Abstract
The seasonal decline in offspring performance is a frequently reported phenomenon in species breeding in a temperate zone, but the potential effect of brood sex ratio on such declines has not been studied. Here, we predicted that this decline may occur if the sex that exhibits the lower immune response or lower survival rate tends to be more frequent among late broods. The seasonal patterns of four performance parameters of collared flycatcher Ficedula albicollis nestlings have been examined during 4 years. Sex was assigned to all studied individuals using molecular techniques. We found significant seasonal decline in cell-mediated immune response, tarsus length and survival of the chicks. The lack of interactions between gender and hatching date revealed that both sexes contributed equally to the observed decline. The brood sex ratio did not vary with the laying date. On the basis of available data, we suggest that the breeding date may only exceptionally induce female-driven sex allocation in species with only slight sexual size dimorphism. In consequence, we suggest that seasonal sex ratio shifts do not account for seasonally declining fitness of nestlings in passerines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The timing of breeding is considered an important determinant of fitness in adult birds and their offspring (e.g. Perrins 1970; Verhulst and Tinbergen 1991; Verboven and Visser 1998; Dubiec and Cichoń 2001). The predominant pattern found is that the chicks hatched late in the season are of lower quality when compared to early ones. Nestling body size and survival rate has repeatedly been shown to decline with the progress of the breeding season (e.g. Verboven and Visser 1998; Lepage et al. 2000). Recently, an increasing number of studies have revealed that immune functions are also impaired in late-hatched nestlings (Sorci et al. 1997; Dubiec and Cichoń 2005; Moreno et al. 2005). The decline of offspring performance is usually attributed to depleting food resources over the season, or to differences in the quality of adult birds commencing their breeding early or late in the season (Nilsson 1999). However, there is no agreement on which of these two factors contributes more to the observed pattern. Another possible explanation is that the seasonal decline in body size and immune function may stem from seasonal variation in brood sex composition in species in which male and female nestlings differ in these traits. We could expect seasonal decline if the smaller sex, or the one showing the lower immune response, tends to be overproduced later in the season. Females have often been reported to bias sex ratio in their clutches (e.g. Ellegren et al. 1996; Komdeur et al. 1997), and they are prone to do so if the reproductive value of daughters and sons differs (Charnov 1982). As male and female nestlings often differ in body size (Råberg et al. 2005), mortality (Bize et al. 2005; Cichoń et al. 2005) and immunocompetence (Fargallo et al. 2002; Tschirren et al. 2003; Dubiec et al. 2006; but see Saino et al. 2002; Bize et al. 2005), sons and daughters may have different fitness returns to their parents. Moreover, the relative value of the male and female offspring may vary depending on the timing of hatching. Consequently, this may induce female-driven sex ratio adjustment according to the breeding date. Indeed, seasonal shifts in sex ratio have been reported in many non-passerine species, especially raptors (e.g. Daan et al. 1996; Tella et al. 1996). Among passerines, some studies have shown seasonal variation in sex ratios (Lessels et al. 1996; Cordero et al. 2001; Rosivall et al. 2004), while others failed to show any consistent trends (Kölliker et al. 1999; Radford and Blakey 2000; Leech et al. 2001; Verboven et al. 2002).
The adjustment of primary sex ratio according to the onset of breeding might be linked to the seasonal variation in mate quality, different costs of raising sons and daughters early or late in the season, or sex-specific sensitivity of nestlings to unfavourable environmental conditions. In the collared flycatcher Ficedula albicollis, sons raised early in the season exhibited bigger sexual ornaments (Qvarnström 1999) and, in this and the sister species, pied flycatcher Ficedula hypoleuca, male offspring experienced higher mortality during embryonic development and suffered more from nest mites (Cichoń et al. 2005; Potti and Merino 1996; but see Potti et al. 2002). Therefore, they might be more affected when hatched late in the season. It is thus possible to assume that female collared flycatchers may tend to invest differentially in the sex of their chicks in relation to the breeding time.
In the present study, we explore the effect of hatching date on cell-mediated immune response and survival rate of collared flycatcher nestlings, and try to relate the observed patterns to seasonal variation in brood sex composition. Specifically, we expect the immunocompetence of the chicks and their probability of survival to decrease over the season. If this is the case, we ask whether the seasonal changes of the sex ratio may account for variation in the studied traits, and whether one of the sexes suffers more from being hatched late in the season.
