Abstract
Quantification of the spatial needs of individuals and populations is vitally important for management and conservation. Geographic information systems (GIS) have recently become important analytical tools in wildlife biology, improving our ability to understand animal movement patterns, especially when very large data sets are collected. This study aims at combining the field of GIS with primatology to model and analyse space-use patterns of wild orang-utans. Home ranges of female orang-utans in the Tuanan Mawas forest reserve in Central Kalimantan, Indonesia were modelled with kernel density estimation methods. Kernel results were compared with minimum convex polygon estimates, and were found to perform better, because they were less sensitive to sample size and produced more reliable estimates. Furthermore, daily travel paths were calculated from 970 complete follow days. Annual ranges for the resident females were approximately 200 ha and remained stable over several years; total home range size was estimated to be 275 ha. On average, each female shared a third of her home range with each neighbouring female. Orang-utan females in Tuanan built their night nest on average 414 m away from the morning nest, whereas average daily travel path length was 777 m. A significant effect of fruit availability on day path length was found. Sexually active females covered longer distances per day and may also temporarily expand their ranges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecologists are interested in animal movement as an important process in population dynamics. Over time the focus has shifted from studying temporal fluctuations in abundance to more spatially explicit approaches of individual movements (Patterson et al. 2008). A central question when analysing animal movements is how observed patterns of animal distribution are determined by interactions between individuals and their environment (Börger et al. 2006). A useful approach is to understand the dynamics of animal movements in relation to social and ecological factors (Benson et al. 2006; Robbins and McNeilage 2003; Harvey et al. 2008). As most animals use the same areas repeatedly over time, movement patterns are often defined using the home range concept. Burt (1943, p. 351) defined the home range as “that area traversed by the individual in its normal activities of food gathering, mating, and caring for young”. The home range can be defined more quantitatively by using the animal’s utilization distribution. Van Winkle (1975) defined this as “the two-dimensional relative frequency distribution for the points of location of an animal over a period of time”. The utilization distribution is an estimate of the probability the animal has been at a certain place and can be used to predict where an animal occurred but was not observed (Horne and Garton 2006b).
Although home range is a common concept in analysing animal space use, there is considerable debate in the scientific literature on how it should be measured (Börger et al. 2006). Several methods for estimating home range size exist and their number is still increasing (Horne and Garton 2006a). However, choosing one model over another is difficult, because all have disadvantages and the resulting estimates of home-range size may vary markedly depending on which method is chosen (Girard et al. 2002; Boyle et al. 2009; Grueter et al. 2009). The importance of objectively selecting models and variables in order to make meaningful comparisons between different studies analysing animals’ spatio-temporal behaviour has been highlighted before (Laver and Kelly 2008) and researchers are therefore urged to carefully report their methods.
Orang-utans primarily feed on fruit, when available, but also consume leaves, bark, flowers and insects (Knott 1998; Morrogh-Bernhard et al. 2009). Requiring large amounts of calories, they spend approximately half of their day feeding, but activity budgets differ between sites. Generally, orang-utans in peat swamp forests spend more than half of their active time feeding whereas those in mixed-dipterocarp forests where masting occurs feed <50% of the time (Morrogh-Bernhard et al. 2009). Apart from mother–infant dyads, Bornean orang-utans (Pongo pygmaeus wurmbii) are fairly solitary animals occupying highly overlapping individual home ranges. Whereas female home ranges are assumed to be affected by ecological factors and reflect the distribution of food sources, male range use is seen as a response to the distribution of females (Singleton et al. 2009). Reliable estimates of male home ranges are difficult to obtain, because the range size generally exceeds the size of study areas. However, even if no estimates are possible, home ranges of adult males (both flanged and unflanged) are several times larger than female ranges in the same population (Singleton et al. 2009). Range use of the Sumatran species (Pongo abelii) has been shown to be linked to seasonal patterns of fruit availability (te Boekhorst et al. 1990; Singleton and van Schaik 2001). Orang-utans at Suaq Balimbing followed fruiting peaks in different types of swamp forest and during mast fruiting events moved into the hills. Their home ranges therefore encompassed a variety of habitats from lowland peat swamp forest to hill forests, and were estimated to be at least 800 ha (Singleton and van Schaik 2001). For Bornean orang-utans, Leighton and Leighton (1983) observed changes in the frequency of sightings of orang-utans that were, at least in part, related to changing food abundance. In general, however, less is known about the seasonal ranging patterns of orang-utans in Borneo than in Sumatra.
The objective of this study was to fill this gap by providing quantitative measures of orang-utan ranging behaviour in a peat swamp forest in Central Kalimantan, Borneo. The central questions addressed are:
-
How can female orang-utan home ranges be effectively modelled?
-
How do range estimates differ according to the home range model chosen?
-
Do environmental factors such as seasonality affect spatio-temporal behaviour of orang-utans?
-
How do female orang-utans change their ranging behaviour with reproductive state?
-
How stable is range use over different years?
This study focused exclusively on female orang-utans, because male orang-utans have much larger home ranges and sample size was not sufficient for accurate range estimates in any of the studies to date.
Methods
Study site
The Tuanan field station is located in the Tuanan Mawas reserve in Central Kalimantan, Indonesia (2.151° South; 114.374° East). The research area lies within a peat swamp forest that was heavily disturbed by selective logging in the early 1990s and by subsequent informal logging, but still supports a relatively high density of orang-utans of ca. 4.25 individuals per km2 (van Schaik and Brockman 2005). The study site consists of about 750 ha of a grid-based trail system of manually cut transects, marked every 50 m.
