Abstract
Objective
To investigate the effects of a range of parameter settings on T1 measurement stability in the portal vein using the T1-mapping sequences Look-Locker (LL) and Modified Look-Locker inversion recovery (MOLLI).
Materials and methods
Ten different versions of LL and MOLLI sequences were tested and compared to a reference sequence provided by the MR manufacturer. Ten healthy volunteers were imaged multiple times on two separate scan days at 3T. The mean T1 values and coefficient of variation (CoV) were calculated for each of the ten sequences and compared to the reference sequence.
Results
Six of the tested sequences had T1 values close to the reference sequence; among those, three sequences achieved lower CoV than the reference sequence. Lowest CoV was achieved using a non-triggered LL sequence with 5 beat readout and a 45o flip angle (mean T1 1733 ms ± 89 ms, CoV 1.3% ± 0.58%).
Conclusion
T1-measurements in the hepatic portal vein can be performed with high precision using either MOLLI or LL sequences provided that LL sampling duration is sufficiently long and flip angle sufficiently high. The advantage of constant timing outweighed the advantage of ECG-triggering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The hepatic portal vein contributes for two-thirds of the total hepatic blood flow and receives blood from the stomach, spleen, pancreas, small intestine, and the colon [1]. Per orally administered substances are transported from the gastrointestinal tract through the portal vein to the liver [1]. The portal vein, therefore, offers a location for detection and evaluation of uptake of substances from the gastrointestinal tract.
The proton T1 relaxation rate R1 (= 1/T1), of a solution is linearly dependent on concentration of dissolved paramagnetic ions [2]. Hence, repeated measurements of T1 in the portal vein before and after ingestion may offer a non-invasive quantitative method for studying uptake of per orally administered substances. The use of T1 measurements for quantification of substance uptake is challenging. Apart from physiologically induced variation in T1, the estimated T1 (\( \widehat{{T_{1} }} \)) may also depend on image noise, heart frequency, sequence-related parameters, and type of T1 mapping technique used [3,4,5,6,7]. Accurate (\( \widehat{{T_{1} }} \)) can be obtained using time-consuming saturation recovery methods; however, the length of these examinations makes them unsuited for in vivo applications, as the long scan times result in unacceptable length of breath hold. Therefore, faster alternative T1 mapping sequences have been introduced, where Look-Locker (LL) [8] and Modified Look-Locker inversion recovery (MOLLI) sequences [9] are currently the most established alternatives for in vivo use.
MOLLI was originally developed for cardiac applications, where cardiac triggering and breath holding are required to avoid severe motion artefacts. The need for cardiac triggering and breath hold put stringent restrictions on sequence parameters and optimal parameters for cardiac T1 mapping which are unlikely to be optimal for non-cardiac applications. Some work has been reported in the literature on optimizing T1 measurements in vessels, like jugular vein [10], carotid artery [11], and sagittal sinus [6]. In these studies, the effects of sequence parameters such as readout duration, heart rate [5, 9, 12], and flip angle [10, 11] have been evaluated.
To our knowledge, there is no literature on T1 measurements in the hepatic portal vein. Such measurements are subject to conditions that differ from cardiac T1 mapping in several aspects. In particular, cardiac triggering may not be critical in this region, which may allow for further improvement on measurement precision, in this study defined as reduced coefficient of variation (CoV), by adjusting relevant acquisition parameters.
The aim of this study was to investigate the effect of acquisition techniques, and relevant sequence parameters on the accuracy and precision of native T1 measurements in the portal vein. We specifically address parameters related to motion and flow, since limitations on these may be less stringent compared to conventional cardiac T1-assessments. We also address parameters related to sampling duration, as precise measurements of the long T1 of native blood may require longer read-out times than for myocardial tissue.
