Abstract
This review is intended to provide plant pathologists and other scientists with a current overview of the most important Fusarium phytopathogens and mycotoxin producers. Knowledge of Fusarium species diversity and their evolutionary relationships has increased dramatically due to the application of multilocus molecular phylogenetics and genealogical concordance phylogenetic species recognition over the past 15 years. Currently Fusarium is estimated to comprise at least 300 genealogically exclusive phylogenetic species; however, fewer than half have been formally described. The most important plant pathogens reside in the following four groups: the F. fujikuroi species complex noted for Bakanae of rice, ear rot of maize, pitch canker of pine and several species that contaminate corn and other cereals with fumonisin mycotoxins; the F. graminearum species complex including the primary agents causing Fusarium head blight of wheat and barley that contaminate grain with trichothecene mycotoxins; the F. oxysporum species complex including vascular wilt agents of over 100 agronomically important crops; and the F. solani species complex, which includes many economically destructive foot and root rot pathogens of diverse hosts. Several other Fusarium phytopathogens reported from Japan and nested within other species complexes are reviewed briefly. With the abandonment of dual nomenclature, a broad consensus within the global community of Fusarium researchers has strongly supported the unitary use of the name Fusarium instead of several teleomorph names linked to it. Plant pathologists and other scientists needing accurate identifications of Fusarium isolates are encouraged to use Fusarium-ID and Fusarium MLST, Internet accessible websites dedicated to the molecular identification of Fusarium species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
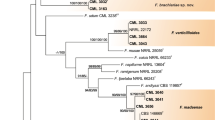
The fungal genus Fusarium Link includes many well-known plant pathogens of agricultural crops, as well as those producing mycotoxins. Collectively, Fusarium comprises ~300 phylogenetically distinct species that have been discovered via molecular phylogenetics; however, most of these species have not yet been described formally (Fig. 1). Many Fusarium species are soil-borne and, depending on the ecological context, may be parasites, endophytes, or pathogens of healthy host plants. Historically, the foundation for Fusarium taxonomy was based on phenotypic characters (Booth 1971; Gerlach and Nirenberg 1982; Nelson et al. 1983; Wollenweber and Reinking 1935). However, over the past two decades, molecular systematics studies that have employed multilocus DNA sequence data to assess species limits (Taylor et al. 2000) have revolutionized our understanding of species diversity and phyletic relationships within Fusarium (reviewed by Geiser et al. 2013; O’Donnell et al. 2013). Here, we review the morphological and molecular systematic status of the most important phytopathogenic groups and briefly summarize some opportunities and challenges that face the field.
Phylogenetic relationships of key Fusarium species based on analyses presented in O’Donnell et al. (2013). As Asian clade, Af African clade, Am American clade of the F. fujikuroi species complex, as defined by O’Donnell et al. (1998a); clades 1, 2 and 3 of the F. solani species complex as defined by O’Donnell (2000), FGSC The F. graminearum species complex as discussed herein. Arrows and question mark represent two hypotheses regarding the phylogenetic limits of Fusarium. The F. dimerum and F. ventricosum species complexes are highlighted in grey due to their uncertain inclusion within Fusarium (Geiser et al. 2013)
Molecular systematics has revolutionized Fusarium taxonomy
Fusarium species are ascomycetous fungi that belong to the Nectriaceae, Hypocreales, Sordariomycetes. The genus Fusarium was first described by Link in 1809 (Mag. Ges. naturf. Freunde, Berlin 3:10) and sanctioned by Fries in 1821 (Systema Mycologicum 1: XLI. Introductio). Before January 1, 2013, when dual nomenclature was abandoned, parallel usage of asexual Fusarium anamorph names was allowed for species with sexual teleomorph stages in Gibberella Sacc. and several other related hypocrealean genera. Because of changes in the new International Code of Nomenclature for algae, fungi, and plants (ICNafp) adopted at the International Botanical Congress in Melbourne, Australia in 2011, creation of both anamorph and teleomorph names are not allowed for Fusarium and other fungi after January 1, 2013 (Hawksworth 2012), and the preexisting anamorph and teleomorph names are to be unified. This change has been welcomed overwhelmingly by plant pathologists and other applied biologists who support the unitary use of Fusarium over the various teleomorphs (Geiser et al. 2013).
Before 1997, Fusarium systematics and taxonomy was based exclusively on phenotypic characters observed in cultures of isolates obtained from diseased plants, animals including humans and diverse substrates such as soil and water. The first significant advance in the taxonomic studies of Fusarium, titled “Die Fusarien”, was published by Wollenweber and Reinking (1935), in which they recognized 16 subgeneric sections (now obsolete because they are artificial or nonmonophyletic), 65 species, 55 varieties and 22 forms. This publication also provided basic methods for collecting and evaluating phenotypic data for taxonomic studies of the genus. Although Wollenweber and Reinking (1935) provided a detailed method for morphological species recognition (MSR, hereafter) within Fusarium, many plant pathologists and other applied-end users, especially outside of Europe, did not adopt this highly differentiated system because it required microscopic study involving detailed measurements of conidia and additional morphological characters. In what is now recognized as a scientifically unfounded counter reaction to Wollenweber and Reinking (1935), Snyder and Hansen (1940, 1941, 1945) promoted a system in which only nine species of Fusarium were recognized. Their system, based on the premise that a plant pathologist should be able to identify Fusarium species by visual inspection of agar cultures in Petri dishes, was clearly an over-simplification. Unfortunately, because of its convenience and ease for putting names on unknowns, Snyder and Hansen’s system was widely accepted among plant pathologists in much of the world. Booth (1971) modified the Wollenweber and Reinking system by proposing an intermediate taxonomy. Subsequently, Gerlach and Nirenberg (1982) expanded the Wollenweber and Reinking system to 78 morphologically distinct species. By contrast, Nelson et al. (1983) conservatively expanded the Snyder and Hansen system as the basis of their taxonomy. The impact of how biological and phylogenetic species recognition has augmented MSR within Fusarium will be discussed in the next paragraph.
Biological species recognition (BSR, hereafter), based on the ability of crosses between isolates to produce fertile ascospore progeny, has been applied with limited success within Fusarium. Laboratory mating studies have identified seven biological species within the Fusarium solani species complex (Matuo and Snyder 1973; VanEtten and Kistler 1988) and 13 within the F. fujikuroi species complex (Hsieh et al. 1977; Kuhlman 1982; Leslie 1995; Lima et al. 2012). While these studies have helped inform taxonomic revision, molecular phylogenetics studies have revealed that fewer than one quarter of the taxa within these species-rich complexes has a known teleomorph (Nirenberg and O’Donnell 1998; O’Donnell 2000; O’Donnell et al. 1998a, 2008a). By contrast, phylogenetic species recognition, based on the discovery of genealogical exclusive species level lineages by GCPSR (genealogical concordance phylogenetic species recognition; Taylor et al. 2000), has had an enormous impact on Fusarium systematics (O’Donnell 2000; O’Donnell and Cigelnik 1997; O’Donnell et al. 1998a, 2000a, b, 2004). Moreover, by employing a GCPSR framework, the strengths and limitations of MSR and BSR can be assessed independently and objectively. Not surprisingly, GCPSR-based studies have revealed that MSR sometimes resolves most or all phylogenetic species (e.g., Aoki and O’Donnell 1999; Aoki et al. 2001, 2003, 2005, 2012a, b; Nirenberg and O’Donnell 1998), whereas in recently evolved groups such as the type B trichothecene toxin-producing Fusarium species (O’Donnell et al. 2013), MSR and BSR fail to resolve most of the phylogenetically distinct species (O’Donnell et al. 2004; Sarver et al. 2011; Starkey et al. 2007). Because molecular phylogenetic studies have consistently revealed that the subgeneric sectional classification of Fusarium, proposed by Wollenweber and Reinking (1935) as followed and modified by Booth (1971) and adopted by Nelson et al. (1983), is highly artificial (i.e., not monophyletic; Kristensen et al. 2005), it has been replaced by monophyletic species complexes discovered in a comprehensive molecular phylogeny (Fig. 1; O’Donnell et al. 2013). Lastly, morphological and molecular phylogenetic data support the transfer of two unrelated, Fusarium-like plant pathogens from the Hypocreales to the Xylariales: Fusarium nivale Ces. ex Berl. & Voglino, now recognized as Microdochium nivale (Fr.) Samuels & I.C. Hallett; and Fusarium tabacinum (J.F.H. Beyma) W. Gams, reclassified as Plectosporium tabacinum (J.F.H. Beyma) M.E. Palm, W. Gams & Nirenberg. In the following section, a brief overview of the four most important phytopathogen-containing species complexes within Fusarium will be presented.
