was calculated to be ∼1,170 g. Taking into account the recorded time that birds spend diving every day, such food intake would require the highest prey-capture rate suggested so far for an avian pursuit diver (∼41 g min−1 underwater). However, little is known about the prey-capture capabilities of avian divers. We used an underwater video array to study the effect of prey density (live juvenile rainbow trout, Oncorhynchus mykiss) on the prey-capture performance of Double-crested Cormorants. We found that cormorant capture success greatly depended on prey density. Prey capture rate was highest at the greatest fish densities and easily surmounted 41 g min−1 underwater. This would suggest that cormorants in Greenland might be able to achieve these high capture rates, if prey densities are sufficiently high. However, what prey items are encountered by cormorants during the winter in Greenland and at what densities remains to be investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cormorants and shags (Phalacrocoracidae) are foot-propelled pursuit divers that forage on both benthic and pelagic prey, predominantly fish. Cormorants are believed to have originated in the tropics (van Tets 1976) from which they spread into all climatic zones, including the polar regions. Today, the Phalacrocoracidae are represented on every continent (Johnsgard 1993). A unique morphological feature, present in at least some Phalacrocorax species, is the reduced water repellency of their plumage. While early studies suggested that cormorant feathers are completely wettable (Rijke 1968), Grémillet et al. (2005a) showed that the feathers of Great Cormorants (Phalacrocorax carbo) are only partially wettable. Their feathers possess a highly waterproof central part and a wettable distal part. This is usually interpreted as an adaptation for diving in shallow water. The amount of air trapped within the plumage of cormorants is lower than in other aquatic birds (Wilson et al. 1992; Grémillet et al. 2005a), which reduces their buoyancy. Consequently, work against buoyancy and, hence, locomotor costs during diving will be reduced when compared with highly buoyant divers (e.g. diving ducks; Lovvorn and Jones 1991). After a dive bout, birds leave the water and typically take up a wing-spreading posture on land, for which multiple functions have been suggested (e.g. feather drying, social display, warming of stomach content; see Grémillet 1995).
However, a wettable or partially wettable plumage reduces the insulation of these birds in water substantially. Unlike other divers, cormorants have very little sub-cutaneous fat (Gremillet et al. 2005b), so they rely almost entirely on the air layer trapped within their plumage for insulation during diving. Insulation will be further reduced when diving to depth, where hydrostatic pressure compresses the air layer. Consequently, birds might lose substantial amounts of heat when diving in cold water through conduction and convection. Given the potentially high thermoregulatory costs, it was not surprising that Schmid et al. (1995) reported the energy expenditure of Great Cormorants (P. carbo sinensis) during shallow horizontal diving to be the highest of any diving bird (see Table 1 in Enstipp et al. 2005).
But if dive costs are so high in cormorants, particularly in thermally challenging locations, how do they balance their energetic demands? In Greenland, a small population of Great Cormorants (P. carbo carbo) lives year-round along the west coast, above the arctic circle. Birds wintering along this coast might experience air temperatures well below freezing (down to −30°C) and water temperatures as low as −1°C. Combining respirometry measurements from Great Cormorants diving in a shallow trench with a physical model of heat loss, Grémillet et al. (2001) estimated the dive costs for wintering cormorants in Greenland to be as high as 64 W kg−1 (diving to 10 m depth in water with a temperature of −1°C). Using the metabolic rate (3.1 W kg−1) reported for Great Cormorants when resting in air (RMR) by Schmid et al. (1995), this would be equal to 21 × RMR. By comparison, dive costs for most avian divers are in the range of 2–5 × RMR (or BMR, basal metabolic rate), albeit at shallow depth and in warmer water (see Table 1 in Enstipp et al. 2005).