Theory
In both LL (Fig. 1a) and MOLLI (Fig. 1b), one or more inversion pulses are followed by a train of read-out segments, sampling the magnetization recovery curves. In LL, a series of low flip angle echo-planar-imaging (EPI) acquisitions is commonly used for readout following an initial IR pulse. Typically, high EPI acceleration and low flip angle are used to avoid saturation of the magnetization recovery. Studies have shown, however, that in flowing blood, the use of higher flip angles can be beneficial due to continuous inflow of unsaturated blood [10, 11].
a Simplified schematic sequence diagram of a Look-Locker (LL) sequence. A 180o inversion pulse used to invert the magnetization from Mz+ to Mz−. Following a waiting time of Timin a readout commences with an excitation pulse “α”, subsequent readout points are performed at Tiinc intervals. In the above case, a total of seven images are acquired with inversion times of Timin + (n – 1 × Tiinc), where n is the image number. Stationary tissue experiences a saturation effect as evident by the solid relaxation curve, while ROIs assessing inflowing blood do not experience this effect, visualised by the dotted line. To change the readout duration the repetition time, in this study given in number of beats, are either increased or decreased. If Tiinc is kept constant, an increase in readout duration also results in an increase in number of sampling points. Increasing the flip angle “α” would increase the saturation effect of stationary tissue, but not that of fast flowing blood. b Simplified sequence diagram of the Modified Look-Locker inversion recovery (MOLLI) sequence. Following a non-selective 180o inversion pulse, the first single shot balanced-SSFP readout is performed at a predefined trigger delay. The resulting image has an inversion time of Timin. For each subsequent cardiac cycle, one image is acquired with the same trigger delay. Tiinc is then defined by the lengths of the following heart cycles. Following the readout of the first inversion pulse (first cycle), a recovery period is allowed, in this example, 2 beats before a second cycle is performed and so on. For each new cycle, position of the inversion pulse is shifted relative to the trigger pulse spreading the sampling points on the recovery curve. In this example, five readouts are performed in the first cycle, followed by a recovery period of 2 beats and then three readouts in the second cycle. This results in the MOLLI scheme of 5(2)3 which has a total scan duration of 10 heartbeats and produces eight images with varying Ti. Increasing the number of beats in the longest cycle effectively increases the readout duration. The density of Ti can be increased, by adding additional cycles and the time allotted for free recovery can be increased by increasing the duration of the recovery period
MOLLI was initially developed to allow for T1 mapping of the heart. The readout is based on the flow-insensitive single-shot-balanced SSFP technique [13] and is fixed in a predefined cardiac phase to allow for pixelwise relaxation assessment. The time between subsequent measurements in MOLLI is then given by the duration of the cardiac cycle. Density of measurements may be increased by adding MOLLI cycles, i.e., repeating the inversion recovery experiment while shifting the timing of the inversion pulse with respect to cardiac phase. The MOLLI scheme 5(3)3 then describes a sequence with five images (one per heart beat) in the first cycle, followed by a pause of 3 heartbeats for magnetization recovery and then a second inversion pulse followed by three images; giving a total of eight inversion recovery sample points, and a breath-hold duration of 11 heartbeats.
Common for both LL and MOLLI sequences is that the magnetization recovery trajectory is influenced by the readout process and this needs to be corrected for to obtain correct \( \widehat{{T_{1} }} \) [8, 9, 14]. In addition, the use of cardiac triggering in both LL and MOLLI can result in a difference in read-out duration and timing of the sampling points based on the patient’s heart rate. For applications outside the heart, cardiac triggering may be less critical, thereby enabling a more flexible timing scheme. Based on the characteristics of the LL and MOLLI sequences, we identify readout duration, sequence timing, and flip angle to be the three main parameters (readily modified by the user) most affecting the quality and accuracy of the resulting T1-maps. These three parameters in the context of T1-mapping sequences will, therefore, be briefly discussed.