Most plant pathogens are nested within four species complexes
-
(1)
Fusarium fujikuroi species complex (FFSC; Fig. 1): These species induce Bakanae disease of rice, ear rot of maize, pitch canker of pine, and contaminate corn and other cereals with fumonisin mycotoxins
Most species within the F. fujikuroi species complex, as we now know it, would have been recognized as F. moniliforme Sheld. in Synder and Hansen’s nine species system (Snyder and Hansen 1941), or as members of Wollenweber and Reinking’s section Liseola. By way of contrast, up to nine species were recognized within section Liseola in other taxonomic systems of Fusarium (Booth 1971; Gerlach and Nirenberg 1982; Nelson et al. 1983; Wollenweber and Reinking 1935). The development of mating type testers (Hsieh et al. 1977; Kuhlman 1982; Leslie 1995; Lima et al. 2012) enabled the identification of 13 biological species within the FFSC. Conidial anamorphs of the biological species within this complex have been described formally: F. verticillioides (Sacc.) Nirenberg, F. sacchari (E.J. Butler & Hafiz Khan) W. Gams, F. fujikuroi Nirenberg, F. proliferatum (Matsush.) Nirenberg ex Gerlach & Nirenberg, F. subglutinans (Wollenw. & Reinking) P.E. Nelson, Toussoun & Marasas, F. thapsinum Klittich, J.F. Leslie, P.E. Nelson & Marasas, F. nygamai L.W. Burgess & Trimboli, F. circinatum Nirenberg & O’Donnell, F. konzum Zeller, Summerell & J.F. Leslie, F. xylarioides Steyaert, F. musae van Hove, Waalwijk, Logrieco & Ant. Moretti, F. temperatum Scaufl. & Munaut., and F. tupiense Lima, Pfenning & Leslie. The practice of referring to these species as mating populations A–L of Gibberella fujikuroi (Sawada) S. Ito should be abandoned because only F. fujikuroi is conspecific with this teleomorph (Seifert et al. 2003). In addition to F. fujikuroi causing bakanae disease of rice and F. circinatum inducing pitch canker disease of Pinus luchuensis Mayr, the following FFSC have been recorded as plant pathogens from Japan: F. fractiflexum T. Aoki, O’Donnell & K. Ichik. for yellow spot of Cymbidium spp. (Aoki et al. 2001), F. guttiforme Nirenberg & O’Donnell for black leaf spot of Odontoglossum spp., F. phyllophilum Nirenberg & O’Donnell for purple spot of Aloe spp., F. proliferatum for purple spot of Basella alba L. and kernel rot of corn, F. sacchari for pokkah boeng of sugarcane, and F. verticillioides for kernel rot of corn (PSJ and NIAS 2012). The 13 FFSC species that are known to reproduce sexually are all heterothallic.
Comparative morphological and molecular phylogenetic studies (Geiser et al. 2005; Nirenberg and O’Donnell 1998; O’Donnell et al.1998a, 2000b) have revealed that the FFSC is represented by over 50 phylogenetically distinct species that comprise three biogeographically structured clades. Because the name F. moniliforme has been applied to numerous species within the FFSC, by broad consensus, the community working with Fusarium recommended that this name not be used (Seifert et al. 2003).
The gibberellin-induced “bakanae” or “foolish seedling” disease of rice (Oryza sativa L.), which is induced by F. fujikuroi, is characterized by symptoms that include exceptionally tall seedlings that fall over before they mature and flower, pale yellow seedlings, and stunted roots. Diseased plants may die in the nursery or paddy, drastically reducing rice yields throughout the Asian continent. The disease was reported originally by Shotaro Hori (1898) in Japan and subsequently by Yosaburo Fujikuro (1916) in Taiwan. Initially, Hori (1898) identified its conidial stage as F. heterosporium Nees, but Kaneyoshi Sawada (1917) subsequently reported and named its teleomorph as Lisea fujikuroi Sawada, in memory of Yosaburo Fujikuro. Subsequently, Seiya Ito (1930) transferred the species to Gibberella as G. fujikuroi, but considered it conspecific with G. moniliformis Wineland (Wineland 1924), the perithecial stage of F. moniliforme. Although Eiichi Kurosawa (1930) suggested that G. fujikuroi and G. moniliformis were related but different species, Wollenweber and Reinking (1935) and others accepted Ito’s synonymy treating them as conspecific. Mating experiments conducted by Hsieh et al. (1977) clearly established that F. fujikuroi (=G. fujikuroi) and F. verticillioides (=G. moniliformis) represented distinct biological species. Molecular phylogenetic analyses have revealed that biological and phylogenetic species recognition within the FFSC yield concordant results (O’Donnell and Cigelnik 1997; O’Donnell et al. 1998a).
Gibberellin phytohormones were first isolated and characterized from isolates of F. fujikuroi (=“Gibberella” fujikuroi) that were inciting “bakanae” disease of rice. Sawada (1912) first suggested that elongation of bakanae rice seedlings was induced by the stimulation of fungal mycelium. This report led to Kurosawa’s discovery (1926) that culture filtrates of a fungus from dried rice seedlings caused marked elongation in rice and other subtropical grasses in Taiwan. Kurosawa reported that a “toxin” secreted by the “bakanae” fungus stimulated shoot elongation, inhibited chlorophyll formation, and suppressed root growth. In a subsequent study, he substituted “toxin” with “growth promoting substance” (Kurosawa 1930). Using strains provided by Kurosawa, Teijiro Yabuta in 1934 isolated a crystalline compound from the fungal culture filtrate that inhibited growth of rice seedlings but was shown to be 5-N-butylpicolinic acid or fusaric acid, not gibberellin. However, the following year, Yabuta (1935) obtained a nearly pure noncrystalline solid from “bakanae”-inducing Fusarium culture filtrates that he named gibberellin, and Yabuta and Sumiki in 1938 finally succeeded in crystallizing a pale yellow solid from their original gibberellin preparations (Yabuta and Hayashi 1939). They named the substance gibberellin A and gibberellin B, but the names were subsequently modified, because their gibberellin A was probably a mixture of the biologically inactive allogibberic acid and biologically active gibberellins, and their gibberellin B was probably a mixture of three biologically active gibberellins. The first chemically pure gibberellin was named gibberellic acid in 1954 by Curtis and Cross (Phinney and West 1960). Since then, more than 100 chemically pure gibberellins have been named, e.g., gibberellin A1, gibberellin A2, gibberellin A3 (=gibberellic acid), gibberellin A4.
Pitch canker disease of pine induced by F. circinatum was originally reported on Virginia pine (Pinus virginiana Mill.) in North Carolina within the United States (Hepting and Roth 1946) and subsequently on other Pinus spp. in other states in the US and in other countries. The pathogen was first identified as F. lateritium Nees f. pini Hepting, reidentified as F. moniliforme var. subglutinans Wollenw. & Reinking, and subsequently reported as F. subglutinans f. sp. pini Correll, Gordon, McCain, Fox, Koehler, Wood & Schultz (Correll et al. 1991). Based on extensive morphological and molecular phylogenetic analyses of the FFSC (Nirenberg and O’Donnell 1998; O’Donnell et al. 1998a), the pathogen was redescribed as the novel species F. circinatum Nirenberg & O’Donnell. The fungus causes pitch canker disease on various species of Pinus from southeastern and western US, Haiti, Japan, Korea, South Africa, Chile, Spain, Mexico, Italy and Portugal (e.g., Correll et al. 1991; Hepting and Roth 1946; Kobayashi and Muramoto 1989; Wingfield et al. 2008). The disease was first reported in Asia on Pinus luchuensis from Tatsugo in the Amami Island, Kagoshima Prefecture of Japan in 1989 (Kobayashi and Muramoto 1989) and then on islands in Okinawa Prefecture. The first report of the disease in Korea came from Kyunggi Province in the mid-1990s. Three reference isolates from Japan were originally deposited in MAFF as F. moniliforme var. subglutinans, but were reidentified as F. circinatum based on morphological data and molecular phylogenetic analyses of DNA sequence data from several genes (Aoki et al. 2001).