Since 2003, numerous researchers and students have contributed to the data pool of the orang-utan network project by collecting data on the wild orang-utans in the area. Data are collected during focal animal follows, if possible from night nest to night nest, using a standardized field procedure. Every 2 min the behaviour of the focal animal is noted (http://www.aim.uzh.ch/orangutannetwork/FieldGuidelines.html). In addition, a map of the animal’s path is drawn by hand, using transect marks and compass directions. The follow maps are then digitized. In order to assess the accuracy of existing follow maps, GPS records and maps of the same follow days were compared and accuracy was found to be satisfactory for subsequent analysis (Wartmann 2008).
Home range models
In the past, the minimum convex polygon (MCP) method was often used in home range modelling. The MCP method geometrically defines the home range as the convex hull around a set of point locations. However, using the MCP method for home range modelling has been criticised (Börger et al. 2006). First and foremost it has the undesirable property that biases increase as sample sizes increase (Burgman and Fox 2003). Another problem is that it assumes uniform space use within the home range boundaries. However, animals are unlikely to use all parts of their home range with the same intensity and thus important information on differential space use within the range is lost (Katajisto and Moilanen 2006). Despite these limitations and a range of alternatives, the MCP method is still widely used (Börger et al. 2006), although few studies report primate ranging based solely on MCP estimates (but see Kaplin 2001; Savini et al. 2008). Most include other home range estimators besides MCP (Grueter et al. 2009; Norscia and Borgognini-Tarli 2008; Neri-Arboleda et al. 2002; Newton-Fisher 2003).
One of these alternatives is the statistical technique of kernel density estimation that was introduced as a home range model by Worton (1989). It provides a probabilistic measure of animal space use (Horne and Garton 2006b) in which the density at any location is an estimate of the amount of time an animal spent there. The input data for a kernel estimator are the recorded animal observations which are assumed to be temporally independent of each other. The objective of kernel density estimation is then to arrive at a density estimate for any location within the bounding box of the observations. First, a grid is superimposed on the study area with a predefined resolution constrained by the density of observations and, for large data sets, computation time. For every grid cell, all observations are averaged within a given kernel bandwidth (radius), whereby typical kernel functions weight the contributions of observations according to distance from the grid point, for example, through a bivariate normal function (Silverman 1986). As kernel density estimations are sensitive to bandwidth (also called “smoothing parameter”), different techniques exist to objectively select this parameter (Kernohan et al. 2001). Narrow kernel bandwidths allow nearby observations to have the greatest influence on the density estimate and thus reveal the small-scale detail in data. Wide kernel bandwidths allow distant observations more influence and show the general shape of the distribution (O’Sullivan and Unwin 2003; Seaman and Powell 1996).
Kernel density estimation thus allows one to distinguish different parts of the animal’s range according to intensity of use. Currently, kernel methods are the prevalent method in wildlife biology for estimating home ranges. In primatology, researchers have also begun to incorporate kernel methods for range estimates, mainly as an addition to MCP or grid cell methods (Neri-Arboleda et al. 2002; Newton-Fisher 2003; Fashing et al. 2007; Norscia and Borgognini-Tarli 2008). In their review of home range studies in wildlife biology, Laver and Kelly (2008) found 60% of studies reporting ranges with kernel methods, with 21% of studies solely relying on kernel methods. The problem for home range estimates based on kernel methods is that a large variety of smoothing factors, kernels, and sample sizes leads to a potentially large number of possible combinations for the kernel method (Gitzen et al. 2006). However, if consistent reporting standards are adhered to, comparability between studies may be ensured (Laver and Kelly 2008). In this paper our objective is to contribute to establishing these reporting guidelines.
Comparing home range estimators
From the maps, the location of an individual focal animal was recorded every half hour during focal follows, yielding a total of between 1016 and 6709 points per individual, for seven focal adult females. Recording of point locations started at the orang-utan nest for individuals that had been followed the previous day or when an individual was found. Recordings ended at the night nest or when the individual was lost. Home range was calculated using fixed kernel methods and the MCP, using data from the four most often observed adult females, with a minimum of 1000 observation hours each. Six different sample sizes (25, 50, 100, 500, 1000, and 2000) were analysed for the different models. A random subsample from all locations obtained for each individual between 2003 and 2007 was selected using Hawth’s analysis tools (Beyer 2004), an extension to ArcGIS v. 9.2 (ESRI, Redlands, CA, USA). To explore the effect of length of study period, we calculated ranges for one individual based on an increasing number of consecutive observations. Thus, as the number of observations increased, we have a proxy for increasingly long study periods and their influence on home range calculation using MCP and kernel methods. To compare the effect of sample size from a long-term study, these ranges were contrasted with those calculated with the same number of observations drawn randomly from all observations. This comparison was carried out using a set of 4000 observations for a single individual (Juni) collected over a total period of 6 years.
The MCP was calculated using the method implemented in the home range tool extension (Rodgers et al. 2007) to ArcGIS that enabled calculation of a range with 95% of all points selected by a “floating mean” algorithm (Carr and Rodgers 1998). The kernel method used was fixed kernel as implemented in the home range tool extension to ArcGIS. Because variance in x and y coordinates of orang-utan location data was unequal, they were automatically rescaled with a unit variance before applying the smoothing parameter selection. Least-squares cross validation (LSCV; Silverman 1986; Worton 1995) smoothing parameter selection is currently the recommended smoothing parameter selection in the ecological literature (Seaman et al. 1999), but it has been found to have several drawbacks (Kernohan et al. 2001). For example, LSCV was criticised for its high variability and its tendency to under-smooth location data (Horne and Garton 2006b). Furthermore, it was reported to fail to compute for large sample sizes (Hemson et al. 2005). This was also the case for orang-utan location data. Biased cross-validation (BCV) proved to be robust for large sample sizes also, and was therefore used as the method to select smoothing parameters. BCV as implemented in the HRT tool extension to ArcGIS calculates a bandwidth value that minimizes the estimated asymptotic mean integrated square error (AMISE) (Carr and Rodgers 1998). The default raster resolution size of 150 m for kernel contours was used, because lower values would have resulted in substantially increased calculation time.