Readout duration
In both LL and MOLLI, one or more inversion pulses are followed by a train of readout segments sampling the magnetization recovery curves. The readout duration is defined by the number of triggering beats in LL and the longest cycle in the MOLLI scheme, as described in Fig. 1a, b. Readout duration should not be confused with; the time between inversions, which in LL is close to the readout duration, but higher in MOLLI due to the recovery phase, or the breath-hold time, which in this study is equal to the total scan time of the sequence and dependent on the number of k-space segments in LL and the MOLLI scheme. Increasing the readout duration, and hence number of readout points, gives additional information on the trajectory of the relaxation curve following the inversion pulse, potentially allowing for a more accurate assessment of T1, at the cost of longer breath holds. It is commonly accepted that the readout duration should ideally be in the order of five times T1 and that insufficient delay between inversion pulses could result in a \( \widehat{{T_{1} }} \) bias [15].
Triggering
The MOLLI Native 5(3)3 sequence is a breath-hold technique developed for T1 quantification of non-gadolinium enhanced cardiac tissue. It is based on a flow-insensitive single-shot true-FISP readout using electrocardiogram (ECG) triggering to lock readout to a predefined cardiac phase [9].
Since the time between measurements in MOLLI, and readout duration for both MOLLI and LL is given by the cardiac frequency, a shift in the frequency will change both the timing of sampling points and read-out duration. Such variation has been reported to make \( \widehat{{T_{1} }} \) heart rate dependent [5, 9, 15]. Deactivating ECG-triggering may prevent this dependency in \( \widehat{{T_{1} }} \) when imaging in areas without cardiac motion.
Flip angle
To minimize saturation effects in LL, a series of low flip angle echo-planar imaging (EPI) acquisitions is commonly used. The low flip angle mitigates the saturation effect, but does result in a lower signal-to-noise ratio (SNR) [10]. Studies have shown, however, that in veins and arteries, the use of higher flip angles to increase signal-to-noise ratio is possible due to continuous inflow of unsaturated blood [6, 10, 11].
Materials and methods
Ten male volunteers average age 28.8 (range 24–33 years) were recruited with the following inclusion criteria: male, age 20–35 years, no known liver or blood disease, no contraindications against MRI, and no medications. A written informed consent was signed by the volunteers, which subsequently attended two MRI sessions with a minimum of 1 week between. No dietary restrictions were given.
Examinations were performed using a Philips 3T Ingenia MR system (Philips Medical systems, Best, The Netherlands) with a 16-channel dStream Torso coil, and an embedded 16-channel posterior coil. Following T2-weighted breath-hold localizers the hepatic portal vein was identified, and the image plane was angulated perpendicular to the portal vein. During MR acquisitions, the volunteers were asked to hold their breath during the expiratory phase.
Initially seven variants of LL (Fig. 1a) and MOLLI (Fig. 1b) were included in the study. In lack of a gold standard, the vendor recommended MOLLI Native [MOLLI scheme 5(3)3] sequence was used as a reference, as this sequence has a proven reproducibility [16]. MOLLI variants were constructed by varying the length of the cycles in the MOLLI scheme while keeping scan duration short for maximum 30 s breath hold. LL variations included comparing readout durations of 3 and 5 beats and flip angles of 7o and 45o. Non-ECG-triggered variants of both MOLLI and LL sequences were implemented by simulating the ECG signal at a rate of 60 beats per min. Table 1 provides an overview of the sequences evaluated. After the fifth volunteer preliminary data were analysed. Four of the sequences were excluded from the study at this time due to resulting \( \widehat{{T_{1} }} \) values being significantly lower than those obtained with the reference sequence as well as values previously reported in the literature. Three additional sequences based on further optimization of the remaining sequences were then added (Table 1).
Phase-sensitive flow measurements (q-flow) were acquired at the start and end of all but five examinations giving a total of 35 flow measurements. Scan geometry was identical to that of the T1-mapping sequences.