Fumonisin mycotoxin contamination of corn and other cereals is primarily from F. verticillioides, which until a decade ago, was typically reported as F. moniliforme. The fumonisins are a family of food-borne carcinogenic mycotoxins, also causing equine leukoencephalomalacia (Proctor et al. 2004; Rheeder et al. 2002). Fumonisin B1 and B2, for example, were originally isolated and characterized from an isolate of F. verticillioides originally identified as F. moniliforme MRC 826 = FRC M-1325 (Gelderblom et al. 1988; Rheeder et al. 2002). Subsequently, several other species within the FFSC and several closely related species in other complexes have been reported to produce forms of fumonisin (Rheeder et al. 2002); however, these results need to be critically evaluated because several of the species reported to produce fumonisins do not appear to possess key genes in the fumonisin gene cluster (Robert H. Proctor, pers. commun.; Proctor et al. 2004). Based on the study by Proctor et al. (2004), fumonisin production was detected in cultures of only six species, i.e., F. fujikuroi, F. globosum Rheeder, Marasas & P.E. Nelson, F. nygamai, F. proliferatum, F. verticillioides in the FFSC, and in the strain FRC O-1890 identified as F. oxysporum Schlecht. from Korea. Among these, F. verticillioides, F. proliferatum and F. nygamai were often found to be high producers of fumonisin. These species produced predominantly the B-series fumonisins (FB), FB1, FB2, FB3 and FB4, with the exception of F. oxysporum, producing predominantly the C-series fumonisins (FC), FC1, hydroxyFC1, isoFC1, FC2, and FC3. Although some species of Fusarium, e.g., F. dlaminii Marasas, P.E. Nelson & Toussoun, F. napiforme Marasas, P.E. Nelson & Rabie, F. subglutinans and F. thapsinum, were reported to produce fumonisins in previous studies (Leslie et al. 1996; Rheeder et al. 2002), the fumonisin (FUM) genes have not yet been detected in these species (Proctor et al. 2004). The study by Proctor et al. (2004) established that the FUM gene cluster is distributed discontinuously within the FFSC, and the presence of FUM genes and the ability to produce fumonisins can vary within a species.
-
(2)
Fusarium graminearum species complex (FGSC; Fig. 1): These species induce Fusarium head blight of wheat and barley and contaminate grain with trichothecene mycotoxins
Fusarium head blight (FHB) or scab of wheat and barley occurs worldwide. It is one of the most economically devastating diseases of wheat, not only because of reduction in yield, but also because of contamination with trichothecene mycotoxins such as deoxynivalenol (DON) and nivalenol (NIV) (Windels 2000) as well as estrogen analogues (Placinta et al. 1999). Several different species of Fusarium have been reported to cause FHB, such as F. graminearum Schwabe, F. culmorum (W.G. Smith) Sacc., F. cerealis (Cooke) Sacc. (syn. F. crookwellense L.W. Burgess, P.E. Nelson & Toussoun), F. avenaceum (Fr.) Sacc., F. acuminatum Ellis & Everh., F. tricinctum (Corda) Sacc., F. sporotrichioides Sherb., F. poae (Peck) Wollenw., but the primary etiological agent has long been considered as a single panmictic species, F. graminearum. However, a series of studies conducted over the past 15 years, employing GCPSR (Taylor et al. 2000), revealed that this morphospecies comprises at least 16 phylogenetically distinct species (Aoki et al. 2012c; O’Donnell et al. 2000a, 2004; Sarver et al. 2011; Starkey et al. 2007). Because this morphologically defined species harbors multiple cryptic species, the clade comprising the 16 FHB species has become known as the F. graminearum species complex (FGSC, O’Donnell et al. 2004). Results of a multilocus phylogeny, based on maximum parsimony and maximum likelihood analyses of 12 combined genes comprising 16.3 kb of aligned DNA sequence data, suggest that subclades of the FGSC evolved in Asia, North America, South America and Australia or Africa. In contrast to the results obtained using GCPSR, MSR using conidial morphology and colony characters was only able to distinguish 6 species and 3–4 species groups among the 16 species recognized within the FGSC (Aoki et al. 2012c; Sarver et al. 2011). The inability of MSR to resolve all of the phylogenetic species within the FGSC is consistent with their hypothesized recent evolutionary origin (O’Donnell et al. 2013). Comparative molecular and morphological analyses are in progress to elucidate the full spectrum of FHB pathogen diversity, their trichothecene toxin potential and geographic distribution. A validated multilocus genotyping assay for species determination and trichothecene toxin chemotype prediction has been extremely useful in the discovery of novel FGSC species and monitoring changes in pathogen populations (Sarver et al. 2011; Starkey et al. 2007; Ward et al. 2008). The 16 species within the FGSC all possess a homothallic mating type locus organization, suggesting that they are self-fertile. The FGSC appears to have evolved from self-sterile ancestors given the heterothallic mating type organization of their close relatives, F. pseudograminearum O’Donnell & T. Aoki, F. cerealis, F. culmorum and F. lunulosporum Gerlach (Aoki et al. 2012c; O’Donnell et al. 2004).
The FGSC species produce trichothecene mycotoxins and estrogenic compounds that often contaminate cereals, making them unsuitable for human and animal consumption, as well as acting as virulence factors on sensitive host plants (Proctor et al. 1995). The trichothecenes produced by Fusarium species are classified as either A- or B-type depending on the absence or presence of a keto group at the C-8 position of the trichothecene ring (Kimura et al. 2007). The FGSC species produce B-type trichothecenes such as deoxynivalenol (DON, known as vomitoxin), nivalenol (NIV) and their acetylated derivatives, i.e., 3-acetyldeoxynivalenol (3ADON) and 15-acetyldeoxynivalenol (15ADON) (Ward et al. 2002). Many trichothecene biosynthesis genes have been identified through biochemical and genetic investigations of F. sporotrichioides (an A-type trichothecene producer) and two FGSC species, F. graminearum and F. asiaticum O’Donnell, T. Aoki, Kistler & Geiser (B-type trichothecene producers). All known trichothecene genes are localized within a gene cluster, except for a 3-O-acetyltransferase (TRI101), TRI1 and TRI16 (Proctor et al. 2009). Three trichothecene chemotypes discovered within the B-type trichothecene-producing species of Fusarium are classified as (1) NIV+the acetylated derivatives (NIV chemotype) (2) DON+3ADON (3ADON chemotype) and (3) DON+15ADON (15ADON chemotype). NIV vs. DON B-type trichothecene chemotype differences are determined by TRI13 (Brown et al. 2002; Lee et al. 2002), and the 3ADON and 15ADON dichotomy is based on differences in TRI3 and TRI8 (Kimura et al. 2003). Although phylogenetic analyses on the individual and the combined 12 gene data set strongly supports the recognition of 16 phylogenetically distinct species under GCPSR, phylogenies inferred from eight trichothecene cluster genes did not track with species phylogeny (O’Donnell et al. 2008b; Sarver et al. 2011; Ward et al. 2002). Genes within the trichothecene toxin gene cluster are evolving under strong balancing selection, and approximately half of the species within the B-type trichothecene toxin-producing clade still segregate for trichothecene toxin chemotype (Aoki et al. 2012c; O’Donnell et al. 2004, 2008b; Sarver et al. 2011; Starkey et al. 2007; Ward et al. 2002).