Based on these estimates, Grémillet et al. (2001) suggested that in order to balance their energy budget, cormorants might try to minimise the time they spend in water, especially during winter, and that they therefore might depend on foraging areas with high fish densities. However, other Phalacrocorax species, such as the European Shag (P. aristotelis) spend up to 7 h per day diving in cold water off Scotland (Daunt et al. 2006). For the Great Cormorants wintering in Greenland, it was recently shown that they continue to dive throughout the winter for up to several hours per day (mean daily dive duration between December and February: 73 ± 19 min; Grémillet et al. 2005b). In addition, these birds increase their foraging depth throughout the winter (Grémillet et al. 2005b), presumably further increasing heat loss and, hence, thermoregulatory costs.
How then do these avian divers with little insulation balance their energy demands in thermally challenging locations, such as Greenland? In this paper we would like to explore the following two scenarios: (1) since the existing estimates of dive costs for Greenlandic conditions are based on a physical model, could it be that they overestimate the actual energy demand of these birds?, and (2) could birds fuel their energetically costly way of life simply by increasing their daily food intake? What prey capture rates might be achievable and, most importantly, what prey densities might be required to sustain them?
The purpose of this study was to: (1) investigate the dive costs of cormorants, with particular emphasis on diving in cold water and to depth, and (2) investigate the prey-capture capabilities of cormorants foraging on live prey at different densities.
Methods
Diving energetics
Energy expenditure during diving in three Phalacrocorax species (P. carbo, P. aristotelis, and P. auritus) was compiled from the literature and corrected for the effect of water temperature. This was done by adjusting all energy expenditure values to a water temperature of 12.6°C (the mean water temperature reported in Schmid et al. 1995) using regression equations provided by the respective study. In the case of P. carbo carbo (Grémillet et al. 2003), a regression equation relating energy expenditure during shallow diving with water temperature was recalculated from the raw data. Experimental set-ups during all shallow dive studies were comparable, with post-absorptive birds conducting horizontal dives in a surface-covered trench/tank of about 1 m depth. Energy expenditure during diving in all studies except for Schmid et al. (1995) was calculated over the period of an entire dive bout (i.e. including several dive cycles of submergence and recovery at the surface). Schmid et al. (1995) estimated dive costs for individual dives assuming the energy expenditure of birds at the surface between dives to be equal to the resting rate in water. Consequently, they added the costs measured at the surface that were in excess of resting, to the costs of the preceeding dive.
The effect of depth on energy expenditure during diving for P. carbo was modelled by Grémillet et al. 2001 (see Fig. 2 therein). These authors combined respirometry measurements with a model established by Grémillet and Wilson (1999), which is based on the theoretical relationship between dive depth and heat flux. Energetic costs during dives to 10 m depth were measured for P. auritus by Enstipp et al. (2006a) for a water temperature range between 6.1 and 15.4°C. The resulting relationship (linear regression) between energy expenditure and water temperature was used to estimate dive costs for wintering conditions in Greenland (i.e. at a water temperature of −1°C).
Prey-capture behaviour
Prey-capture rates for various avian pursuit divers were compiled from the literature. All studies included in our investigation assessed prey-capture rates indirectly by combining bird activity recordings (i.e. from radio tags or data loggers) with food intake estimates. The latter were based on nest balance recordings, time-energy budgets, or stomach temperature recordings.
We used an underwater video array positioned inside a deep dive tank (10 m depth, 5 m diameter) to study the effect of prey density on the prey-capture performance of Double-crested Cormorants (for details, see Enstipp et al. 2007). In brief, every morning live juvenile rainbow trout (Oncorhynchus mykiss) were weighed [body mass (M b) range 23–92 g] and a fixed number of trout with a similar mass was introduced into the dive tank. Trout swam freely throughout the water column for at least 2 h before a bird was introduced and started to forage. Each bird (n = 9 birds) participated in one 30-min trial per day (individual trials; n = 82). All predator-prey interactions were filmed and subsequently analysed. Fish density encountered by a particular bird at the start of a trial (taken as the total number/mass of fish in the tank at that time divided by tank volume) was altered by changing its position within the daily trial order. To assess cormorant prey-capture rate (expressed as “catch per unit effort”, CPUE, in g fish caught per min spent submerged), we marked down the total time spent submerged and the number of fish caught during a trial. The latter was multiplied with the mean M b of trout for that day and divided by the time a bird spent submerged during the trial.