Data analysis
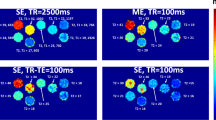
All T1 relaxation time calculations were performed in nordicICE (NordicNeuroLab, Bergen, Norway). Elliptical ROIs were placed in the portal vein in the raw images. The average signal from three ROI placements was used as input to the curve fit function in nordicICE to obtain \( \widehat{{T_{{1{\text{s}}}} }} \) for each scan. Figure 2 shows an example raw image with an ROI placed in the portal vein. When performing ROI placement as much as possible of the vein was included in the ROI, while visual artefacts and vessel wall were avoided.
Flow measurements were analysed using SEGMENT version 2.1 R 6078 (Medviso, Lund Sweden).
Statistical analysis
For each sequence s and examination e, an average of estimated T1 was calculated by the expression:
where i denotes the ith repetition of a given sequence, and n is the number of repetitions of a given sequence in an examination. Over all mean \( \overline{{T_{{1{\text{s}}}} }} \) was then calculated as the average of \( \overline{{T_{{1 {\text{es}}}} }} \) over all examinations. For all but one examination each sequence was repeated six times, in the final examination, only five repetitions were acquired.
To compare the longitudinal stability in the T1 measurements across all volunteers the mean, standard deviation (SD), and standard error of mean (SEM) for all sequences in all sessions were calculated. CoV was calculated for each sequence at each examination by
where s = sequence, e = examination.
The mean CoVs (\( \overline{{CoV_{\text{s}} )}} \) and SEM across all volunteers and examinations were then calculated for all sequences.
In cases where the veins could not be positively recognized, due to low SNR or artefacts in the image, the data point was defined as non-readable and removed from the data set.
Paired samples t test with Bonferroni correction was used to compare \( \overline{{T_{{1{\text{s}}}} }} \) and \( \overline{{CoV_{\text{s}} }} \) of the test sequences against the reference MOLLI Native sequence with the null hypothesis being that there are no significant differences between the test sequences and the reference sequence. The adjusted level of significance from Bonferroni correction was 0.005 for mean T1 and 0.01 for CoV. In addition, paired samples t test were performed to evaluate the effect of each parameter on CoV.
Flow curves were averaged over all scans to give average minimum, maximum, and mean velocity.
Results
Mean T1 values for all sequences analysed are shown in Fig. 3 and are summarized in Table 2. Six of the tested sequence variants reported T1 values that were not significantly different from the reference sequence (1759 ms, SEM = 26 ms). All variants of the LL with 3 beat readout sequences and one MOLLI sequence reported a significantly lower T1 than the reference sequence.
Plot of mean T1 values measured in volunteer’s hepatic portal vein for the ten sequences tested. Mean of the reference sequence with SEM is shown as solid grey line and dotted, respectively. Five sequences had \( \widehat{{T_{1} }} \) values similar to that of the reference sequence: LL 5 beat ECG-triggered 7o flip angle 1757 ms (± 26, p = 0.95), LL 5 beat ECG-triggered 45o flip angle 1720 ms (± 21, p = 0.14), LL 5 beat non-triggered 45o flip angle 1734 ms (± 28, p = 0.92), MOLLI 10(5)5; 1756 ms (± 19, p = 0.035), and MOLLI 10(5)5 non-triggered; 1771 ms (± 17, p = 0.018). The other five sequences had lower T1 than the reference sequence. LL 3 beat ECG-triggered 7o flip angle 1357 ms (± 19) p < 0.001, LL 3 beat ECG-triggered 45o flip angle 1340 ms (± 15, p < 0.001), LL 3 beat non-triggered 7o flip angle 1553 ms (± 15, p < 0.001), and LL 3 beat non-triggered 45o flip angle 1525 ms (± 16, p < 0.001). And MOLLI 10(1)1 ECG-triggered; 1702 ms (± 25, p = 0.0006)
\( \overline{{CoV_{\text{s}} }} \) for all sequence variants are shown in Fig. 4 and are summarized in Table 2. Among the sequences with T1 values close to the reference sequence, three sequence estimated \( \overline{{CoV_{\text{s}} }} \) which was lower than the reference sequence CoV of 2% (SEM = 0.171%) (Table 2), but the difference was not statistically significant 29. The lowest CoV was achieved using a non-triggered LL with 5 beat readout and 45o flip angle, with a CoV of 1.29%.