Three FGSC species, F. asiaticum, F. graminearum and F. vorosii B. Tóth, Varga, Starkey, O’Donnell, H. Suga & T. Aoki, have been recorded from Japan and their geographic distribution has been elucidated (Starkey et al. 2007; Suga et al. 2008). Fusarium graminearum is distributed mainly in the cooler northern areas of Japan, whereas F. asiaticum is restricted to the warmer southern areas. The third FGSC species in Japan, F. vorosii, is currently only known from Naganuma-cho, Hokkaido, the northernmost island. The former two species are phenotypically indistinguishable based on a detailed morphological comparison (O’Donnell et al. 2004). Therefore, molecular systematic data are essential to accurately identify the FGSC pathogens in Japan. Suga et al. (2008) elucidated trichothecene production profiles of F. graminearum (3ADON and 15ADON) and F. asiaticum (NIV and 3ADON) in Japan.
-
(3)
Fusarium oxysporum species complex (FOSC; Fig. 1): These species cause vascular wilts
Members of the F. oxysporum species complex (FOSC) are some of the most economically important and widespread plant pathogenic species of Fusarium. Distributed worldwide, they are extremely common in various soils and plants, causing vascular wilts, damping-off, and crown and root rots on a wide range of economically important hosts. In Japan, members of the FOSC have been recorded as pathogens on more than 130 hosts (PSJ and NIAS 2012). Over 100 formae speciales (ff. sp.) and races of F. oxysporum have been recognized and reported, which illustrates the importance of this complex to phytopathology (Armstrong and Armstrong 1981; Booth 1971; Snyder and Hansen 1940). The concept of forma specialis under the rank of species was first introduced into Fusarium taxonomy by Snyder and Hansen (1940, 1941, 1945), based on the pathogenicity of strains to specific host plants. Although the 10th International Botanical Congress in Edinburgh, UK, 1964 decided that the forma specialis classification was not governed by the botanical nomenclature code, the idea to determine formae speciales has been widely adopted among the majority of plant pathologists working especially on the FOSC and the Fusarium solani species complex (FSSC) (Armstrong and Armstrong 1981; Booth 1971).
Multigene genealogies have revealed that many formae speciales within the FOSC are nonmonophyletic (Baayen et al. 2000; O’Donnell et al. 1998b; Skovgaard et al. 2001). The etiological agent of Panama disease of banana, F. oxysporum f. sp. cubense Snyder & Hansen, for example, was shown to have evolved at least five independent times, indicating that pathogenicity to this host has evolved convergently (O’Donnell et al. 1998b). Other formae speciales that appear to have evolved multiple independent times include F. oxysporum ff. sp. asparagi Cohen, dianthi Snyder & Hansen, gladioli Snyder & Hansen, lini Snyder & Hansen, opuntiarum Gordon (Baayen et al. 2000), and vasinfectum Snyder & Hansen (Skovgaard et al. 2001). The discovery of horizontal transfer of genes and chromosomes within the FOSC provides a possible means by which host specificity may have evolved convergently within this agronomically important complex (Ma et al. 2013).
To date a sexual cycle has not been discovered within the FOSC; however, isolates that possess and express MAT1-1 or MAT1-2 mating type idiomorphs have been discovered (Arie et al. 2000; Yun et al. 2000), suggesting that a cryptic sexual cycle is present within the FOSC. In contrast to the forma specialis naming system that can obscure evolutionary relationships, vegetative compatibility groups (VCGs) appear to accurately reflect genetic relatedness of phytopathogenic races in many formae speciales (Kistler et al. 1998). Isolates belonging to the same VCG typically possess very similar or identical multilocus haplotypes that belong to the same clonal lineage (Baayen et al. 2000; Kistler 1997). The VCG therefore appears to represent a useful tool for identifying unique genetic entities. The standard method for VCG determination within the FOSC was originally proposed by Puhalla (1985) using nitrate non-utilizing (nit) mutants. The method, as improved by Correll et al. (1987), has been adopted by many workers to analyze and classify VCGs according to Puhalla’s system for VCG classification. Since Puhalla’s original publication in which 16 VCGs were typed, over 125 VCGs have been identified among plant pathogenic isolates of the FOSC that correspond to more than 30 formae speciales. Standardization of the VCG numbering system, including numbering of formae speciales, has been proposed to accurately communicate genetic diversity within the FOSC (Kistler et al. 1998). The relationship between races and VCG has also been studied in several phytopathogenic FOSC. Race 2 of f. sp. apii Snyder & Hansen (Correll et al. 1986) and race 3 of f. sp. vasinfectum (Katan and Katan 1988) each comprise a single VCG. In F. oxysporum f. sp. melonis Snyder & Hansen (Jacobson and Gordon 1988) and f. sp. lycopersici Snyder & Hansen (Elias and Schneider 1991), however, the relationship between race and VCG is complicated. In F. oxysporum f. sp. lactucae Hubb. & Gerik, the three races each correspond to separate VCGs (Fujinaga et al. 2005).
Although phylogenetic structure has been discovered via molecular phylogenetics within the FOSC, many additional phylogenetically informative loci are needed to resolve species-level lineages within this complex. Given that 11 genome sequences are available publically, it should be possible to mine these data to identify a set of loci for species-level studies. Molecular phylogenetic studies have led to the recognition of several FOSC-like species, including its sister taxon Fusarium foetens Schroers, O’Donnell, Baayen & Hooftman (Schroers et al. 2004). The latter species, which is pathogenic to a begonia hybrid (Schroers et al. 2004), was described based on subtle phenotypic differences that distinguish it from F. oxysporum, including a distinctive, pungent odor in culture. By way of contrast, cultures of F. redolens Wollenw. and F. hostae Geiser & Juba (Geiser et al. 2001) produce a lilac-like odor. F. foetens causing leaf and stem rot of begonia elatior hybrids was also reported from Japan (PSJ and NIAS 2012). In addition, several species related phylogenetically to the FOSC have also been described. Although F. redolens [≡ F. oxysporum var. redolens (Wollenw.) Gordon] and F. udum Butl. [≡ F. oxysporum f. udum (Butl.) Snyder & Hansen] have been classified with F. oxysporum within section Elegans (Booth 1971; Gerlach and Nirenberg 1982; Snyder and Hansen 1940; Wollenweber and Reinking 1935), they are currently recognized as separate species within closely related species complexes. Fusarium redolens is placed in the F. redolens species complex (FRSC) together with F. hostae (Geiser et al. 2001), and F. udum is nested within the African clade of the FFSC (O’Donnell et al. 2013). Fusarium nisikadoi T. Aoki & Nirenberg, F. commune Skovgaard, Nirenberg & O’Donnell and F. miscanthi W.Gams, Klamer & O’Donnell are phylogenetically closely related to the FOSC and FFSC and together form part of the F. nisikadoi species complex (FNSC) (O’Donnell et al. 2013).
-
(4)
Fusarium solani species complex (FSSC; Fig. 1): These pathogens cause foot and root rot of diverse hosts
Fusarium solani (Mart.) Appel & Wollenw. has been reported widely as a pathogen on various vegetables, fruits and flowers, as well as being responsible for fusarioses of humans and other animals. However, this morphospecies is estimated to comprise at least 60 phylogenetically distinct species (Nalim et al. 2011; O’Donnell et al. 2008a). When Wollenweber and Reinking (1935) first circumscribed this group of Fusarium as section Martiella, 5 species, 10 varieties and 4 forms were recognized. However, Snyder and Hansen (1941) treated all of these taxa, including species in section Ventricosum, as F. solani. Although 7 heterothallic biological species were discovered and characterized within the FSSC (Matuo and Snyder 1973), they were recognized as mating populations (MPs I–VII) of Nectria haematococca Berk. & Br. (Sakurai and Matuo 1960) or as formae speciales of F. solani (Matuo 1972). The latter included F. solani f. sp. cucurbitae Snyder & Hansen Race 1 [mating population I (MP I)], causing foot rot and fruit rot of cucurbits; f. sp. batatas McClure (MP II), causing foot rot of sweet potato; f. sp. mori Sakurai & Matuo (MP III), causing stem blight of mulberry tree; f. sp. xanthoxyli Sakurai & Matuo (MP IV), causing trunk-blight of Japanese pepper (Zanthoxylum piperitum (L.) De Candolle); f. sp. cucurbitae Race 2 (MP V), causing fruit rot of cucurbits; f. sp. pisi Snyder & Hansen (MP VI), causing foot rot of pea, root rot of pea and ginseng, and stem blight of mulberry tree; and f. sp. robiniae Matuo & Sakurai (MP VII), causing twig-blight of black locust (Robinia pseudoacacia L.). Several additional formae speciales with no known teleomorph have been described, including f. sp. eumartii Snyder & Hansen, causing Eumartii wilt of potatoes and foot rot of tomatoes; f. sp. phaseoli Snyder & Hansen, causing foot rot of bean; f. sp. radicicola Snyder & Hansen, causing tuber rot of potatoes and bulb rot of tulip; f. sp. piperis Albuq., causing root rot of pepper; and f. sp. glycines Roy, causing soybean SDS.