Methods
Study species and study area
The research was conducted in Grobelczyk Wood, NE part of Niepolomice Forest (50°06′N, 20°24′E), southern Poland. The study plot, which comprises ca. 250 nest boxes, is situated in hornbeam-oak forest, where 30–60 pairs of collared flycatchers breed every year. The collared flycatcher is a small (13–15 g), insectivorous, and long-distance migratory passerine. It is a monogamous species (only one case of polygyny was observed during 4 years of study), with a moderate level of extra-pair copulations at this site (ca. 25% of broods; unpublished data). In the studied population, it raises one brood per year, with mean clutch size of 6.41 (SD = 0.05) and modal clutch size of 7 (n = 246). The data from four breeding seasons (2000 and 2003–2005) are presented. This dataset covers 93 nests in which the chicks were sexed, and in 90 of them data on the offspring immune response, condition parameters and survival were available.
General procedures
The boxes were checked every 2–3 days since ca. 25 April, to record the start of egg laying (laying date = date of first egg), clutch size and hatching date (day of hatching = day 0). Nestlings were marked individually 2 days after hatching by clipping their nails, and blood samples were taken by puncturing the brachial or femoral vein and then stored in 95% ethanol. As we aimed at assigning the primary sex ratio, all dead embryos or nestlings were collected, if present. On day 11, the nestlings were weighed (to the nearest 0.1 g), the tarsus length was measured with digital calliper (0.1 mm accuracy), and they were banded with aluminium rings.
Cell-mediated immunity
The 11-day-old nestlings were inoculated with antigen (phytohaemagglutinin, PHA; Sigma–Aldrich, USA) to induce cell-mediated immune response (Smits et al. 1999). The centre of the right wing web was marked with a permanent marker, and the wing web thickness was measured in the marked place 3 times with a pressure sensitive spessimeter with 0.01-mm accuracy (Mitutoyo, SM-12). Antigen (0.2 mg of PHA in 0.04 ml of phosphate-buffered saline, PBS) was then injected in the centre of the wing. After 24 h (±1 h), the swelling of the wing web was measured in the same way. The measurements taken after injection were highly repeatable: r = 0.96; F 414,830 = 78.15; p < 0.001 (Lesells and Boag 1987). The magnitude of immune response was calculated as the difference between the mean wing web thickness after and before treatment (wing-web index). The PHA-induced immune reaction is regarded as a reliable indicator of individuals’ cell-mediated immunocompetence (Lochmiller et al. 1993; Martin et al. 2006). It has been frequently shown to be a condition-dependent trait (e.g. Hõrak et al. 1999; Alonso-Álvarez and Tella 2001) and to predict the survival probability (Cichoń and Dubiec 2005; Moreno et al. 2005).
Molecular analyses of sex
The sex of nestlings was determined molecularly based on differences in intron size in CHD gene located in the sex chromosomes. The DNA was extracted from blood samples (or other tissues in the case of dead nestlings), using Chelex with addition of Proteinase K. The standard 10-μl PCR mix was applied, containing two sets of primers to amplify the CHD introns; 3007 and 3112 for CHD-Z intron, and 2987 and 3112 for CHD-W intron (Ellegren and Fridolfsson 1997), except for the samples from 2005, which were amplified with P2 and P8 primers (Griffiths et al. 1998). The PCR conditions were as follows: initial step of 94°C for 5 min, 35 cycles of 94°C for 30 s, 50°C for 30 s and 72°C for 45 s, and final step of 72°C for 4 min. The PCR products were separated on 1.4 or 2% agarose gel with ethidium bromide, then visualised under the UV light and photographed. The sexing of adult individuals of known sex (n = 30) confirmed the accuracy of this method. In total, we assigned the sex of 560 nestlings from 93 nests. In one nest, we were not able to determine the sex of two chicks, probably because of the DNA degradation, and in the other nine nests, we failed to collect single samples of blood. These nests were included in the analysis of the seasonal shift in brood sex ratios, as we believe that the missing samples randomly represented male and female offspring. Nestlings with unknown sex were excluded from any other analysis.
Statistics
The effect of hatching date on nestlings’ tarsus length, mass and immune response (wing-web index) was analysed with Proc Mixed procedure assuming REML method, with sex as a fixed factor and hatching date as a covariate. Year and brood identity (code of the nest) nested in year were included in the model as random factors. The same model was applied to analyse changes in sex ratio throughout the season, with the laying date as a covariate and year as a random factor. The survival rate (ratio: fledged young/clutch size) was analysed using the generalised mixed linear model, with a logit link function assuming binomial variance, with sex as a fixed factor, hatching date as a covariate and year and brood identity nested in year as random factors. Least squares means ± SE presented throughout the paper were derived from the above models. The analyses were performed with SAS 8.2.