Annual ranges
To assess whether ranges remained stable over multiple years for female orang-utans, annual ranges were calculated for five females from 2003 to 2007. A total of more than 29000 locations (~14500 observation hours) were used. Based on comparisons of different home range estimators with real location data of orang-utans, using the information-theoretic approach (Horne and Garton 2006a), the method selected to define the annual range was fixed kernel density estimation. Range sizes reported are based on 90% and core areas based on 50% volume contours, as 95% volume contours were found to overestimate range sizes by increasing range estimates based on few observations. Commonly the 50% contour is chosen as an objective boundary in home range studies to delineate areas of higher use referred to as core areas. For example, 89% of evaluated home range studies using kernel estimates reported core areas based on 50% contours (Laver and Kelly 2008).
As orang-utans are extremely long-lived animals (Wich et al. 2004), studies covering a complete lifetime of ranging do not exist to date. Therefore, it is important to clearly state the time frame of the study for which ranging analyses were conducted. In this study, years were used as a time frame, allowing for comparisons with other studies. Furthermore, seasons that reflected fruit abundance in the area were used as a more biologically informed time frame to analyse orang-utan ranging with regard to food sources. Shorter time frames, for example weeks or months, would not relate so directly to fruiting, and in the case of weeks would have limited numbers of observations available. The sample size for each female and year was on average 1210 points (±440).
The issue of autocorrelation for home range studies has led to considerable debate in the scientific literature. Autocorrelation is said to pose a problem in home range studies because n autocorrelated observations are less informative than n independent observations, because in autocorrelated data variances will be underestimated and thus statistically derived home range estimates will also be underestimated (Swihart and Slade 1985). However, based on simulated data De Solla et al. (1999) concluded that independence of observations is not a prerequisite for kernel estimations and counselled against “destructive random subsampling” until statistical independence is reached, because they found this also removed biologically meaningful information.
In this study, subsets of up to 300 observation points were tested for autocorrelation before home ranges were calculated, using an autocorrelation index developed by Swihart and Slade (1985). This index was then used to compare the sensitivity of home ranges based on different sample sizes and thus also subject to varying degrees of autocorrelation.
Range overlaps
Annual range and core area sizes alone do not necessarily convey a complete picture of orang-utan ranging over the years, because years may not be ecologically valid time units for these long-lived animals with birth intervals of 7 years or more (Wich et al. 2004), and because home ranges may gradually shift over time. Range overlaps for the same individual between different years show which parts of the range were used over two or more consecutive years. Average range overlap for the same individual was calculated as the percentage of the annual range in year t contained in range in year t + 1. Moreover, overlaps between individuals show how much of the range is shared with other females. Dyadic overlaps between individuals were calculated as the intersection between the two respective annual ranges and core areas.
Comparison with other sites
To facilitate comparisons with studies from other sites where home ranges were calculated for the entire study period, ranges are also reported based on all collected point location data from 2003 to 2007 with kernel, MCP, and grid cell count methods. In the grid cell method, a grid is overlaid on the study site and the sum of the grid cells where observations were recorded provides an estimate of range size. For the grid cell counts two different grid sizes were used, namely 25 × 25 m and 50 × 50 m.
Travel distances
The calculation of daily path lengths and distances between consecutive nests yields important information on animal space use on a daily scale. Daily path length is defined as the total distance an individual orang-utan travels per day, from the moment it leaves its nest in the morning to the moment it builds the nest for the next night. In this study, daily path lengths are approximated by summing the distances between all half-hour locations for a follow day. Nest distance is defined as the Euclidian distance between two consecutive night nests. Given the large number of orang-utan location data that have been collected so far, a manual approach to data analysis was not feasible. Therefore, a software solution was designed and a programme implemented for this work in the Java programming language (Arnow et al. 2004) to automatically calculate daily path lengths and nest distances for individual orang-utans. Only full follow days (n = 972) were considered in the analysis to avoid bias due to incomplete, and therefore shorter path lengths.
Reproductive state of female orang-utans
Periods of sexual activity of female orang-utans were estimated from the likely or known dates of birth of their offspring (van Noordwijk and van Schaik 2005), and from data on sexual behaviour, defined as females engaging in voluntary or female-initiated sexual activity in any given month (Mitra Setia and van Schaik 2007). Following this definition, the female Kerry was sexually active from March 2004 to July 2005 and from March 2006 to June 2006. The female Juni was sexually active from January 2004 to May 2005.
Seasonality
In a phenology plot, 1611 numbered trees have been surveyed by various members of the project team once a month since 2003 to assess the productivity of the forest. As an index of habitat-wide fruit abundance, the fruit availability index (FAI) was used (FAI = 100 × number of trees carrying fruit/total number of trees in the plot), i.e. the percentage of trees in a plot that carry fruit in a specific month. The monthly FAI values were automatically classified into three classes using quantiles (low FAI = 0.066–3.148, medium FAI = 3.148–6.090, high FAI = 6.091–13.986). The three classes of fruit availability were later used to analyse daily path lengths. To analyse seasonality in range use however, “low” and “medium” fruit availability were aggregated into one class. These categories produced fairly long and continuous periods of the two different levels of fruit abundance, rather than short-term alterations, enabling us to calculate ranges for each class. Habitat-wide fruit availability was then used to define two levels of fruit availability in Tuanan: a period of low to medium fruit abundance indicating food scarcity and a period of high fruit availability indicating food abundance.