Plot of the average coefficient of variation (CoV) in % of the sequences. Mean of the reference sequence are presented as solid grey line with dotted lines representing SD. Among the sequences that had T1 similar to the reference sequence, \( \overline{{ CoV_{\text{s}} }} \) of three sequences were lower than the reference, but the difference was not statistically significant. These were: LL 5 beat non-triggered 45o flip angle; 1.29% (± 0.183%, p = 0.0597), MOLLI 10(5)5 ECG-triggered; 1.88% (± 0.245%, p = 0.73), MOLLI 10(5)5 non-triggered, and 1.86% (± 0.262%, p = 0.75)
Comparing the LL sequences with 3 beat readout to those with 5 beat readout (Fig. 5a), we found a lower \( \overline{{T_{{1{\text{s}}}} }} \) (1408 ms vs. 1737 ms, p < 0.001) and a non-significant increase in \( \overline{{CoV_{\text{s}} }} \) (3.24% vs. 2.83%, p = 0.22). Non-triggered sequences performed better in terms of precision than corresponding sequences with ECG-triggering with a significant increase in \( \overline{{T_{{1{\text{s}}}} }} \) (1643 ms vs. 1580 ms, respectively, p < 0.001) and a reduction in \( \overline{{CoV_{\text{s}} }} \) (2.2% vs. 2.9%, respectively, p = 0.0023) (Fig. 5b). Finally, increasing the flip angle from 7o to 45o resulted in an increase in precision with a \( \overline{{CoV_{\text{s}} }} \) reduction from 4.35 to 2.5%. (p < 0.001) (Fig. 5c) and a decrease in T1 from 1593 to 1574 ms, (p = 0.01).
Comparison of the CoV by individual parameters. The difference in CoV caused by readout duration a timing method, b flip angle, c shows a significant decrease in CoV for timing method (CoV 2.9% vs. 2.2%, paired samples t test p = 0.0023) and flip angle (CoV 4.35% vs. 2.5% p < 0.001). The decrease caused by increasing readout duration was, however, not significant (CoV 3.24% vs. 2.83%, paired samples t test p = 0.22)
A total of 13 scans were removed from the study due to artefacts caused by breath-hold challenges.
The flow results revealed a large range in flow measurements both at minimum 12 cm/s (range 8–21 cm/s) and at maximum 18 cm/s (range 13–26 cm/s) and at mean velocity 15.43 cm/s (range 10–24 cm/s). Paired samples t test among the examinations, where two flow measurements were acquired showed no significant differences in Vmin (p = 0,33), Vmax (p = 0.15), or Vmean (p = 0.23) between the two measurements.
Discussion
The results of the present study suggest that T1 of hepatic portal vein blood can be determined with high precision (\( \overline{{CoV_{\text{s}} }} \) < 1.3%). MOLLI acquisitions with longer cycles, and an optimized LL sequence performed slightly better than the reference sequence. We further found that improvements in precision can be made by increasing flip angle in LL, and by not using ECG-triggering in both LL and MOLLI.
If T1-mapping is to be used to quantify absorbed agents in portal vein, it is of importance that the T1 values measured are correct and reproducible. The measured T1 in blood of our reference sequence falls well within the range reported by the previous studies of T1 in blood at 3T (range 1618–1878 ms, average 1746 ms) [6, 10, 11, 17,18,19,20].