Results
Male and female collared flycatcher nestlings did not differ in either body size measured as body mass (males: 14.27 ± 0.40; females: 13.95 ± 0.40) and tarsus length (males: 17.17 ± 0.09; females: 17.18 ± 0.09) or cell-mediated immune response (males: 79.84 ± 12.86; females: 76.97 ± 12.88; Table 1).
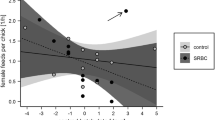
Body mass did not show any seasonal variation, while both tarsus length and cell-mediated immune response declined with the progress of the breeding season after controlling for the between-brood variation and year (Table 1; Fig. 1). The observed decline was consistent between years, as revealed by non-significant interaction between hatching date and season (results not shown). Male and female nestlings contributed equally to the observed seasonal decline in tarsus length and immune response since interaction between hatching date and sex was not significant (Table 1).
The relationship between hatching date and two parameters of collared flycatcher Ficedula albicollis nestling performance. The dates on the X-axis are median dates calculated by subtracting actual date from median date of hatching in the specific year. The points are least squares means of the given trait in the nest, calculated separately for each sex. The regression lines are presented to illustrate the trend
The survival of nestlings also declined with the progress of breeding season (after controlling for between-nest and between-year variation; Table 1), and again non-significant interaction between hatching date and gender revealed that the decreasing probability of survival among late-hatched nestlings affected both sexes equally.
In the studied population, sex ratio was not related to laying date and this pattern was consistent between years (F 1,88 = 0.16; p = 0.69).
Discussion
Our study revealed that, in the examined population of the collared flycatcher, the immune response of nestlings and their probability of fledging decline with the progress of breeding season, and that this decline is consistent between years. These results corroborate the findings of some earlier studies showing a similar pattern of impaired immunocompetence in the offspring of late breeders (Sorci et al. 1997; Dubiec and Cichoń 2005; Moreno et al. 2005). The decline in nestling performance is usually attributed to seasonal changes in either intrinsic quality of parents or environmental conditions (Nilsson 1999). We could therefore expect that seasonally declining offspring immune response might be caused by lower genetic quality of late-breeding individuals or by depleting food resources over the season. We are not able to tell which of the two factors plays a role in the studied population, but other data suggest that the functioning of the immune system of the hole-nesting passerines might be both genetically (Cichoń et al. 2006), and environmentally (Dubiec and Cichoń 2005) determined. Here, we predicted that seasonal variation in sex ratio may potentially contribute to the observed decline. Specifically, we expected that brood sex ratio may change with the progress of the season towards the sex showing lower immunocompetence or lower probability of survival. We found, however, that in our population sex ratio did not vary with the time of the season, despite the existence of factors potentially promoting seasonal sex ratio adjustment. For instance, early hatched sons develop larger forehead patches (Qvarnström 1999), which later on act as a badge of male status (Pärt and Qvarnström 1997) and correlate with reproductive success (Sheldon and Ellegren 1999). Additionally, Cichoń et al. (2005) detected higher mortality of male embryos, and Potti and Merino (1996) found, in the congeneric species, pied flycatcher, that sons suffered more from blood-sucking mites. Females would therefore benefit from producing male-biased broods at the beginning of the season, and female-biased later on, as daughters may presumably cope better with adverse environmental conditions. In fact, Rosivall et al. (2004) reported sex ratio to be related to the date of clutch initiation in the Hungarian population of the collared flycatcher, which is opposite to our results and to the Swedish data (Ellegren et al. 1996). Such between-studies differences in seasonal sex ratio shifts have also been recorded in the great tit Parus major (Lessells et al. 1996; Radford and Blakey 2000; Verboven et al. 2002), which may suggest different sex adjustment optima in different populations (Griffith et al. 2003).