Results
Comparison of MCP and kernel methods
With the MCP method, home range size estimates increased with increasing sample size. Mean range size for four females increased from 138 ha (±69) calculated with 25 sub-sampled observation points to 287 ha (±103) with 2000 sub-sampled observation points. For example, for the female Mindy, home range size almost tripled from smallest to largest sample size (Table 1). For three out of four females, no asymptote of range size was reached, even with 2000 points. Variation due to sample size was much reduced when using fixed kernel estimates. On average, the smallest ranges were estimated with a subsample of 25 points (242 ha ± 86) and the largest with 100 points used (299 ha ± 83). With kernel methods range sizes decreased slightly at higher sample sizes.
Comparison of the two sub-sampling regimes (one sub-sampled from all observations and one cumulative number of subsequent observations) in Fig. 1 shows that the increase in estimated range size is much more pronounced if cumulative observations are used rather than locations sub-sampled from a longer period of time. Neither kernel nor MCP methods can therefore substitute for a long-term data collection procedure for these orang-utans.
According to Swihart and Slade’s (1985) index, all samples >50 were significantly autocorrelated. If only night nests are used and time steps between successive observations were larger than 24 h, autocorrelation was still present in the data, but only for sample sizes larger than 100. Thus if only night nests were used as sub-samples, values of Swihart and Slade’s index of autocorrelation were reduced, but data was still significantly autocorrelated according to these indices. Ranges calculated with a fixed kernel for the more autocorrelated samples yielded larger home ranges (301.79 ha ± 118.00, n = 12) than ranges calculated with less autocorrelated or independent locations (278.09 ha ± 90.87, n = 12), but differences were not significant (Mann–Whitney U, Z = −0.404, p > 0.05). There was thus no significant effect of autocorrelation on range size estimates found using kernel methods.
Statistical analysis of estimated range sizes across models, individuals and sample sizes showed that differences in home range size estimates between individuals were significant across models and sample sizes (Kruskal–Wallis, χ 2 = 40.744, p < 0.05). Differences between home range models were significant (Kruskal–Wallis, χ 2 = 19.766, p < 0.05). Sample size correlated with home range estimates for the MCP method (Spearman’s rho = 0.569, p < 0.05), but not for kernel methods (Spearman’s rho = −0.101, p > 0.05). In general, model type and the individual study animal were thus important factors in explaining differences in home range sizes. Sample size was an important factor in the MCP method, but not in fixed kernel estimates.
Annual ranges and range overlap
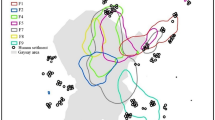
During the course of any year, female orang-utans in Tuanan used an area of approximately 200 ha (90% contour).
The size of annual home ranges did not differ between years (Kruskal–Wallis, χ 2 = 1.719, p > 0.05) but they were significantly different between individuals (Kruskal–Wallis, χ 2 = 11.213, p < 0.05). The females with the largest ranges and also the largest variation in annual range sizes were those that had been sexually active during the study period (Kerry and Juni, Fig. 2). Mindy consistently had the smallest annual ranges. Spearman’s correlation showed no effect of total sample size on annual area estimates (Spearman’s rho = 0.13, p > 0.05). Core areas (defined as the continuous area(s) in which an individual spends half its time) were on average 65 ha large, amounting to 33% of the annual range. Thus, during half the time, female orang-utans occupied only a third of their annual range.
Average range overlap for the same individual between two consecutive years was high at 76.38% (±13.19). We could not demonstrate that home ranges gradually shifted over the years, as the correlation between range overlap and time interval did not reach significance, despite adequate sample size (Spearman’s rho = −0.287, n = 40, p = 0.073). This suggests that adult female ranges remain relatively stable over a period of several years.
Comparison with other sites
To compare results with those from other study sites for which different estimators were used, we also calculated home ranges for the entire study period with three different methods (Table 2). For three out of four females, grid cell counts provided the smallest and most conservative estimates of home range size with both grid sizes (50 × 50 m and 25 × 25 m). For the female Mindy range estimates were larger with grid cell counts (50 m cell size) than with kernel or MCP, because the grid cell count included infrequently visited areas in the home range that were not included in the 90% kernel estimate. MCP range sizes were largest for the three females and overestimated range size by including large unused areas.
Total sample size did not have a direct effect on range estimates, because Mindy, with small range estimates, was the second most observed female.
Daily path lengths and nest distances
Distances between morning and night nest on the same day were measured as the direct line between the two nests. On average, orang-utan females in Tuanan built their night nest 413.85 m away from the morning nest (±220.58, n = 972; Table 3). Significant individual variation among nest distances was observed (Kruskal–Wallis, χ 2 = 42.523, p < 0.05).
On average, a female in Tuanan travelled 777.21 m per day (±402.39, n = 972, min = 84 m, max = 2691 m). Differences between individuals were significant (Kruskal–Wallis, χ 2 = 59.655, p < 0.05). There was no significant correlation between a female’s annual home range size and her mean daily path length per year (Spearman’s rho = 0.321, p > 0.05, n = 24).
Seasonality in range use
Mean range size for individuals seemed smaller when fruit was abundant (158.23 ha ± 58) than when it was scarce (197.34 ha ± 85), but differences were not statistically significant (Mann–Whitney U, Z = −1.703, p > 0.05). This was confirmed by a general linear model (GLM) with the factors “fruit availability” and “individual” and their interactions. The model was significant (ANOVA, F = 3.335, p < 0.05) with an R 2 value of 0.509. The factor individual was significant (F = 5.347, p < 0.05), with a high partial eta-squared value of 0.424 (the partial eta-squared value is an indicator of the relative importance of a factor, with values between 0 and 1). The factor “fruit availability” with the two levels “high” and “medium to low” was not significant in the model (F = 3.124, p > 0.05), neither was the interaction of individual and level of fruit availability (F = 0.897, p > 0.05). The GLM indicates that the individual variation in ranges is more important than seasonal influences. Average overlap of seasonal ranges between individuals seemed higher when fruit was scarce (72.98 ha ± 41.29, n = 45) than when fruit was abundant (60.43 ha ± 33.36, n = 26), but again these differences were not significant (ANOVA, F = 1.740, p > 0.05). Core range overlap was larger when fruit was scarce (8.05 ha ± 10.99, n = 45) than when fruit was abundant (5.24 ha ± 7.30, n = 26), but not significantly (Kruskal–Wallis, χ 2 = 0.729, p > 0.05). In general, orang-utan females share almost a third of their seasonal range with any other female, but use intensively used core areas more exclusively.