The shorter T1 estimates obtained with the LL sequences with 3 beat readout make these sequences unsuited for evaluation of T1 in blood. From visual inspection of the relaxation curves (data not shown), it is probable that the shorter T1 measured with 3 beats is a result of insufficient sampling time in relation with the target T1 relaxation times. As stated by both McRobbie et al. and Taylor et al., the readout duration should be in the order of 5 times T1 [15, 21]. It should also be recognized that the time between inversions in our LL sequences are close to equal to the readout duration and thus not allowing for a substantial time of free recovery, also in the MOLLI sequence the time allotted for free recovery is only equal to that of the recovery duration.
With the measured \( \overline{{T_{{1{\text{s}}}} }} \) from our reference sequence of 1759 ms, this results in a suggested readout duration of 8795 ms. A change from 5(3)3 in the reference sequence to 10(5)5 results in a doubling of the readout duration and an increase in free recovery from 3 to 5 heart beats. Assuming non-triggered acquisition with a simulated heart rate of 60 bpm, the time between inversion pulses will be 8 s for the benchmark sequence and 15 s for the 10(5)5 scheme. Especially, for long T1, values and faster heart rates, there will be a bias in T1 due to an incomplete relaxation recovery between the inversion pulses [15]. The sequences used in this study had scan durations ranging from 10.8 to 20.2 s and readout durations ranging from 3 to 10 s. It appears from the results that an increase in both scan and readout duration resulted in an increase of \( \bar{T}_{1} \). Increasing the readout duration, and thus the scan duration, allows for a higher number of sampling points in both LL and MOLLI sequences, which is likely to increase the precision of the T1 measurements. In a clinical setting, however, increasing scan time may provide increasing motion artefacts due to prolonged breath-hold requirements, which could offset the benefit of increasing readout duration observed in healthy volunteers. One may also argue that the through plane flow situation ensure-free recovery also during imaging and hence the need for additional time after readout only affect scans with short read-out durations. This may allow further improvement in scan efficiency when measurements are restricted to flowing blood.
With the slice thickness of 5 mm in our LL sequences, the observed flow velocities indicate a necessary spacing of approximately 40 ms in average and 62 ms for the lowest flow measured to allow for sufficient inflow of fresh blood to allow for the use of high flip angles.
As can be seen from both Fig. 4 and the statistical analysis, the LL with 5 beat readout and 45o flip angle trended towards a lower \( \overline{{CoV_{\text{s}} }} \) than the reference sequence. The lower \( \overline{{CoV_{\text{s}} }} \) of this sequence indicates a higher precision, making it more adept for longitudinal studies in the hepatic portal vein.
Some general assumptions on T1 measurements might be drawn from our data. Sufficient sampling duration is reported to be of great importance to avoid bias in \( \widehat{{T_{1} }} \). Non-triggered acquisitions result in both a fixed readout duration and a fixed spacing between readout timepoints. As shown by others [5, 9, 12], \( \widehat{{T_{1} }} \) is to some degree dependent on heart rate. Changes in patient heart rate during acquisition may, therefore, result in a change in \( \widehat{{T_{1} }} \) that could obscure, or mimic, real changes in T1. Patient compliance could potentially also be increased by not using ECG-triggering, since potential challenges with triggering signal are avoided. This does, however, not allow for a compensation of pulsatile flow and motion from the cardiac cycle. According to Gallix et al. [22], there are some uncertainties whether or not portal vein flow is continuous or pulsatile among healthy volunteers. As evident from our results, when performing T1 mapping in hepatic portal vein, the potential effect of pulsatile flow and motion is mitigated by the positive effects of fixed readout duration and timing. If LL is used, then increasing the flip angle appears to reduce \( \overline{{CoV_{\text{s}} }} \). This is in agreement with other studies such as Qin et al. [10] that uses a flip angle of 90o in their study. A prerequisite for using the higher flip angle is necessarily that the spacing of readout segments is higher than the time required for through slice flow. The spacing between readout segments in our LL protocols was 120 ms for the LL 3 beat and 200 ms for the LL 5 beat which is higher than the required 62 ms from the volunteer with the lowest flow velocity justifying the use of high flip angles.