Results
Diving energetics
When accounting for water temperature, energy expenditure in P. carbo carbo (Grémillet et al. 2003; 22.8 W kg−1), P. aristotelis (Enstipp et al. 2005; 20.0 W kg−1), and P. auritus (Enstipp et al. 2006a; 21.6 W kg−1) during shallow horizontal diving was similar (Fig. 1). By contrast, energy expenditure of P. carbo sinensis under equivalent conditions was measured to be 31.4 W kg−1 (Schmid et al. 1995) and, hence, considerably higher (by ∼46%; Fig. 1). When expressing dive costs as multiples of RMR, the latter measurement reaches 10 × RMR.
Energy expenditure (W kg−1) during shallow diving (1 m) in three Phalacrocorax species. Mean water temperature (T W) in the first study on P. carbo sinensis was 12.6°C (Schmid et al. 1995) and energy expenditure measured in all other studies was adjusted to that temperature using linear regression equations established by each study. Values are from Schmid et al. (1995) (1), Grémillet et al. (2003) (2), Enstipp et al. (2005) (3), and Enstipp et al. (2006a) (4). BMR values for P. aristotelis and P. auritus were taken from the indicated studies, while RMR values for both sub-species of P. carbo are from Schmid et al. (1995)
When diving at the same water temperature (12.6°C) to 10 m depth, energy expenditure in P. auritus was measured to have increased by ∼19% (25.6 W kg−1; Fig. 2; Enstipp et al. 2006a). In contrast, based on the respirometry measurements of Grémillet et al. (2001) and the physical model of Grémillet and Wilson (1999), dive costs for P. carbo carbo were estimated to have increased by ∼154% (58.0 W kg−1; Fig. 2). A decrease in water temperature to wintering conditions encountered in Greenland (i.e. −1°C), would further increase the dive costs during these 10-m dives in both species. Extrapolating from the measured relationship between energy expenditure and water temperature for P. auritus (Enstipp et al. 2006a; temperature range 6.1–15.4°C) to such a low temperature (assuming the relationship remains linear) would result in an estimated increase in dive costs of ∼22% (31.3 W kg−1). Using the physical model of heat loss by Grémillet and Wilson (1999), dive costs for P. carbo carbo are estimated to reach 64.0 W kg−1 (Fig. 2).
Energy expenditure (W kg−1) during dives to 10 m depth in P. carbo and P. auritus. Values for P. carbo are based on a model by Grémillet and Wilson (1999); see Grémillet et al. (2001, Fig. 2). Energy expenditure for P. auritus was measured for a temperature range of 6.1–15.4°C (Enstipp et al. 2006a) and extrapolated to −1°C using the established linear regression equation
Prey-capture behaviour
Estimates of prey-capture rates (CPUE) for avian divers reported in the literature are rare and typically well below 10 g min−1 underwater (Fig. 3). However, capture rates in cormorants and shags have been estimated to be considerably higher than in other avian divers. The greatest capture success suggested so far is for Great Cormorants wintering in Greenland. Based on time-activity data and a bioenergetics model, Grémillet et al. (2005b) calculated that Great Cormorants require a daily food intake (DFI) of ∼1,170 g, more than one-third of their body mass. To catch such quantity of fish within the timeframe that birds spend foraging, they require a theoretical prey-capture rate of ∼41 g min−1 underwater (range 22–80 g min−1; Grémillet et al. 2005b).