However, total daily travel path lengths correlated positively with FAI (Spearman’s rho, correlation coefficient = 0.225, p < 0.05), indicating that the more fruit was available, the further orang-utans travelled during the day. With fruit availability was low, mean daily travelled distance was 694.80 m (±348.49, n = 393). In months with medium fruit availability, distances were, on average, 822.04 m (±456.85, n = 297). In months with high fruit availability, distances travelled per day were largest with 844.84 m (±392.46, n = 282). Differences in travel distance between the three levels of fruit availability were significant (Kruskal–Wallis, χ 2 = 33.780, p < 0.05).
Reproductive state and ranging
Daily path lengths and nest distances were analysed according to reproductive state of the females, divided into two categories of sexually active/not active. The only two females that were sexually active during the study period were Juni and Kerry, and only these two individuals were analysed. Differences between these two females in total daily travelled paths were not significant (Mann–Whitney U, Z = −0.428, p > 0.05). On the other hand, differences in daily path lengths between reproductive states were remarkable. When not sexually active, the females travelled 703.76 m on average (±342.46, n = 206), whereas when they were sexually active they travelled 1124.21 m per day (±502.25, n = 101), which is an increase of 60% in daily path length. Differences between daily path length in different reproductive states were significant (Mann–Whitney U, Z = −7.539, p < 0.05). Orang-utan females in Tuanan thus covered substantially greater distances when sexually active.
Discussion
Estimating home range size
In this study, we compared two home range methods (MCP and fixed kernel) by analysing the effect of sample sizes on model results. The problem associated with the MCP method was clearly apparent. With the MCP method, range sizes increased with increasing sample sizes. The MCP method underestimated range size for small sample sizes and overestimated ranges for large sample sizes by including unused areas in the convex hull.
In the kernel method we used BCV as an objective, automated method to select smoothing parameters. We found BCV to strike a balance between oversmoothing and undersmoothing and it was also robust at large sample sizes. Using this automated approach, kernels smooth locations more at small sample sizes and less with increasing sample size. This procedure resulted in more stable range estimates irrespective of sample size. Indeed, range sizes decreased slightly for the highest sample sizes. This effect can, in part, be attributed to autocorrelation, which is known to lead to underestimated range sizes (Swihart and Slade 1985). We found that different levels of autocorrelation did not significantly affect home range size estimates. The choice of 150 m as the kernel grid size was based on considerations of data accuracy on the one hand, as the cell size for the kernel grid should not be lower than the accuracy of the data, and computation time on the other hand. In our case, this choice yielded satisfactory results, but other cell sizes may also be used, taking into account the properties of the data used and the total home range size for the study animal.
Comparison of results from different home range models, parameters, and sample sizes showed that all factors affected range estimates and introduced uncertainties into model estimates. However, differences between individuals remained consistent regardless of sample size or method (MCP versus kernel). This indicates that comparisons between studies are possible, but only if prerequisites for comparative studies are met, i.e. that similar models and sample sizes are used, emphasising the need to present detailed information on ranging data and analytical methods.
The MCP method has been shown to have several severe methodological shortcomings (Burgman and Fox 2003). Nevertheless, it is still used, most often in combination with other models (Laver and Kelly 2008). First, it needs a large sample size to reach asymptotic home range sizes. However, in this study asymptotic home range sizes were not reached, even with sample sizes as high as 2000 points, and despite the fact that home ranges did not shift significantly over time. This finding indicates that orang-utans use their home range rather extensively, as expected given the high spatio-temporal variability of fruit availability. Second, the MCP method assumes uniform range use within the convex hull, and is therefore unable to account for multiple centres of activity. Third, it relies on outlying, extreme points as parts of the convex hull, leading to inclusion of rare “excursions” outside the regular home range. Researchers have tried to solve these problems by excluding outlying points with various methods. These techniques exclude a percentage of outlying points based on a distance criterion (e.g. distance from arithmetic mean of all point locations). However, the biological rationale for these “point-peeling-techniques” is weak, and Kernohan et al. (2001) recommend kernel estimators as a technique less sensitive to outliers and therefore preferable. Finally, the MCP method yielded suboptimal home range estimates, even if subsampling from a larger data set (Fig. 1). The various constraints of the MCP method have led researchers to advise against its use as a home range size estimator (Börger et al. 2006).
The grid cell method (White and Garrot 1990), like the MCP method, has long been favoured for its simplicity. Although grid cell count methods are capable of accounting for multiple centres of activity and are not affected by autocorrelation (Kernohan et al. 2001), they are sensitive to outliers and dependant on cell size. As opposed to the grid cell counts, kernel estimates are based on a utilization distribution that describes the frequency distribution over a specific time (van Winkle 1975). Regardless of the method, sample size plays a major role in the adequacy of the home range estimate (Fig. 1). There is no analytical substitute for adequate sample size, i.e. length of study period. For instance, increasing the cell size in the grid cell method will not increase the adequacy of the home range estimate.