Although not addressed directly in this study, there are some limitations when using a high flip angle for LL imaging. While this gave a significant reduction in \( \overline{{CoV_{\text{s}} }} \) in our study, it must be stressed that the high flip angle will result in increased saturation of stationary tissue and this sequence might, therefore, not be applicable if T1 measurements of both stationary tissue and blood are of interest. In this study, ROI measurements and subsequent T1 estimations were based on the raw data images and not parametric maps. We have not performed a stringent analysis of the differences in \( \widehat{{T_{1} }} \) and CoV depending on the method of analysis; however, a parametric map-based analysis was also performed on these data and a scatterplot comparing the two methods can be seen in Fig. 1 in supplementary materials.
Limitations
Although the scan protocol was repeated in each volunteer several times in succession, and on two separate days, only ten volunteers were included, limiting the statistical power. Furthermore, the volunteers were young and healthy and may not be directly comparable to older patients, where, e.g., breath-hold capacity may be an issue. In addition, the volunteers were not asked to follow any dietary restrictions. This may result in a change of the true T1 during scanning; however, such an effect should be equal for all sequences and, therefore, not significantly alter the results of our variation analysis. A further limitation is the absence of a gold standard sequence for validation of the accuracy of the T1 measurements. We do, however, believe that the choice of using the vendor supplied MOLLI 5(3)3 as a benchmark sequence for comparison is a good compromise as this sequence is well established in clinical practice and has been thoroughly validated [16].
Conclusion
In conclusion, T1-measurements in the hepatic portal vein may be performed with comparable accuracy and precision using either the MOLLI sequences or LL sequences described in this study. Portal vein blood flow is sufficiently fast with little pulsation to allow the use of high flip angle readout without cardiac triggering. Increasing read-out duration beyond that of the standard MOLLI showed only a small effect on both \( \widehat{{T_{1} }} \) and CoV. Among the sequences tested in this study, a non-triggered Look-Locker with 5 beat readout, 45o flip angle had the lowest CoV.
References
Ganong WF (1999) Review of medical physiology, 19th edn. Appleton & Lange, Stamford
Shen Y, Goerner FL, Snyder C, Morelli JN, Hao D, Hu D, Li X, Runge VM (2015) T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol 50(5):330–338
Liu P, Chalak LF, Krishnamurthy LC, Mir I, Peng SL, Huang H, Lu H (2016) T1 and T2 values of human neonatal blood at 3 Tesla: dependence on hematocrit, oxygenation, and temperature. Magn Reson Med 75(4):1730–1735
Zhang X, Petersen ET, Ghariq E, De Vis JB, Webb AG, Teeuwisse WM, Hendrikse J, van Osch MJ (2013) In vivo blood T(1) measurements at 1.5 T, 3 T, and 7 T. Magn Reson Med 70(4):1082–1086
Piechnik SK, Ferreira VM, Lewandowski AJ, Ntusi NA, Banerjee R, Holloway C, Hofman MB, Sado DM, Maestrini V, White SK, Lazdam M, Karamitsos T, Moon JC, Neubauer S, Leeson P, Robson MD (2013) Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson 15(13):11
Varela M, Hajnal JV, Petersen ET, Golay X, Merchant N, Larkman DJ (2011) A method for rapid in vivo measurement of blood T1. NMR Biomed 24(1):80–88
Treibel TA, Nasis A, Fontana M, Maestrini V, Castelletti S, Bhuva AN, Rosmini S, Abdel-Gadir A, Bulluck H, Kellman P, Piechnik SK, Robson MD, Moon J (2015) An instantaneous ECV with no blood sampling: using native blood T1 for hematocrit is as good as standard ECV. J Cardiovasc Magn Reson 17(1):Q129