Prey capture rates (“catch per unit effort” in g min−1 submerged) for various seabird species (means ± SD). P. c. (Phalacrocorax carbo), P. a. (P. aristotelis), P. n. (P. neglectus), U. a. (Uria aalge), S. d. (Speniscus demersus). Wi. and Su. refer to winter and summer, respectively. In all studies, prey-capture rates were assessed indirectly by combining bird activity recordings with food intake estimates. The latter were based on either nest balance recordings, time-energy budgets, or stomach temperature recordings. Values are from Grémillet et al. (2005b) (1); Grémillet (1997) (2); Lorentsen et al., unpublished data, in Grémillet et al. (2004) (3); Grémillet et al. (2003) (4); Enstipp et al. (2006b) (5); Wanless et al. (1998) (6); Wilson and Grémillet (1996) in Grémillet (1997) (7); Enstipp et al. (2006b) (8); Wilson and Grémillet (1996) in Grémillet (1997) (9)
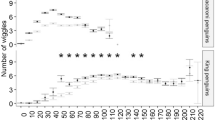
In our captive experiments, fish density ranged from 0.17 to 7.27 g m−3, equivalent to 1–23 trout within the tank. Foraging success of Double-crested Cormorants ranged from 0 to 402 g min−1 underwater and strongly depended on prey density (Fig. 4). We found a significant, apparently linear, relationship between fish density and cormorant prey capture rate (CPUE), where high fish densities were associated with greater prey capture rates (Fig. 4). The range of theoretical prey capture rates required by Great Cormorants wintering in Greenland (indicated by the box in Fig. 4) falls within the lower range of capture rates achieved by the cormorants in our experiments.
Prey-capture rates of captive Double-crested Cormorants (CPUE) foraging on live rainbow trout at various densities (n = 9 birds and 82 trials). CPUE increased significantly with an increase in fish density and was best described by y = 26.04x + 9.6, where y is CPUE and x is fish density (F = 6.84, P < 0.0001, r 2 = 0.45). The box indicates the range of theoretical prey-capture rates required by Great Cormorants wintering in Greenland. Note that they fall within the lower range of the capture rates achieved by the captive birds. This suggests that cormorants might be able to achieve the required high capture rates, if prey densities are sufficiently high
Discussion
Our investigation started with the following two questions: (1) does the existing dive cost estimate for Great Cormorants wintering in Greenland overestimate the actual energy demand of these birds? (2) could birds fuel their costly way of life during the winter by increasing their DFI? We will discuss our findings in the context of these two questions.
Diving energetics
Estimated energy expenditures during shallow horizontal diving in the three cormorant species at 12°C (P. carbo carbo, P. aristotelis, P. auritus) were similar (Fig. 1). They were, however, considerably below the value reported for P. carbo sinensis by Schmid et al. (1995). The reason for this is most likely the different methods used to calculate energy expenditure (see Butler 2000). First, in the study by Schmid et al. (1995), energy expenditure was calculated on the basis of single dives, while all other studies used entire dive bouts (or at least multiple dive cycles). Secondly, and more important, all studies except Schmid et al. (1995) calculated energy expenditure by measuring the oxygen consumed while a bird was at the surface and dividing this value by the duration of the surface interval plus the preceding dive [VO2total/(t surface + t dive); “method 1”; see Castellini et al. 1992]. In fact, since multiple consecutive dive cycles were considered, total oxygen consumption during all surface periods was divided by the sum of all surface and dive durations. Schmid et al. (1995), however, calculated energy expenditure by subtracting resting oxygen uptake at the surface from the total oxygen consumed at the surface and dividing the remainder by the duration of the preceding dive [(VO2total − VO2rest)/t dive; “method 2”]. The rationale for doing so is based upon the assumption that if metabolic rate during the surface period between dives is elevated above the resting rate in water, this is due to the exercise of the previous dive (i.e. repaying the oxygen debt) or a consequence of it (i.e. elevated metabolic rate to minimise the surface period) (Culik et al. 1996). Culik et al. (1996) compared different methods to calculate the energy expenditure of King Penguins (Aptenodytes patagonicus) during diving in a shallow swim channel. Analysis was based on single dives and, when energy expenditure was calculated by the two methods described above, the second method produced a dive cost estimate ∼26% greater than the estimate derived from the first method (8.4 vs 10.6 W kg−1 for the first and second method, respectively) (Culik et al. 1996). This is reasonably similar to the measured difference in dive costs of Great Cormorants reported by Grémillet et al. (2003) and Schmid et al. (1995), which amounts to an elevation of ∼37% (22.8 vs 31.4 W kg−1).