A series of multilocus molecular phylogenetic analyses of the FSSC resolved three biogeographically structured clades designated 1–3 (Nalim et al. 2011; O’Donnell 2000; O’Donnell et al. 2008a; Zhang et al. 2006). These studies supported the genealogical exclusivity of the seven biological species and most but not all of the formae speciales. Three nonmonophyletic host specific forms include ff. sp. cucurbitae, phaseoli and glycines, which comprise 2, 2 and 4 phylogenetic species, respectively (Aoki et al. 2003, 2005, 2012a; Covert et al. 2007; O’Donnell 2000). Because most of the species within the FSSC lack Latin binomials, an informal nomenclature was developed in which a unique Arabic number and lowercase Roman letter were used to distinguish all of the species/haplotypes within the medically and phytopathologically species-rich clade 3 (O’Donnell et al. 2008a). Because strains reported in the literature as F. solani are nested within all three clades, use of the name F. solani should be avoided until the name is assigned to an appropriate phylogenetic species. Fortunately, with the broad acceptance of the unitary use of Fusarium by the plant pathology community, only the Fusarium morph name needs to be used in a publication (Geiser et al. 2013). Even so, it is important to note that the teleomorphic genus Neocosmospora E. F. Smith is nested within the clade 3 of the FSSC (O’Donnell 2000). The type species, N. vasinfecta E. F. Smith, was recently recombined within Fusarium as F. neocosmosporiellum O’Donnell & Geiser to promote its clear connection to Fusarium (Geiser et al. 2013). Because the current circumscription of Nectria (Fr.) Fr. restricts this genus to species that have Tubercularia Tode anamorphs, several species within the FSSC, including the FSSC-associated Nectria haematococca, were transferred to the new teleomorphic genus, Haematonectria Samuels & Nirenberg (Rossman et al. 1999). Subsequently, H. haematococca (Berk. & Br.) Samuels & Rossman was recombined as Neocosmospora haematococca (Berk. & Br.) Samuels, Nalim & Geiser, and its anamorph was described as Fusarium haematococcum Nalim, Samuels & Geiser, which is phylogenetically distinct from F. solani (Nalim et al. 2011). Several species from Sri Lanka that belong to FSSC clade 2, i.e., F. kurunegalense Samuels, Nalim & Geiser, F. rectiphorus Samuels, Nalim & Geiser, F. mahasenii Samuels, Nalim & Geiser, and F. kelerajum Samuels, Nalim & Geiser were also described by Nalim et al. (2011). Collectively these studies revealed that species within clade 2 of the FSSC are united by the production of multiseptate conidia on tall aerial conidiophores, which were referred to as “Acremonium-like conidiophores” (Nalim et al. 2011).
Another series of important phenotypic, phylogenetic and phytopathological studies were conducted on the Fusarium species responsible for soybean sudden death syndrome (SDS) and bean root rot, all of which are members of clade 2 of the FSSC (Aoki et al. 2003, 2005, 2012a, b; O’Donnell et al. 2010a). Prior to these studies, these pathogens were typically reported as formae speciales of F. solani, i.e., f. sp. glycines for soybean SDS, f. sp. phaseoli for kidney bean or mung bean root rot, and f. sp. adukicola Yasue, Tanaka, Suga, Kageyama & Hyakumachi for azuki bean root rot. Detailed morphological and molecular phylogenetic analyses of these fusaria revealed that they comprise seven morphologically and phylogenetically distinct species, including four that can induce soybean SDS in the field (F. tucumaniae T. Aoki, O’Donnell, Yosh. Homma & Lattanzi, F. virguliforme O’Donnell & T. Aoki, F. brasiliense T. Aoki & O’Donnell and F. crassistipitatum Scandiani, T. Aoki & O’Donnell), two that cause bean root rot [F. phaseoli (Burkh.) T. Aoki & O’Donnell and F. cuneirostrum O’Donnell & T. Aoki], and one responsible for azuki bean root rot (F. azukicola T. Aoki, H. Suga, F. Tanaka, Scandiani & O’Donnell) (Aoki et al. 2003, 2005, 2012a, b). These seven species possess several unique phenotypic features that may characterize Fusarium within clade 2 of the FSSC: (1) multiseptate conidia on tall aerial conidiophores (equivalent to “Acremonium-like conidiophores” sensu Nalim et al. 2011), (2) 0(–1)-septate ellipsoidal conidia on short aerial conidiophores, (3) multiseptate conidia in sporodochia, (4) 1(–2)-septate short-clavate (or comma-shaped) conidia in sporodochia, and (5) extremely slow mycelial growth rates (1–3 mm/day) on PDA. The seven pathogens can be differentiated by conidial and conidiophore morphology and by phylogenetic analysis of DNA sequence data from one or more informative loci.
Most of the known species of the FSSC that form teleomorphs are heterothallic, including the seven biological species recognized as the MPs I–VII of F. solani/N. haematococca within clade 3, F. tucumaniae within clade 2 of the FSSC (Covert et al. 2007), and F. illudens C. Booth and N. plagianthi Dingley within clade 1 of the FSSC (O’Donnell 2000). Several homothallic species, e.g., F. striatum Sherb. [syn. = Haematonectria ipomoeae (Halst.) Samuels & Nirenberg] and F. neocosmosporiellum (syn. = Neocosmospora vasinfecta), appear to have evolved independently within the FSSC (O’Donnell 2000).
Other phytopathogenic Fusarium species
Significant Fusarium pathogens in other species complexes include F. avenaceum in the F. tricinctum species complex (FTSC, O’Donnell et al. 2013) and F. lateritium in the F. lateritium species complex (FLSC, O’Donnell et al. 2013); (Fig. 1). Fusarium avenaceum was previously classified in the artificial section Roseum based on phenotypic features (Gerlach and Nirenberg 1982; Wollenweber and Reinking 1935); however, recent molecular phylogenetic analyses support placing it within the FTSC (O’Donnell et al. 2013). Fusarium avenaceum has been reported to be a saprophyte as well as an aggressive pathogen responsible for damping-off, root rots, stalk rots and fruit rots of a wide range cereals, legume, and vegetable crops (Gerlach and Nirenberg 1982; Nalim et al. 2009). This species has also been reported to be associated with F. graminearum or F. culmorum ecologically (Cook 1967) and in association with Fusarium head blight of wheat and barley (Bottalico and Perrone 2002). In Japan, F. avenaceum has been recorded as the causal pathogen for many diseases, including crown rot of red clover and alfalfa, seedling blight of rice, foxtail millet and maize, root rot of pea, and head blight of wheat, barley and timothy-grass among others (PSJ and NIAS 2012). In addition to F. oxysporum f. sp. pisi W.C. Snyder & H.N. Hansen and F. solani f. sp. pisi, several other Fusarium species cause root rot of pea, including F. avenaceum, F. arthrosporioides Sherb., and F. anguioides Sherb. in the FTSC and F. sporotrichioides in the F. sambucinum species complex.