In their review, Kernohan et al. (2001) compared the most common home range estimators based on different criteria such as sensitivity to sample size and outliers. They found kernels to outperform other estimators such as MCP and grid cell counts. However, the drawback of kernel methods is their lack of comparability, which was said to be an advantage of MCP methods (Laver and Kelly 2008). Therefore, many studies have applied two home range estimators (for recent examples see Moyer et al. 2007, Molinari-Jobin et al. 2007, and Fashing et al. 2007). However, there is an emerging consensus that use of the MCP method in wildlife biology and ecology as a home range size estimator has little future (Börger et al. 2006).
For comparisons across studies the focus should lie on devising reliable guidelines and standards for kernel methods as has previously been suggested (Laver and Kelly 2008). These guidelines should be biologically informed, taking into account the mobility of animals, the tendency for home ranges to shift, possible seasonal shifts in home range location, the animals’ tendency to move out of regular range when in estrus, etc. Researchers studying the same species should try to agree on methods used so that comparisons across studies will be possible. As a minimum, every study using kernel home range method should:
-
report sample size used for home range estimates;
-
use fixed rather than adaptive kernels (Seaman et al. 1999; Kernohan et al. 2001);
-
use automated methods for smoothing parameter selection, and report smoothing parameter values;
-
estimate ranges over biologically meaningful temporal scales and include temporally consistent periods (e.g. annual range); and
-
report resolution of the kernel grid used.
In this study we used a sample size of 300 locations for home range estimates, with a fixed kernel and 90% volume contour. BCV was used as the automated method to select the kernel smoothing parameter. We used a resolution of 150 m for the kernel grid. Ranges were estimated both for years and seasons that were defined according to an FAI (Fig. 3).
Comparison with other sites
The results from this study fit well with reported variation in orang-utan subspecies, with Pongo pygmaeus morio (Borneo) having the smallest ranges, Pongo pygmaeus wurmbii (Borneo) having intermediate ranges, and Pongo abelii (Sumatra) having the largest (Table 4).
For example, in Sumatra at the Suaq Balimbing study site, Singleton and van Schaik (2001) reported estimated female home range sizes of 850 ha based on the MCP method. In contrast, mean home range in Tuanan was 280 ha (range 172–379 ha, if estimated with MCP).
Home range sizes seem to be considerably smaller in Tuanan than they are in Suaq. This can be attributed to different factors. It was argued that the low species richness of the Suaq swamp results in a clumped distribution of fruiting tree species, leading orang-utans to use a larger area to maintain an adequate diet (Singleton and van Schaik 2001), e.g. the orang-utan diet at Suaq contains 61 plant species, whereas the swamp forest in Tuanan contains around 125 species (C. P. van Schaik and I. Singleton, unpublished data).
Knott et al. (2008) reported home ranges from Gunung Palung, Borneo with different grid-cell methods and MCP. Polygons based on 100% of locations gave estimates of 595 ha for Gunung Palung. For Tuanan, polygons based on 95% of points gave estimates of 280 ha. Because it is impossible that the remaining 5% of observations in Tuanan would double the estimated home range size, this difference between Gunung Palung and Tuanan is real. However, to develop reliable estimates of the actual differences in range size, we would need to analyse the raw data sets with the same method.
Differences between the reported means may be attributed to differences in habitat quality and population density between the sites. For some sites, much larger home ranges are reported, even if they harbour the same subspecies. For example Gunung Palung has larger range estimates than Tuanan and Sabangau (all P. p. wurmbii) (Singleton et al. 2009). The most likely explanation for this variation is the nature of the habitat mosaic. Whereas habitats are rather homogeneous in Tuanan and Sabangau, the habitat mosaic is more heterogeneous in both Gunung Palung and Suaq Balimbing. The Suaq and Gunung Palung sites both contain several distinct habitat types, i.e. swamp and dryland forests on a mosaic scale that can be traversed by individuals with one or 2 days’ travel (Singleton et al. 2009).
Differences in home range sizes between sites are therefore likely to be due to factors such as fruit species-richness of the habitat and nature of the heterogeneity of the habitat mosaic.
Sexual activity and range use
As had been noted before for Sumatran orang-utans (van Schaik 2004), sexually active females strongly increased their activity level and also moved outside their regular home range. This may imply that sexually active females range more widely in order to ensure meeting the best possible mates, or alternatively that being sexually active, and thus assured of male interest, allows them to move into areas they cannot normally visit.
Seasonality and range use
A key point of this study was to apply spatio-temporal models to analyse orang-utan movements. Orang-utans primarily feed on fruit when it is abundant (Knott 2005). Therefore, seasons were divided according to fruit availability. As was shown by comparing seasonal ranges, ranges remained rather stable irrespective of fruit abundance. However, marked differences were found between seasons of high and low fruit abundance in the daily travel distance and distance between consecutive night-nests. When fruit was scarce, orang-utans foraged more on vegetative matter and travelled shorter distances. On the other hand when fruit was abundant, they significantly increased travel distances. Orang-utan females thus do show seasonal changes in their feeding and ranging behaviour. It is well known that in times of relative food abundance, orang-utans travel more, visiting different trees when they bear fruit or flowers, which results in larger travel and nest distances (Knott 2005; Wich et al. 2006). They can afford to eat less vegetative matter because they have better, energy-rich food available. In times of fruit scarcity, on the other hand, they feed more on relatively low-energy foods such as leaves, pith, and inner bark (Knott 1998). Those food sources are less spatially dispersed and can therefore be exploited by spending comparatively less energy on travel. What this study showed, however, is that those responses are not reflected in range size, but rather in how the range is used. Thus, at higher food abundance, individuals travel further within the same home range. This study provides an example of integrating both spatial and behavioural data to analyse orang-utan movement patterns.