Which calculation method should be used might depend on the questions we are asking. The second method might be of interest when calculating physiological parameters like the calculated aerobic dive limit (cADL). However, from an ecological point of view, dive and surface intervals are not separate entities but part of the same activity. Hence, if we are interested to know the overall energetic costs of diving, the first method might be more appropriate. In the context of ecological energetics, it might also be preferable to estimate dive costs over longer periods (e.g. entire dive bouts), since birds might not always recover completely from a preceding dive during the short surface period (see Ydenberg and Forbes 1988). This is also supported by De Leeuw’s argument that an analysis based on single dive events would mostly exclude thermoregulatory costs, which in his study with Tufted Ducks (Aythya fuligula) were largely paid off at the end of a dive bout (De Leeuw 1996).
Expressing energy expenditure during diving as multiples of RMR or BMR (Fig. 1) becomes somewhat misleading in the case of the Great Cormorant (P. c. carbo and P. c. sinensis) because of the very low RMR measured by Schmid et al. (1995). Their value is well below the rate predicted from allometry and another measurement for the Japanese sub-species (P. carbo hanedae; see Enstipp et al. 2005 for discussion).
Our investigation shows that when the differences in experimental conditions (e.g. water temperature) and calculation methods are accounted for, energy expenditure during shallow horizontal diving in the cormorant species investigated so far are fairly similar and, in fact, not considerably different from other avian divers (see Table 1 in Enstipp et al. 2005 and Fig. 7 in Enstipp et al. 2006a).
The measured effect of depth on the energetic costs of diving in Double-crested Cormorants (Enstipp et al. 2006a) was less drastic than suggested by the thermodynamic model of Grémillet and Wilson (1999) (Fig. 2). Using this model, Grémillet et al. (2001) estimated that dive costs of Great Cormorants when diving to 10 m depth in 12.6°C water would increase to 58 W kg−1. This represents an increase of ∼154%, when compared with birds diving in a shallow trench at the same water temperature (22.8 W kg−1). In Double-crested Cormorants, however, the measured increase in dive costs under identical conditions was only ∼19% (from 21.6 to 25.6 W kg−1; Enstipp et al. 2006a). Similarly, with a decline in water temperature to wintering conditions in Greenland (−1°C), the model of Grémillet and Wilson (1999) produces a much higher estimate for Great Cormorants (64 W kg−1) than the extrapolation from the measurement range to such a low temperature in Double-crested Cormorants (31.3 W kg−1; Fig. 2). There are a number of reasons why the thermodynamic model might overestimate the actual dive costs of cormorants diving to depth (see Enstipp et al. 2006a for discussion). This illustrates how crucial the actual measurement of parameters is for the improvement of existing bioenergetic models. In this context, Grémillet et al. (2003) established a different model to calculate the energy expenditure during diving in cormorants, also incorporating the effect of depth (their Eq. 2). This model produces considerably lower estimates than the model in Grémillet et al. (2001), with 39.4 versus 47.5 W kg−1 for dives to 10 m in water of 12.6 and −1°C, respectively.
In the Introduction, we raised the question whether the energy demand of cormorants wintering in Greenland might have been overestimated. Based on our investigation, we conclude that the use of Grémillet and Wilson’s thermodynamic model (Grémillet and Wilson 1999) would most likely overestimate the dive costs of cormorants and, therefore, overestimate their daily energy expenditure and DFI during wintering in Greenland. However, the estimated DFI of ∼1,170 g fish, calculated by Grémillet et al. (2005b) for Great Cormorants wintering in Greenland, is based on the improved model (Grémillet et al. 2003) that also incorporates the observed dive characteristics of cormorants during that time (e.g. dive-pause-ratio) and, hence, produces a much lower estimate for the dive costs.