Fusarium lateritium in the FLSC is a tree pathogen responsible for dieback, twig blight, collar rot, canker, leaf spot and fruit rot (Gerlach and Nirenberg 1982). Traditionally it has been placed in section Lateritium together with the morphologically similar species, F. sarcochroum (Desm.) Sacc., F. stilboides Wollenw., as well as the coffee wilt pathogen, F. xylarioides Steyaert (Booth 1971; Gerlach and Nirenberg 1982). However, molecular phylogenetic data support the transfer of F. xylarioides to the FFSC (Geiser et al. 2005). Isolates identified phenotypically as F. lateritium in Japan were responsible for damping-off of Pinus spp., Japanese cedar [Cryptomeria japonica (Linn.fil.) D. Don], black locust and silktree (Albizia julibrissin Durazz.), twig blight of mulberry trees, dieback of black locust, and stem rot of feather cockscomb (Celosia cristata L.) (PSJ and NIAS 2012).
Future challenges
While Fusarium systematics has been advanced greatly by molecular phylogenetics, a phylogenetically informed synthesis has yet to be completed. With the large amount of data available and a high demand for reliable identification methods, electronically portable Internet-accessible databases and other resources will undoubtedly play a leading role in providing user communities with the tools they need. In addition, genomic data are accumulating at a logarithmic rate; complete genome data are expected to be available for all of the important phytopathogenic Fusarium species in the not-too-distant future. One obvious expected benefit is the discovery of additional phylogenetically informative loci that will improve inferences about relationships and species boundaries within the genus. Surely, this deluge of data will facilitate a second molecular revolution in Fusarium systematics, but the technology for generating data is improving more quickly than the technology for handling it. Many computational and analytical challenges remain for interpreting phylogenomic data sets, especially when they apply to large numbers of taxa. Despite these challenges, we envision a twenty-first century synthesis of Fusarium systematics that will encompass not only phylogenetic trees based on many loci, species descriptions and information about ecology, pathology and physiology, but also Internet-based tools for reliable and accurate species identification, comparative genomics and communication among users (Park et al. 2011).
Challenges are also visible on the classical taxonomic front. As mentioned earlier, the use of dual nomenclature is not allowed after January 1, 2013 under the new International Code of Nomenclature for algae, fungi, and plants (Melbourne Code, 2012). Thus, only a single scientific name can now be applied to a holomorphic fungus such as a Fusarium that produces a teleomorph. However, anamorphic or teleomorphic scientific names published before 1 January 2013 compete for priority (Hawksworth 2012). For example, the anamorphic name Fusarium Link 1809: Fries 1821 (based on F. roseum Link, syn. = F. sambucinum Fuckel) competes with the teleomorphic name Gibberella Sacc. 1877 [based on Gibberella pulicaris (Fr.) Sacc.]. In addition, the teleomorph genera Cyanonectria Samuels & P. Chaverri, Albonectria Rossman & Samuels, Geejayessia Schroers, Gräfenhan & Seifert, and Haematonectria have a strong connection to Fusarium; these teleomorphs together with Neocosmospora and other Fusarium species form a strongly supported monophyletic group (O’Donnell et al. 2013). Based on broad and robust support from plant pathologists and other users, Geiser et al. (2013) proposed the unitary use of Fusarium over all of these competing teleomorph names. The logic for promoting the unitary use of the name Fusarium is that it is monophyletic as currently circumscribed (Geiser et al. 2013; O’Donnell et al. 2013). This anamorph name is used considerably more than all of the teleomorph names combined; fewer than 20 % of Fusarium species have a known teleomorph, and most of these are never encountered by applied biologists. The name Fusarium was published more than a half century before any of the teleomorph names. The molecular phylogenetic results reported by O’Donnell et al. (2013) and Geiser et al. (2013) support circumscribing Fusarium such that either the F. dimerum–F. ventricosum species complexes or the F. albidum species complex represents the earliest-diverging lineage within the genus. Both hypotheses circumscribe the genus such that it includes all or virtually all phytopathogenic and medically important Fusarium species.
While this proposal effectively deals with the generic concept, there are some 1,500 species epithets associated with the genus that are typified in several genera. Sorting through these names and building a phylogenetically informed “one name–one fungus” Fusarium is a daunting task, but one that will be facilitated by the excellent molecular and genomic resources already available. For plant pathologists and other scientists who require accurate identifications of Fusarium isolates, usage of Fusarium-ID (Geiser et al. 2004; Park et al. 2011) and Fusarium MLST (O’Donnell et al. 2010b), two Internet-accessible websites dedicated to the molecular identification of Fusarium species, are fully encouraged.
References
Aoki T, O’Donnell K (1999) Morphological and molecular characterization of Fusarium pseudograminearum sp. nov., formerly recognized as the group 1 population of F. graminearum. Mycologia 91:597–609
Aoki T, O’Donnell K, Ichikawa K (2001) Fusarium fractiflexum sp. nov. and two other species within the Gibberella fujikuroi species complex recently discovered in Japan that form aerial conidia in false heads. Mycoscience 42:461–478
Aoki T, O’Donnell K, Homma Y, Lattanzi AR (2003) Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex—F. virguliforme in North America and F. tucumaniae in South America. Mycologia 95:660–684
Aoki T, O’Donnell K, Scandiani MM (2005) Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense sp. nov., F. cuneirostrum sp. nov., F. tucumaniae, and F. virguliforme. Mycoscience 46:162–183
Aoki T, Scandiani MM, O’Donnell K (2012a) Phenotypic, molecular phylogenetic, and pathogenic characterization of Fusarium crassistipitatum sp. nov., a novel soybean sudden death syndrome pathogen from Argentina and Brazil. Mycoscience 53:167–186
Aoki T, Tanaka F, Suga H, Hyakumachi M, Scandiani MM, O’Donnell K (2012b) Fusarium azukicola sp. nov., an exotic azuki bean root-rot pathogen in Hokkaido, Japan. Mycologia 104:1068–1084
Aoki T, Ward TJ, Kistler HC, O’Donnell K (2012c) Systematics, phylogeny and trichothecene mycotoxin potential of Fusarium head blight cereal pathogens. Mycotoxins 62:91–102
Arie T, Kaneko I, Yoshida T, Noguchi M, Nomura Y, Yamaguchi I (2000) Mating-type genes from asexual phytopathogenic ascomycetes Fusarium oxysporum and Alternaria alternata. Mol Plant Microbe Interact 13:1330–1339
Armstrong GM, Armstrong JK (1981) Formae speciales and races of Fusarium oxysporum causing wilt disease. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: disease, biology, and taxonomy. Pennsylvania State University Press, University Park, Pennsylvania, pp 391–399
Baayen RP, O’Donnell K, Bonants PJM, Cigelnik E, Kroon LPNM, Roebroeck EJA, Waalwijk C (2000) Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 90:891–900
Booth C (1971) The genus Fusarium. Commonwealth Mycological Institute, Kew
Bottalico A, Perrone G (2002) Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur J Plant Pathol 108:611–624
Brown DW, McCormick SP, Alexander NJ, Proctor RH, Desjardins AE (2002) Inactivation of a cytochrome P-450 is a determinant of trichothecene diversity in Fusarium species. Fungal Genet Biol 36:224–233
Cook RJ (1967) Gibberella avenacea sp. n., perfect stage of Fusarium roseum f. sp. cerealis “Avenaceum”. Phytopathology 57:732–736