Look DC, Locker DR (1970) Time saving in measurement of NMR end EPR relaxation times. Rev Sci Instrum 41:250–251
Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP (2004) Modified Look-Locker inversion recovery (MOLLI) for high-resolution T 1 mapping of the heart. Magn Reson Med 52(1):141–146
Qin Q, Strouse JJ, van Zijl PC (2011) Fast measurement of blood T 1 in the human jugular vein at 3 Tesla. Magn Reson Med 65(5):1297–1304
Li W, Liu P, Lu H, Strouse JJ, van Zijl PC, Qin Q (2016) Fast measurement of blood T 1 in the human carotid artery at 3T: accuracy, precision, and reproducibility. Magn Reson Med 77(6):7
Vassiliou V, Heng EL, Nyktari E, Greiser A, Firmin D, Pennell DJ, Gatehouse P, Prasad SK (2015) Effect of temperature and heart rate variability on Phantom T1 maps. J Cardiovasc Magn Reson 17(1):W24
Scheffler K, Lehnhardt S (2003) Principles and applications of balanced SSFP techniques. Eur Radiol 13(11):2409–2418
Deichmann RH, Haase A (1992) Quantification of T1 Values by SNAPSHOT-FLASH NMR Imaging. J Magn Reson 96(3):608–612
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M (2016) T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging 9(1):67–81
Kellman P, Hansen MS (2014) T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 16(1):2
Kalavagunta C, Michaeli S, Metzger GJ (2014) In-vitro Gd-DTPA relaxometry studies in oxygenated venous human blood and aqueous solution at 3 and 7T. Contrast Media Mol Imaging 9(2):169–176
Shimada K, Nagasaka T, Shidahara M, Machida Y, Tamura H (2012) In vivo measurement of longitudinal relaxation time of human blood by inversion-recovery fast gradient-echo MR imaging at 3T. Magn Reson Med Sci 11(4):265–271
Wu W-C, Jain V, Li C, Giannetta M, Hurt H, Wehrli FW, Wang DJJ (2010) In vivo venous blood T(1) measurement using inversion recovery true-FISP in children and adults. Magn Reson Med 64(4):1140–1147
Zhang X, Petersen ET, Ghariq E, Vis JB, Webb AG, Teeuwisse WM, Hendrikse J, Osch MJP (2013) In vivo blood T1 measurements at 1.5 T, 3 T, and 7 T. Magn Reson Med 70(4):1082–1086
McRobbie DW (2007) MRI from picture to proton, 2nd edn. Cambridge University Press, Cambridge
Gallix BP, Taourel P, Dauzat M, Bruel JM, Lafortune M (1997) Flow pulsatility in the portal venous system: a study of Doppler sonography in healthy adults. Am J Roentgenol 169(1):141–144
Acknowledgements
Grant support: this project is funded by the Research Council of Norway.
Author information
Authors and Affiliations
Contributions
SASV design and planning of the study, acquisition of data, analysis and interpretation of data, drafting of manuscript, and critical revision. PKH design and planning of the study, drafting of manuscript, and critical revision. AB design and planning of the study, drafting of manuscript, and critical revision. MA-M design and planning of the study, drafting of manuscript, and critical revision. CH design and planning of the study, drafting of manuscript, and critical revision. THS design and planning of the study, drafting of manuscript, and critical revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10334_2018_731_MOESM1_ESM.jpg
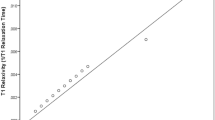
Supplementary Figure 1 Comparison of the apparent T1 and CoV depending on the method of analyzation. Y-axis are measurements made by the method described in the methods section. X-axis are measurements made in parametric maps. As evident from the figure, the measurements are well comparable between the two methods of analyzation.

Rights and permissions
About this article
Cite this article
Vatnehol, S.A.S., Hol, P.K., Bjørnerud, A. et al. Precision of T1-relaxation time measurements in the hepatic portal vein: influence of measurement technique and sequence parameters. Magn Reson Mater Phy 32, 359–368 (2019). https://doi.org/10.1007/s10334-018-00731-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-018-00731-1