Seasonal variation in sex ratio has been repeatedly reported in many non-passerine birds, especially birds of prey, which differ not only in body size, but also in life history tactics (e.g. Daan et al. 1996; Tella et al. 1996). In passerines, only four studies showed early broods being female-biased (Weatherhead 1983; Lessells et al. 1996; Cordero et al. 2001; Rosivall et al. 2004), and numerous studies failed to find any link between breeding onset and sex ratio (e.g. Kölliker et al. 1999; Radford and Blakey 2000; Leech et al. 2001; Verboven et al. 2002; this study). These findings, showing seasonal variation in sex ratio being more often observed among sexually dimorphic species, may indicate that the crucial factor involved is the different cost of raising sons and daughters. Consequently, this may suggest that the timing of breeding may not exert a strong selection on sex allocation in passerines, which usually exhibit only slight sexual size dimorphism.
In our study, we did not find that male and female nestlings differed in their response to phytohaemagglutinin. Sexual differences in nestling immunocompetence have been studied several times, but the published results are not consistent. Males have been shown to have lower immunocompetence in the European kestrel Falco tinnunculus (Fargallo et al. 2002) and great tit (Tschirren et al. 2003), while in contrast, males exhibit higher immunocompetence in blue tit Parus caeruleus (Dubiec et al. 2006), and some studies did not find any differences (Saino et al. 2002; Bize et al. 2005, this study). Sexual dimorphism in immune response of nestlings is expected due to immunosupressive influence of androgens (Grossman 1989). However, Silverin and Sharp (1996) found the level of testosterone to differ among male and female great tit nestlings only in the very first days after hatching, so its immunosuppressive effect might be absent later in the nestlings’ life.
Additionally, we expected sex-related differences to come into play among late-hatched nestlings, as they may face a harsher environment (due to, e.g., lower food abundance). Some studies found sexual differences in nestlings’ environmental sensitivity (e.g. Tschirren et al. 2003; Fargallo et al. 2002; Dubiec et al. 2006). We found, however, both sexes contributing equally to the observed decline of immunocompetence, tarsus length and survival rate. Sheldon et al. (1998), by enlarging broods of collared flycatcher, imposed an adverse environment on the nestlings, putting them in a context similar to that they may encounter when hatched late in the season, and also failed to find differences in body size of male and female chicks. As suggested by Råberg et al. (2005), the level of the sex-specific sensitivity might be related to sexual size dimorphism, which, as we showed, is slight in the collared flycatcher.
To conclude, our study revealed seasonal decline in cell-mediated immune response, tarsus length and survival of the collared flycatcher nestlings. Both sexes contributed equally to that pattern, revealing no gender-specific environmental susceptibilities. Contrary to expectations, there was no seasonal shift in sex ratio which, as we predicted, could explain the observed seasonal variation of performance. The available data may suggest that laying date is not likely to affect the females’ decision about the sex allocation in passerines. We suggest that this scenario is more likely to occur in species with a greater sexual dimorphism. This in turn leads to the conclusion that we could expect the seasonal decline in the nestlings’ fitness to be explained by variation in sex ratio only in species with more pronounced differences between male and female nestlings.
Zusammenfassung
Der jahreszeitlich bedingte Rückgang zellvermittelter Immunität bei Halsbandschnäpper-Nestlingen Ficedula albicollis: Spielt das Geschlecht der Nachkommen eine Rolle?
Häufig wurde bei Arten, die in den gemäßigten Breiten brüten, ein jahreszeitlich bedingter Rückgang der Nachkommenqualität festgestellt, wohingegen der mögliche Einfluss des Geschlechterverhältnisses in der Brut auf einen solchen Rückgang unbeachtet blieb. Hier stellen wir die Hypothese auf, dass ein Qualitätsrückgang dann auftreten könnte, wenn das Geschlecht, das eine niedrigere Immunkompetenz oder geringere Überlebensrate aufweist, in späten Bruten häufiger auftritt. Über vier Jahre wurde das jahreszeitliche Muster von vier Qualitätsmerkmalen von Halsbandschnäppernestlingen untersucht und mit Hilfe molekularer Techniken das Geschlecht aller untersuchten Individuen bestimmt. Wir stellten einen signifikanten, jahreszeitlich bedingten Rückgang der zellvermittelten Immunantwort, der Tarsuslänge und der Überlebenswahrscheinlichkeit der Küken fest. Eine fehlende Kopplung zwischen Geschlecht und Schlupfdatum zeigte, dass beide Geschlechter gleichermaßen zum beobachteten Rückgang beitrugen. Das Geschlechterverhältnis innerhalb der Brut veränderte sich nicht mit dem Legedatum. Auf Grund der verfügbaren Daten verfechten wir die These, dass bei Arten mit geringem Geschlechtsdimorphismus in der Körpergröße das Brutdatum womöglich nur in Ausnahmefällen eine weibchengesteuerte Regulation des Geschlechterverhältnisses induziert. Daraus resultierend behaupten wir, dass bei Singvögeln eine jahreszeitliche Veränderung im Geschlechterverhältnis den jahreszeitlichen Rückgang der Nestlingsqualität nicht zu erklären vermag.