Correll JC, Puhalla JE, Schneider RW (1986) Identification of Fusarium oxysporum f. sp. apii on the basis of colony size, virulence and vegetative compatibility. Phytopathology 76:396–400
Correll JC, Klittich CJR, Leslie JF (1987) Nitrate nonutilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology 77:1640–1646
Correll JC, Gordon TR, McCain AH, Fox JW, Koehler CS, Wood DL, Schultz ME (1991) Pitch canker disease in California: pathogenicity, distribution, and canker development on Monterey pine (Pinus radiata). Plant Dis 75:676–682
Covert SF, Aoki T, O’Donnell K, Starkey D, Holliday A, Geiser DM, Cheung F, Town C, Strom A, Juba J, Scandiani M, Yang XB (2007) Sexual reproduction in the soybean sudden death syndrome pathogen Fusarium tucumaniae. Fungal Genet Biol 44:799–807
Elias KS, Schneider RW (1991) Vegetative compatibility groups in Fusarium oxysporum f. sp. lycopersici. Phytopathology 81:159–162
Fujikuro Y (1916) On Bakanae-disease of rice (in Japanese). Formosan Agr Rev 121:5–12
Fujinaga M, Ogiso H, Shinohara H, Tsushima S, Nishimura N, Togawa M, Saito H, Nozue M (2005) Phylogenetic relationships between the lettuce root rot pathogen Fusarium oxysporum f. sp. lactucae races 1, 2, and 3 based on the sequence of the intergenic spacer region of its ribosomal DNA. J Gen Plant Pathol 71:402–407
Geiser DM, Juba JH, Wang B, Jeffers SN (2001) Fusarium hostae sp. nov., a relative of F. redolens with a Gibberella teleomorph. Mycologia 93:670–678
Geiser DM, del Mar Jiménez-Gasco M, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, Zhang N, Kuldau GA, O’Donnell K (2004) FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol 110:473–479
Geiser DM, Lewis Ivey ML, Hakiza G, Juba JH, Miller SA (2005) Gibberella xylarioides (anamorph: Fusarium xylarioides), a causative agent of coffee wilt disease in Africa, is a previously unrecognized member of the G. fujikuroi species complex. Mycologia 97:191–201
Geiser DM, Aoki T, Bacon CW, Baker SE, Bhattacharyya MK, Brandt ME, Brown DW, Burgess LW, Chulze S, Coleman JJ, Correll JC, Covert SF, Crous PW, Cuomo CA, De Hoog GS, Di Pietro A, Elmer WH, Epstein L, Frandsen RJN, Freeman S, Gagkaeva T, Glenn AE, Gordon TR, Gregory NF, Hammond-Kosack KE, Hanson LE, del Mar Jímenez-Gasco M, Kang S, Kistler HC, Kuldau GA, Leslie JF, Logrieco A, Lu G, Lysøe E, Ma L-J, McCormick SP, Migheli Q, Moretti A, Munaut F, O’Donnell K, Pfenning L, Ploetz RC, Proctor RH, Rehner SA, Robert VARG, Rooney AP, Bin Salleh B, Scandiani MM, Scauflaire J, Short DPG, Steenkamp E, Suga H, Summerell BA, Sutton DA, Thrane U, Trail F, Van Diepeningen A, VanEtten HD, Viljoen A, Waalwijk C, Ward TJ, Wingfield MJ, Xu J-R, Yang X-B, Yli-Mattila T, Zhang N (2013) One fungus, one name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 103:400–408
Gelderblom WCA, Jaskiewicz K, Marasas WFO, Thiel PG, Horak RM, Vleggaar R, Kriek NPJ (1988) Fumonisins—novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol 54:1806–1811
Gerlach W, Nirenberg HI (1982) The genus Fusarium—a pictorial atlas. Mitt Biol Bundesanst Land Forstwirtsch Berlin Dahlem 209:1–406
Hawksworth DL (2012) Managing and coping with names of pleomorphic fungi in a period of transition. IMA Fungus 3:15–24
Hepting GH, Roth ER (1946) Pitch canker, a new disease of some southern pines. J For 44:742–744
Hori S (1898) Experiment on Bakanae of rice (in Japanese). Agricult Exp Stat Rec Ministry Agricul Comm 12:110–119
Hsieh WH, Smith SN, Snyder WC (1977) Mating groups in Fusarium moniliforme. Phytopathology 67:1041–1043
Ito S (1930) Miscellaneous talk on rice disease (in Japanese). Ann Phytopath Soc Jpn 2:276–277
Jacobson DJ, Gordon TR (1988) Vegetative compatibility and self-incompatibility within Fusarium oxysporum f. sp. melonis. Phytopathology 78:668–672
Katan T, Katan J (1988) Vegetative-compatibility grouping of Fusarium oxysporum f. sp. vasinfectum from tissue and the rhizosphere of cotton plants. Phytopathology 78:852–855
Kimura M, Tokai T, O’Donnell K, Ward TJ, Fujimura M, Hamamoto H, Shibata T, Yamaguchi I (2003) The trichothecene biosynthesis gene cluster of Fusarium graminearum F15 contains a limited number of essential pathway genes and expressed non-essential genes. FEBS Lett 539:105–110
Kimura M, Tokai T, Takahashi-Ando N, Ohsato S, Fujimura M (2007) Molecular and genetic studies of Fusarium trichothecene biosynthesis: pathways, genes, and evolution. Biosci Biotechnol Biochem 71:2105–2123
Kistler HC (1997) Genetic diversity in the plant-pathogenic fungus Fusarium oxysporum. Phytopathology 87:474–479
Kistler HC, Alabouvette C, Baayen RP, Bentley S, Brayford D, Coddington A, Correll J, Daboussi M-J, Elias K, Fernandez D, Gordon TR, Katan T, Kim HG, Leslie JF, Martyn RD, Migheli Q, Moore NY, O’Donnell K, Ploetz RC, Rutherford MA, Summerell B, Waalwijk C, Woo S (1998) Systematic numbering of vegetative compatibility groups in the plant pathogenic fungus Fusarium oxysporum. Phytopathology 88:30–32
Kobayashi T, Muramoto M (1989) Pitch canker of Pinus luchuensis, a new disease in Japanese forests (in Japanese). For Pests 38:169–173
Kristensen R, Torp M, Kosiak B, Holst-Jensen A (2005) Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1α gene sequences. Mycol Res 109:173–186
Kuhlman EG (1982) Varieties of Gibberella fujikuroi with anamorphs in Fusarium section Liseola. Mycologia 74:759–768
Kurosawa E (1926) Experimental studies on the substance secreted by the ‘bakanae’ fungus of rice (a preliminary report) (in Japanese). Trans Nat Hist Soc Taiwan 16(87):213–227
Kurosawa E (1930) On the overgrowth phenomenon of rice seedlings by the excretion of the cultures of Lisea Fujikuroi Sawada and related organism (in Japanese). Trans Nat Hist Soc Taiwan 20:218–239
Lee T, Han Y-K, Kim K-H, Yun S-H, Lee Y-W (2002) Tri13 and Tri7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl Environ Microbiol 68:2148–2154
Leslie JF (1995) Gibberella fujikuroi: available populations and variable traits. Can J Bot 73(suppl 1):282–291
Leslie JF, Marasas WFO, Shephard GS, Sydenham EW, Stockenström S, Thiel PG (1996) Duckling toxicity and the production of fumonisin and moniliformin by isolates in the A and F mating populations of Gibberella fujikuroi (Fusarium moniliforme). Appl Environ Microbiol 62:1182–1187
Lima CS, Pfenning LH, Costa SS, Abreu LM, Leslie JF (2012) Fusarium tupiense sp. nov., a member of the Gibberella fujikuroi complex that causes mango malformation in Brazil. Mycologia 104:1408–1419
Ma L-J, Geiser DM, Proctor RH, Rooney AP, O’Donnell K, Trail F, Gardiner DM, Manners JM, Kazan K (2013) Fusarium pathogenomics. Annu Rev Microbiol 67:399–416
Matuo T (1972) Taxomonic studies of phytopathogenic fusaria in Japan. Rev Plant Protec Res 5:34–45
Matuo T, Snyder WC (1973) Use of morphology and mating populations in the identification of formae speciales in Fusarium solani. Phytopathology 63:562–565
Nalim FA, Elmer WH, McGovern RJ, Geiser DM (2009) Multilocus phylogenetic diversity of Fusarium avenaceum pathogenic on lisianthus. Phytopathology 99:462–468
Nalim FA, Samuels GJ, Wijesundera RL, Geiser DM (2011) New species from the Fusarium solani species complex derived from perithecia and soil in the old world tropics. Mycologia 103:1302–1330
Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium species. An illustrated manual for identification. Pennsylvania State University Press, University Park
Nirenberg HI, O’Donnell K (1998) New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 90:434–458
O’Donnell K (2000) Molecular phylogeny of the Nectria haematococca–Fusarium solani species complex. Mycologia 92:919–938
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylo Evol 7:103–116
O’Donnell K, Cigelnik E, Nirenberg HI (1998a) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465–493