Prey-capture behaviour
The experiments with captive Double-crested Cormorants foraging on live rainbow trout illustrate the importance of prey density for the foraging success of a predator (Fig. 4). Perhaps not surprisingly, cormorants achieved the highest capture rates (mean: ∼200 g min−1 submerged) at the greatest fish densities tested in our experiments (7.27 g m−3). Conversely, at low fish densities, cormorant foraging success was reduced sometimes to the point that birds did not catch any fish. Based on activity recordings and a bioenergetics model, Grémillet et al. (2005b) suggested that Great Cormorants wintering in Greenland would require a theoretical prey-capture rate of ∼41 g min−1 underwater (range 22–80 g min−1). While this represents the highest prey-capture rate suggested for an avian pursuit-diver so far (Fig. 3), this estimate falls within the lower range of capture rates achieved by the captive birds in our experiments (Fig. 4). Hence, this would suggest that cormorants in Greenland might be able to achieve these high capture rates if prey densities are sufficiently high. Cormorants might therefore be able to balance their increased energetic demand during the winter simply by eating more. However, this explanation might be an oversimplification, since our captive experiments can only serve as a proxy for the natural situation. Clearly, the predatory performance of cormorants might be different under natural conditions. In our experiments, cormorants foraged within a confined space, where tank walls restricted the movements of both predator and prey. While tank dimensions were relatively large, it is difficult to judge to what degree the confined space might have worked in favour of the predator. Furthermore, nothing is known about the prey densities that cormorants encounter during the winter in Greenland. In fact, presently we do not even know what cormorants feed on during the winter. During the summer, birds predominantly forage on sculpins, capelins, and gadids (Grémillet et al. 2004). Density assessments during this time, conducted within small patches where cormorants were observed to forage, revealed a relatively low fish density, well below 1 g m−3 (∼0.03 prey items m−2; Grémillet et al. 2004). Hence, if densities are similar during the winter, birds might not be able to achieve these high prey-capture rates. Furthermore, at low prey densities, birds might have to spend an increased amount of time and energy to locate and capture sufficient food, which would tend to further increase their energy demand and, hence, food requirements (Enstipp et al. 2006b).
Cormorants are perceived as very efficient predators and the estimates of prey-capture rates illustrated in Fig. 3 certainly support this view. One factor that might contribute to their enormous foraging success is their great plasticity when it comes to food choice. Cormorants in general are highly opportunistic foragers that feed on both benthic and pelagic prey. Some species forage in freshwater and marine habitats. While the species investigated in our study are principally piscivorous, there has been the suggestion that Great Cormorants might occasionally take invertebrate prey (e.g. polychaetes; Leopold and van Damme 2003). This is also supported by the dive patterns that Grémillet et al. (2005b) observed in Greenlandic Great Cormorants. Their year-round recordings revealed that dive bout organisation changed drastically in early spring. While dive bouts consisted of relatively few dives to greater depth during the winter (typically less than 50 dives to a maximum depth of 40 m), birds conducted many shallow dives in quick succession during early spring (up to ∼200 dives to a maximum depth of less than 5 m; Enstipp and Grémillet, unpublished data). Such a dive pattern would be consistent with the successful capture of small prey items of little energetic value (i.e. invertebrates). Hence, a great variety in dietary choice, which allows birds to switch prey when some species become less abundant, might contribute significantly to the success of cormorants. Another factor might be their good visual resolution underwater, when compared with other aquatic predators (Strod et al. 2004). Despite the challenge of living in two different media (air/water) that require compensatory mechanisms, cormorant vision underwater is better than in most fishes and marine mammals (Strod et al. 2004). However, when compared with aerial avian predators, visual resolution in Great Cormorants was recently found to be very inferior (White et al. 2007). Furthermore, the visual environment of cormorants underwater is strongly affected by water turbidity, which decreases image resolution and this might limit their visual foraging. Other means of locating and catching prey have also been suggested (e.g. using tactile or acoustic cues; Grémillet et al. 2005c) but remain to be investigated. Hence, how Great Cormorants in Greenland manage to find so much fish, especially in the darkness of the polar night, remains a mystery.