Because male orang-utans have much larger ranges than females and are difficult to follow, little is known about their movements. Moreover, because sexually mature males can be flanged or unflanged, which is accompanied by major differences in mating strategy (van Schaik 2004), another remaining question is how flanged and unflanged males differ in their ranging behaviour. The objective of future research should thus be to fill this gap in our knowledge by integrating behavioural and movement analyses of male orang-utans.
References
Arnow D, Dexter S, Weiss G (2004) Introduction to programming using java: an object-oriented approach, 2nd edn. Pearson Education, Boston
Benson JF, Chamberlain MJ, Leopold BD (2006) Regulation of space use in a solitary felid: population density or prey availability? Anim Behav 71:685–693
Beyer HL (2004) Hawth’s analysis tools for ArcGIS. http://www.spatialecology.com/htools. Accessed 24 Apr 2008
Börger L, Franconi N, De Michele G, Gantz A, Meschi F, Manica A, Lovari S, Coulson T (2006) Effects of sampling regime on the mean and variance of home range size estimates. J Anim Ecol 75:1393–1405
Boyle SA, Lourenço WC, da Silva LR, Smith AT (2009) Home range estimates vary with sample size and methods. Folia Primatol 80:33–42
Burgman MA, Fox JC (2003) Bias in species range estimates from minimum convex polygons: implications for conservation and options for improved planning. Anim Conserv 6:19–28
Burt WH (1943) Territoriality and home range concepts as applied to mammals. J Mammol 24:346–352
Carr AP, Rodgers AR (1998) HRT: the home range extension for ArcView™ (beta test version 0.9, July 1998), users’ manual. (http://planet.uwc.ac.za/nisl/computing/HRE/Tutorial%20Guide.pdf. Accessed 8 May 2008)
De Solla S, Bondurianskz R, Brooks RJ (1999) Eliminating autocorrelation reduces biological relevance of home range estimates. J Anim Ecol 68:221–234
Fashing PJ, Mulindahabi F, Gakima JB, Masozera M, Mununura I, Plumptre AJ, Nguyen N (2007) Activity and ranging patterns of Colobus angolensis ruwenzorii in Nyungwe Forest, Rwanda: possible costs of large group size. Int J Primatol 28:529–550
Girard I, Ouellet JP, Courtrois R, Dussault C, Breton L (2002) Effects of sampling effort based on GPS telemetry on home-range size estimations. J Wildl Manag 66:1290–1300
Gitzen RA, Millspaugh JJ, Kernohan BJ (2006) Bandwidth selection for fixed-kernel analysis of animal utilization distributions. J Wildl Manag 70:1334–1344
Grueter CC, Li D, Ren B, Wei F (2009) Choice of analytical method can have dramatic effects on primate home range estimates. Primates 50:81–84
Harvey V, Côté SD, Hammill MO (2008) The ecology of 3-D space use in a sexually dimorphic mammal. Ecography 31:371–380
Hemson G, Johnson P, South A, Kenward R, Ripley R, MacDonald D (2005) Are kernels the mustard? Data from global positioning system (GPS) collars suggests problems for kernel home-range analyses with least-squares cross-validation. J Anim Ecol 74:455–463
Horne JS, Garton EO (2006a) Selecting the best home range model. An information-theoretic approach. Ecology 87:1146–1152
Horne JS, Garton EO (2006b) Likelihood cross-validation versus least squares cross-validation for choosing the smoothing parameter in kernel home-range analysis. J Wildl Manag 70:641–648
Kaplin BA (2001) Ranging behavior of two species of guenons (Cercopithecus lhoesti and C. mitis doggetti) in the Nyungwe Forest Reserve, Rwanda. Int J Primatol 22:521–548
Katajisto J, Moilanen A (2006) Kernel-based home range method for data with irregular sampling intervals. Ecol Model 194:405–413
Kernohan BJ, Gitzen RA, Millspaugh JJ (2001) Analysis of animal space use and movements. In: Millspaugh JJ, Marzluff JM (eds) Radio tracking and animal populations. Academic Press, San Diego, pp 125–166
Knott CD (1998) Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int J Primatol 19:1061–1079
Knott CD (2005) Energetic responses to food availability in the great apes: implications for Hominin evolution. In: Brockman DK, van Schaik CP (eds) Primate seasonality: implications for human evolution. Cambridge University Press, London, pp 351–378
Knott CD, Beaudrot L, Snaith T, White S, Tschauner H, Planasky G (2008) Female-female competition in Bornean orangutans. Int J Primatol 29:975–997
Laver PN, Kelly MJ (2008) A critical review of home range studies. J Wildl Manag 72:290–298
Leighton M, Leighton DR (1983) Vertebrate responses to fruiting seasonality within a Bornean rain forest. In: Sutonn SL, Whitmore TC, Chadwick AC (eds) Tropical rain forest: ecology and management. Special Publication No. 2 of the British Ecological Society, Blackwell, Oxford, pp 181–195
Mitani JC (1989) Orangutan activity budgets: monthly variations and the effects of body size, parturition and sociality. Am J Primatol 18:87–100
Mitra Setia T, van Schaik CP (2007) The response of adult orang-utans to flanged male long calls: Inferences about their function. Folia Primatol 78:215–226
Molinari-Jobin A, Zimmermann F, Ryser A, Breitenmoser-Würsten A, Capt S, Breitenmoser U, Molinari P, Haller H, Eyholzer R (2007) Variation in diet, prey selectivity and home-range size of Eurasian lynx (Lynx lynx) in Switzerland. Wildl Biol 13:393–405
Morrogh-Bernhard HC, Husson SJ, Knott CD, Wich SA, van Schaik CP, van Noordwijk MA, Lackman-Ancrenaz I, Marshall AJ, Kanamori T, Kuze N, Bin Sakong R (2009) Orangutan activity budgets and diet. A comparison between species, populations and habitats. In: Wich SA, Utami Atmoko SS, Mitra Seta T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, Oxford, pp 119–133