References
Alonso-Álvarez C, Tella JL (2001) Effects of experimental food restriction and body-mass changes on the avian T-cell-mediated immune response. Can J Zool 79:101–105
Bize P, Roulin A, Tella JL, Richner H (2005) Female-biased mortality in experimentally parasitized alpine swift Apus melba nestlings. Funct Ecol 19:405–413
Charnov EL (1982) The theory of sex allocation. Princeton University Press, N.J., USA
Christe P, Møller AP, de Lope F (1998) Immunocompetence and nestling survival in the house martin: the tasty chick hypothesis. Oikos 83:175–179
Cichoń M, Dubiec A (2005) Cell-mediated immunity predicts the probability of local recruitment in nestling blue tits. J Evol Biol 18:962–966
Cichoń M, Sendecka J, Gustafsson L (2005) Male-biased sex ratio among unhatched eggs in great tit Parus major, blue tit P. caeruleus, collared flycacther Ficedula albicollis. J Avian Biol 36:386–390
Cichoń M, Sendecka J, Gustafsson L (2006) Genetic and environmental variation in immune response of collared flycatcher nestlings. J Evol Biol 19:1701-1706
Cordero PJ, Viñuela J, Aparicio JM, Veiga JP (2001) Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J Evol Biol 14:829–834
Daan S, Dijkstra C, Weissing FJ (1996) An evolutionary explanation for seasonal trends in avian sex ratios. Behav Ecol 7:426–430
Dubiec A, Cichoń M (2001) Seasonal decline in health status of great tit (Parus major) nestlings. Can J Zool 79:1829–1833
Dubiec A, Cichoń M (2005) Seasonal decline in nestling cellular immunocompetence results from environmental factors—an experimental study. Can J Zool 83:920–925
Dubiec A, Cichoń M, Deptuch K (2006) Sex-specific development of cell-mediated immunity under experimentally altered rearing conditions in blue tit nestlings. Proc R Soc Lond B 273: 1759-1764
Ellegren H, Gustafsson L, Sheldon BC (1996) Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. Proc Natl Acad Sci USA 93:11723–11728
Ellegren H, Fridolfsson A-K (1997) Male-driven evolution of DNA sequences in birds. Nat Genet 17:182–184
Fargallo JA, Laaksonen T, Pöyri V, Korpimäki E (2002) Inter-sexual differences in the immune response of Eurasian kestrel nestlings under food shortage. Ecol Lett 5:95–101
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Griffith SC, Örnborg J, Russell AF, Andersson S, Sheldon BC (2003) Correlations between ultraviolet coloration, overwinter survival and offspring sex in the blue tit. J Evol Biol 16:1045–1054
Grossman C (1989) Possible underlying mechanisms of sexual dimorphism in the immune-response, fact and hypothesis. J Ster Biochem Mol Biol 34:241–251
Hõrak P, Tegelmann L, Ots I, Møller AP (1999) Immune function and survival of great tit nestlings in relation to growth conditions. Oecologia 121:316–322
Kölliker M, Heeb P, Werner I, Mateman AC, Lessels CM, Richner H (1999) Offspring sex ratio is related to male body size in the great tit (Parus major). Behav Ecol 10:68–72
Komdeur J, Daan S, Tinbergen J, Mateman AC (1997) Extreme adaptive modification in sex ratio of the Seychelles warbler’s eggs. Nature 385:522–525
Leech DI, Hartley IR, Steward IRK, Griffith SC, Burke T (2001) No effect of parental quality or extra-pair paternity on brood sex ratio in the blue tit (Parus caeruleus). Behav Ecol 12:674–680
Lepage D, Gauthier G, Menu S (2000) Reproductive consequences of egg-laying decisions in snow geese. J Anim Ecol 69:414–427
Lessels CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lessells CM, Mateman AC, Visser J (1996) Great tit hatchling sex ratios. J Avian Biol 27:135–142
Lochmiller RL, Vestey MR, Boren JC (1993) Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. Auk 110:503–510
Martin II LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M (2006) Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol 20:290–299
Moreno J, Merino S, Sanz JJ, Arriero E, Morales J, Tomás G (2005) Nestling cell-mediated immune response, body mass and hatching date as predictors of local recruitment in the pied flycatcher Ficedula hypoleuca. J Avian Biol 36:251–260