In conclusion, our study shows that the increase in energetic costs of Double-crested Cormorants diving to depth is less drastic than suggested by the physical model of Grémillet and Wilson (1999), which was used by Grémillet et al. (2001) to estimate dive costs for Great Cormorants wintering in Greenland. Using this model to calculate the daily energy and food requirements of cormorants would most likely lead to an overestimation. Results of our captive experiments demonstrate that cormorants are able to achieve high prey-capture rates if prey densities are sufficiently high. Cormorants in the wild might therefore be able to balance their increased energetic demand during the winter by eating more.
References
Butler PJ (2000) Energetic costs of surface swimming and diving of birds. Physiol Biochem Zool 73:699–705
Castellini MA, Kooyman GL, Ponganis PJ (1992) Metabolic rates of freely diving Weddell seals: correlations with oxygen stores, swim velocity and diving duration. J Exp Biol 165:181–194
Culik BM, Pütz K, Wilson RP, Allers D, Lage J, Bost C-A, Le Maho Y (1996) Diving energetics in king penguins (Aptenodytes patagonicus). J Exp Biol 199:973–983
Daunt F, Afanasyev V, Silk JRD, Wanless S (2006) Extrinsic and intrinsic determinants of winter foraging and breeding phenology in a temperate seabird. Behav Ecol Sociobiol 59:381–388
De Leeuw JJ (1996) Diving costs as a component of daily energy budgets of aquatic birds and mammals: generalizing the inclusion of dive-recovery costs demonstrated in tufted ducks. Can J Zool 74:2131–2142
Enstipp MR, Grémillet D, Lorentsen S-H (2005) Energetic costs of diving and thermal status in European shags (Phalacrocorax aristotelis). J Exp Biol 208:3451–3461
Enstipp MR, Grémillet D, Jones DR (2006a) The effect of depth, temperature and food ingestion on the foraging energetics of a diving endotherm, the double-crested cormorant (Phalacrocorax auritus). J Exp Biol 209:845–859
Enstipp MR, Daunt F, Wanless S, Humphreys EM, Hamer KC, Benvenuti S, Grémillet D (2006b) Foraging energetics of North Sea birds confronted with fluctuating prey availability. In: Boyd IL, Wanless S, Camphuysen CJ (eds) Top predators in marine ecosystems. Symposium of the Zoological Society London, Cambridge University Press, Cambridge, pp 191–210
Enstipp MR, Grémillet D, Jones DR (2007) Investigating the functional link between prey abundance and seabird predatory performance. Mar Ecol Progr Ser 331:267–279
Grémillet D (1995) ‘Wing drying’ in cormorants. J Avian Biol 26:176
Grémillet D (1997) Catch per unit effort, foraging efficiency, and parental investment in breeding great cormorants (Phalacrocorax carbo carbo). ICES J Mar Sci 54:635–644
Grémillet D, Wilson RP (1999) A life in the fast lane: energetics and foraging strategies of the great cormorant. Behav Ecol 10:516–524
Grémillet D, Wanless S, Carss DN, Linton D, Harris MP, Speakman JR, Le Maho Y (2001) Foraging energetics of arctic cormorants and the evolution of diving birds. Ecol Lett 4:180–184
Grémillet D, Wright G, Lauder A, Carss DN, Wanless S (2003) Modelling the daily food requirements of wintering great cormorants: a bioenergetics tool for wildlife management. J Appl Ecol 40:266–277
Grémillet D, Kuntz G, Delbart F, Mellet M, Kato A, Robin J-P, Chaillon P-E, Gendner J-P, Lorentsen S-H, Le Maho Y (2004) Linking the foraging performance of a marine predator to local prey abundance. Funct Ecol 18:793–801
Grémillet D, Chauvin C, Wilson RP, Le Maho Y, Wanless S (2005a) Unusual feather structure allows partial plumage wettability in diving great cormorants (Phalacrocorax carbo). J Avian Biol 36:1–7