Moyer MA, McCown JW, Oli MK (2007) Factors influencing home-range size of female Florida black bears. J Mammol 88:468–476
Neri-Arboleda I, Stott P, Arboleda NP (2002) Home ranges, spatial movements and habitat associations of the Philippine tarsier (Tarsius syrichta) in Corella, Bohol. J Zool 257:387–402
Newton-Fisher NA (2003) The home range of the Sonso community of chimpanzees from the Budongo Forest, Uganda. Afr J Ecol 41:150–156
Norscia I, Borgognini-Tarli SM (2008) Ranging behavior and possible correlates of pair-living in southeastern Avahis (Madagascar). Int J Primatol 29:153–171
O’Sullivan D, Unwin DJ (2003) Geographic information analysis. Wiley, Hoboken
Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J (2008) State–space models of individual animal movement. Trends Ecol Evol 23:87–94
Robbins MM, McNeilage A (2003) Home range and frugivory patterns of mountain gorillas in Bwindi Impenetrable National Park, Uganda. Int J Primatol 24:467–491
Rodgers AR, Carr AP, Beyer HL, Kie JG (2007) HRT: home range tools for ArcGIS. Version 1.1. Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research, Thunder Bay, Ontario, Canada. http://blue.lakeheadu.ca/hre/. Accessed 29 Apr 2008
Savini T, Boesch C, Reichard UH (2008) Home-range characteristics and the influence of seasonality on female reproduction in white-handed gibbons (Hylobates lar) at Khao Yai National Park, Thailand. Am J Phys Anthropol 135:1–12
Seaman DE, Powell RA (1996) An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 77:2075–2085
Seaman DE, Millspaugh JJ, Kernohan BJ, Brundige GC, Raedeke KJ, Gitzen RA (1999) Effects of sample size on kernel home range estimates. J Wildl Manag 63:739–747
Silverman BW (1986) Density estimation for statistics and data analysis. Chapman & Hall, London
Singleton I, van Schaik CP (2001) Orangutan home range size and its determinants in a Sumatran swamp forest. Int J Primatol 22:877–911
Singleton I, Knott CD, Morrogh-Bernard HC, Wich SA, van Schaik CP (2009) Ranging behaviour of orangutan females and social organization. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, Oxford, pp 205–213
Swihart RK, Slade NA (1985) Testing for independence of observations in animal movements. Ecology 66:1176–1184
te Boekhorst IJA, Schürmann CL, Sugardjito J (1990) Residential status and seasonal movements of wild orang-utans in the Gunung Leuser Reserve (Sumatera, Indonesia). Anim Behav 39:1098–1109
van Noordwijk MA, van Schaik CP (2005) Development of ecological competence in Sumatran orangutans. Am J Phys Anthropol 127:79–94
van Schaik CP (2004) Among orangutans: red apes and the rise of human culture. Harvard University Press, Belknap
van Schaik CP, Brockman D (2005) Seasonality in primate ecology, reproduction, and life history: an overview. In: Brockman DK, van Schaik CP (eds) Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge University Press, London, pp 3–20
van Winkle W (1975) Comparison of several probabilistic home-range models. J Wildl Manag 39:118–123
Wartmann FM (2008) Seasonality in spatio-temporal behaviour of female orangutans. A case study in Tuanan Mawas, Central Kalimantan, Indonesia. Master thesis, University of Zurich, Switzerland (unpublished)
White G, Garrot R (1990) Analysis of wildlife radio tracking data. Academic Press, San Diego
Wich SA, Utami-Atmoko SS, Mitra Setia T, Rijksen HD, Schürmann C, van Hoof JA, van Schaik CP (2004) Life history of wild Sumatran orangutans (Pongo abelii). J Hum Evol 47:385–398
Wich SA, Geurts M, Setia TM, Utami-Atmoko SS (2006) Influence of fruit availability on Sumatran orangutan sociality and reproduction. In: Hohmann G, Robbins MM, Boesch C (eds) Feeding ecology in apes and other primates. Ecological, physical and behavioural aspects. Cambridge University Press, London, pp 335–356
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Worton BH (1995) Using Monte Carlo simulation to evaluate kernel-based home range estimators. J Wildl Manag 59:794–800
Acknowledgments
This study was conducted in the framework of the Memorandum of Understanding between Universitas Nasional Jakarta (UNAS) and the Anthropological Institute and Museum of the University of Zurich. Travel costs and fieldwork were financially supported by the A.H. Schultz Foundation. We acknowledge the Director General of PHKA, BKSDA Palangkaraya, the Direktorat Fasilitasi Organisasi Politik dan Kemasyarakatan, Departemen Dalam Negeri, the Indonesian Institute of Science (LIPI), the Institute of Research and Technology (RISTEK) and the Indonesian Embassy in Switzerland for granting research permission, the Bornean Orang-Utan Survival Foundation (BOS) and MAWAS, Palangkaraya, for hosting the project in the MAWAS reserve, and our colleagues at UNAS for support and collaboration. Many thanks to all field assistants: Hadi, Kumpo, Pak Rahmat, Tono, and Yandi for sharing their knowledge and to all previous students and assistants for data collection. We thank Maria van Noordwijk for the many interesting discussions and Claude Rosselet for his perseverance in entering maps. We thank three anonymous reviewers for comments on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wartmann, F.M., Purves, R.S. & van Schaik, C.P. Modelling ranging behaviour of female orang-utans: a case study in Tuanan, Central Kalimantan, Indonesia. Primates 51, 119–130 (2010). https://doi.org/10.1007/s10329-009-0186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-009-0186-6