Nilsson J-Å (1999) Fitness consequences of timing of reproduction. In: Adams NJ, Slotov RH (eds) Proc Int Ornithol Congr 22:234–247
Pärt T, Qvarnström A (1997) Badge size in collared flycatcher predicts outcome of male competition over territories. Anim Behav 54:893–899
Perrins CM (1970) The timing of birds’ breeding seasons. Ibis 112:242–255
Potti J, Merino S (1996) Parasites and the ontogeny of sexual size dimorphism in a passerine bird. Proc R Soc Lond B 263:9–12
Potti J, Dávilla JA, Tella JL, Frias Ó, Villar S (2002) Gender and viability selection on morphology in fledgling pied flycatchers. Mol Ecol 11:1317–1326
Qvarnström A (1999) Genotype-by-environment interactions in the determination of the size of a secondary sexual character in the collared flycatcher (Ficedula albicollis). Evolution 53:1564–1572
Råberg L, Stjernman M, Nilsson J-Å (2005) Sex and environmental sensitivity in blue tit nestlings. Oecologia 145:496–503
Radford AN, Blakey JK (2000) Is variation in brood sex ratios adaptive in the great tit (Parus major)? Behav Ecol 11:294–298
Rosivall B, Török J, Hasselquist D, Bensch S (2004) Brood sex ratio adjustment in collared flycatchers (Ficedula albicollis): results differ between populations. Behav Ecol Sociobiol 56:346–351
Saino N, Ambrosini R, Martinell R, Calza S, Møller AP, Pilastro A (2002) Offspring sexual dimorphism and sex-allocation in relation to parental age and paternal ornamentation in the barn swallow. Mol Ecol 11:1533–1544
Sheldon BC, Merilä J, Lindgren G, Ellegren H (1998) Gender and environmental sensitivity in nestling collared flycatchers. Ecology 79:1939–1948
Sheldon BC, Ellegren H (1999) Sexual selection resulting from extra-pair paternity in collared flycatcher. Anim Behav 57:285–298
Silverin B, Sharp P (1996) The development of the hypothalamic-pituitary-gonadal axis in juvenile great tits. Gen Comp Endocrinol 103:150–166
Smits JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
Sorci G, Soler JJ, Møller AP (1997) Reduced immunocompetence of nestlings in replacement clutches of the European magpie (Pica pica). Proc R Soc Lond B 264:1593–1598
Tella JL, Donazar JA, Negro JJ, Hiraldo F (1996) Seasonal and interannual variations in the sex ratio of lesser kestrel Falco naumanni broods. Ibis 138:342–345
Tschirren B, Fitze PS, Richner H (2003) Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. J Anim Ecol 72:839–845
Verboven N, Visser ME (1998) Seasonal variation in local recruitment of Great Tits: the importance of being early. Oikos 81:511–524
Verboven N, Käkelä M, Orell M (2002) Absence of seasonal variation in great tit offspring sex ratios. J Avian Biol 33:138–142
Verhulst S, Tinbergen JM (1991) Experimental evidence for a casual relationship between timing and success of reproduction in the great tit Parus major. Ecology 60:269–282
Weatherhead PJ (1983) Secondary sex ratio adjustment in red-winged blackbirds (Agelaius phoeniceus). Behav Ecol Sociobiol 12:57–61
Acknowledgments
We are grateful to Nikolina Bąbała and Ludmiła Blachnicka for help during the fieldwork. Joanna Rutkowska gave valuable comments on an earlier version of the manuscript. The research project was funded by The State Committee for Scientific Research, Republic of Poland, in years 2004–2006, and some financial support was obtained from DS/WbiNoZ/InoS/757/06. The molecular analyses of sex were mostly performed at the University of Newcastle during a Marie Curie MOTIVE Scholarship to T.W., under the kind supervision of Kirsten Wolff. The study was conducted under the licence no. 33/OP/2004 from the Ethical Committee of Jagiellonian University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Wilk, T., Dubiec, A. & Cichoń, M. Seasonal decline in cell-mediated immunity of collared flycatcher Ficedula albicollis nestlings: does the sex of offspring matter?. J Ornithol 148, 199–205 (2007). https://doi.org/10.1007/s10336-006-0121-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-006-0121-1