Grémillet D, Kuntz G, Woakes AJ, Gilbert C, Robin J-P, Le Maho Y, Butler PJ (2005b) Year-round recordings of behavioural and physiological parameters reveal the survival strategy of a poorly insulated diving endotherm during the Arctic winter. J Exp Biol 208:4231–4241
Grémillet D, Kuntz G, Gilbert C, Woakes AJ, Butler PJ, Le Maho Y (2005c) Cormorants dive through the Polar night. Biol Lett 1:469–471
Johnsgard PA (1993) Cormorants, darters, and pelicans of the world. Smithsonian Institution Press, Washington
Leopold MF, van Damme CJG (2003) Great cormorants Phalacrocorax carbo and polychaetes: can worms sometimes be a major prey of a piscivorous seabird? Mar Ornithol 31:83–87
Lovvorn JR, Jones DR (1991) Body mass, volume, and buoyancy of some aquatic birds, and their relation to locomotor strategies. Can J Zool 69:2888–2892
Rijke AM (1968) The water repellency and feather structure of cormorants Phalacrocoracidae. J Exp Biol 48:185–189
Schmid D, Grémillet DJH, Culik BM (1995) Energetics of underwater swimming in the great cormorant (Phalacrocorax carbo sinensis). Mar Biol 123:875–881
Strod T, Arad Z, Izhaki I, Katzir G (2004) Cormorants keep their power: visual resolution in a pursuit-diving bird under amphibious and turbid conditions. Curr Biol 14:R376–R377
Van Tets GF (1976) Australasia and the origin of shags and cormorants, Phalacrocoracidae. Proc Int Ornithol Congr 16:121–124
Wanless S, Grémillet D, Harris MP (1998) Foraging activity and performance of shags Phalacrocorax aristotelis in relation to environmental characteristics. J Avian Biol 29:49–54
White CR, Day N, Butler PJ, Martin GR (2007) Vision and foraging in cormorants: more like herons than hawks? PLoS ONE 2:e639
Wilson RP, Grémillet D (1996) Body temperatures of free-living African penguins (Spheniscus demersus) and Bank cormorants (Phalacrocorax neglectus). J Exp Biol 199:2215–2223
Wilson RP, Hustler K, Ryan PG, Burger AE, Nöldeke EC (1992) Diving birds in cold water: do Archimedes and Boyle determine energetic costs? Am Nat 140:179–200
Ydenberg RC, Forbes LS (1988) Diving and foraging in the western grebe. Ornis Scand 19:129–133
Acknowledgments
Research on European Shags and Double-crested Cormorants was conducted and funded within the framework of the European Commission project ‘Interactions between the marine environment, predators and prey: implications for sustainable sandeel fisheries' (IMPRESS; QRRS 2000-30864). Financial support was also provided by a “Discovery Grant” and equipment grants from NSERC to D.R.J. The British Columbia Ministry of Environment granted holding permits for the Double-crested Cormorants. All experimental procedures with the Double-crested Cormorants were approved by the UBC Animal Care Committee (Animal Care Certificate # A02-0122) and were in compliance with the principles promulgated by the Canadian Council on Animal Care. We wish to thank Pat Butler and Akiko Kato for organising such an excellent symposium (“Behaviour and physiology of under-water foraging in diving birds”) at the 24th International Ornithological Congress and for giving us the opportunity to present our work. Thanks to two referees for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Enstipp, M.R., Jones, D.R., Lorentsen, SH. et al. Energetic costs of diving and prey-capture capabilities in cormorants and shags (Phalacrocoracidae) underline their unique adaptation to the aquatic environment. J Ornithol 148 (Suppl 2), 593–600 (2007). https://doi.org/10.1007/s10336-007-0203-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-007-0203-8