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998b) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci USA 95:2044–2049
O’Donnell K, Kistler HC, Tacke BK, Casper HH (2000a) Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc Natl Acad Sci USA 97:7905–7910
O’Donnell K, Nirenberg HI, Aoki T, Cigelnik E (2000b) A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41:61–78
O’Donnell K, Ward TJ, Geiser DM, Kistler HC, Aoki T (2004) Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet Biol 41:600–623
O’Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, Zhang N, Geiser DM (2008a) Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J Clin Microbiol 46:2477–2490
O’Donnell K, Ward TJ, Aberra D, Kistler HC, Aoki T, Orwig N, Kimura M, Bjørnstad A, Klemsdal SS (2008b) Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genet Biol 45:1514–1522
O’Donnell K, Sink S, Scandiani MM, Luque A, Colletto A, Biasoli M, Lenzi L, Salas G, González V, Ploper LD, Formento N, Pioli RN, Aoki T, Yang XB, Sarver BAJ (2010a) Soybean sudden death syndrome species diversity within North and South America revealed by multilocus genotyping. Phytopathology 100:58–71
O’Donnell K, Sutton DA, Rinaldi MG, Sarver BAJ, Balajee SA, Schroers H-J, Summerbell RC, Robert VARG, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee Y-H, Kang S, Park B, Geiser DM (2010b) Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 48:3708–3718
O’Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, Ward TJ, Frandsen RJN, Lysøe E, Rehner SA, Aoki T, Robert VARG, Crous PW, Groenewald JZ, Kang S, Geiser DM (2013) Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet Biol 52:20–31
Park B, Park J, Cheong K-C, Choi J, Jung K, Kim D, Lee Y-H, Ward TJ, O’Donnell K, Geiser DM, Kang S (2011) Cyber infrastructure for Fusarium: three integrated platforms supporting strain identification, phylogenetics, comparative genomics and knowledge sharing. Nucl Acids Res 39:D640–D646
Phinney BO, West CA (1960) Gibberellins as native plant growth regulators. Annu Rev Plant Physiol 11:411–436
Phytopathological Society of Japan (PSJ); National Institute of Agrobiological Sciences (NIAS) (2012) Common names of plant diseases in Japan, 2nd edn. Phase Out Inc., Nagoya
Placinta CM, D’Mello JPF, Macdonald AMC (1999) A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim Feed Sci Technol 78:21–37
Proctor RH, Hohn TM, McCormick SP (1995) Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact 8:593–601
Proctor RH, Plattner RD, Brown DW, Seo J-A, Lee Y-W (2004) Discontinuous distribution of fumonisin biosynthetic genes in the Gibberella fujikuroi species complex. Mycol Res 108:815–822
Proctor RH, McCormick SP, Alexander NJ, Desjardins AE (2009) Evidence that a secondary metabolic biosynthetic gene cluster has grown by gene relocation during evolution of the filamentous fungus Fusarium. Mol Microbiol 74:1128–1142
Puhalla JE (1985) Classification of strains of Fusarium oxysporum on the basis of vegetative compatibility. Can J Bot 63:179–183
Rheeder JP, Marasas WFO, Vismer HF (2002) Production of fumonisin analogs by Fusarium species. Appl Environ Microbiol 68:2101–2105
Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999) Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud Mycol 42:1–248
Sakurai Y, Matuo T (1960) Studies on the intraspecific group in Fusarium solani. (1) On mating populations and morphologic groups in the species (in Japanese). Res Rep Fac Text Sericult Shinshu Univ 10:21–32
Sarver BAJ, Ward TJ, Gale LR, Broz K, Kistler HC, Aoki T, Nicholson P, Carter J, O’Donnell K (2011) Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance. Fungal Genet Biol 48:1096–1107
Sawada K (1912) Diseases of agricultural crops in Taiwan (in Japanese). Formosan Agr Rev 63:9–17
Sawada K (1917) Contributions on Formosan fungi, Part 14 (in Japanese). Trans Nat Hist Soc Taiwan 7:128–135
Schroers HJ, Baayen RP, Meffert JP, de Gruyter J, Hooftman M, O’Donnell K (2004) Fusarium foetens, a new species pathogenic to begonia elatior hybrids (Begonia × hiemalis) and the sister taxon of the Fusarium oxysporum species complex. Mycologia 96:393–406
Seifert KA, Aoki T, Baayen RP, Brayford D, Burgess LW, Chulze S, Gams W, Geiser D, de Gruyter J, Leslie JF, Logrieco A, Marasas WFO, Nirenberg HI, O’Donnell K, Rheeder J, Samuels GJ, Summerell BA, Thrane U, Waalwijk C (2003) The name Fusarium moniliforme should no longer be used. Mycol Res 107:643–644
Skovgaard K, Nirenberg HI, O’Donnell K, Rosendahl S (2001) Evolution of Fusarium oxysporum f. sp. vasinfectum races inferred from multigene genealogies. Phytopathology 91:1231–1237
Snyder WC, Hansen HN (1940) The species concept in Fusarium. Amer J Bot 27:64–67
Snyder WC, Hansen HN (1941) The species concept in Fusarium with reference to section Martiella. Amer J Bot 28:738–742
Snyder WC, Hansen HN (1945) The species concept in Fusarium with reference to Discolor and other sections. Amer J Bot 32:657–666
Starkey DE, Ward TJ, Aoki T, Gale LR, Kistler HC, Geiser DM, Suga H, Tóth B, Varga J, O’Donnell K (2007) Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet Biol 44:1191–1204
Suga H, Karugia GW, Ward T, Gale LR, Tomimura K, Nakajima T, Miyasaka A, Koizumi S, Kageyama K, Hyakumachi M (2008) Molecular characterization of the Fusarium graminearum species complex in Japan. Phytopathology 98:159–166
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–31
VanEtten HD, Kistler HC (1988) Nectria haematococca, mating populations I and VI. Adv Plant Pathol 6:189–206
Ward TJ, Bielawski JP, Kistler HC, Sullivan E, O’Donnell K (2002) Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc Natl Acad Sci USA 99:9278–9283
Ward TJ, Clear RM, Rooney AP, O’Donnell K, Gaba D, Patrick S, Starkey DE, Gilbert J, Geiser DM, Nowicki TW (2008) An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet Biol 45:473–484
Windels CE (2000) Economic and social impacts of Fusarium head blight: changing farms and rural communities in the Northern Great Plains. Phytopathology 90:17–21
Wineland GO (1924) An ascigerous stage and synonomy for Fusarium moniliforme. J Agric Res 28:909–922
Wingfield MJ, Hammerbacher A, Ganley RJ, Steenkamp ET, Gordon TR, Wingfield BD, Coutinho TA (2008) Pitch canker caused by Fusarium circinatum—a growing threat to pine plantations and forests worldwide. Australasian Plant Pathol 37:319–334
Wollenweber HW, Reinking OA (1935) Die Fusarien, ihre Beschreibung. Schadwirkung und Bekämpfung, Paul Parey, Berlin
Yabuta T (1935) Biochemistry of the ‘bakanae’ fungus of rice (in Japanese). Agr Hort (Tokyo) 10:17–22
Yabuta T, Hayashi T (1939) Biochemical studies on the bakanae fungus of rice. II. Isolation of ‘gibberellin’, the active principle which makes the rice seedlings grow slenderly (in Japanese). J Agric Chem Soc Jpn 15:257–266
Yun SH, Arie T, Kaneko I, Yoder OC, Turgeon BG (2000) Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genet Biol 31:7–20
Zhang N, O’Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, Geiser DM (2006) Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol 44:2186–2190
Acknowledgments
The authors are indebted to numerous colleagues, and individuals and culture collections that have provided us with cultures and related information on phytopathogenic species of Fusarium. The mention of firm names or trade products does not imply that they are endorsed or recommended by the US Department of Agriculture over other firms or similar products not mentioned. The USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aoki, T., O’Donnell, K. & Geiser, D.M. Systematics of key phytopathogenic Fusarium species: current status and future challenges. J Gen Plant Pathol 80, 189–201 (2014). https://doi.org/10.1007/s10327-014-0509-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-014-0509-3