Abstract
The genetic diversity of 120 Ralstonia solanacearum strains isolated from a variety of host plants across Japan was assessed on the basis of hypersensitive response (HR) in tobacco leaves and phylogenetic analyses of endoglucanase gene egl, hrpB, and gyrB. Phylogenetic analysis of egl revealed that only three strains belonged to phylotype IV, and 117 strains belonged to phylotype I. Partial sequences of HrpB were identical among phylotype I strains except for one strain. Analyses using the partial nucleotide sequences of the gyrB and egl gene fragments grouped phylotype I strains into 11 gyrB and 8 egl types, respectively, whereas analyses using the partial amino acid sequences of GyrB and Egl grouped phylotype I strains into 4 GyrB and 5 Egl types, respectively. Using multilocus sequence typing of GyrB and Egl, we identified 10 unique sequence types within the Japanese phylotype I strains. Strains belonging to the GyrB42 or GyrB66 type caused wilt in tobacco, and strains belonging to GyrB2 or GyrB9 type elicited HR, demonstrating that HR induction in tobacco is genetically differentiated in the Japanese strains of R. solanacearum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ralstonia solanacearum is a soilborne phytopathogenic bacterium in the β-subdivision of the Proteobacteria (Yabuuchi et al. 1995). R. solanacearum, distributed in tropical, subtropical, and some warm temperature regions of the world, causes bacterial wilt in an unusually broad host range of plants in more than 200 species from highly diverse botanical monocot and dicot families (Hayward 1991). In addition, recent reports indicate that the range of plants infected by R. solanacearum is increasing (Wicker et al. 2007). This bacterium has a high level of phenotypic (metabolic and pathogenic) and genotypic diversity.
Four monophyletic groups of strains, termed phylotypes, have been distinguished on the basis of nucleotide sequences of multiple genes, the ITS region, the hrpB gene, and the endoglucanase gene egl (Fegan and Prior 2005). These phylotypes correlate with the geographical origins of the strains. Phylotype I is composed of strains originating primarily from Asia, phylotype II from America, phylotype III from Africa and its surrounding islands in the Indian Ocean, and phylotype IV from Indonesia, including Pseudomonas syzygii and the blood disease bacterium (BDB) of banana (Prior and Fegan 2005; Villa et al. 2005). Although R. solanacearum can be distinguished from other bacteria by phylogenetic analysis of 16S rDNA sequences (Taghavi et al. 1996), DNA–DNA hybridization analysis has revealed that the relatedness between isolates of R. solanacearum is often less than the expected limit of more than 70% within a species. Because of this high genetic variation between isolates, R. solanacearum is considered a “species complex” (Gillings and Fahy 1994).
R. solanacearum and other gram-negative plant pathogens such as Pseudomonassyringae, Xanthomonas campestris, and X. oryzae infect their hosts by through wounds or natural openings. Once in the intercellular spaces (the apoplast), they produce virulence proteins called type III effectors that are secreted directly into host cells using the type III secretion system (TTSS) to enhance microbial fitness (Alfano and Collmer 2004). Interaction of the effectors and cognate host factors determine the host plant response to virulent and avirulent pathogens (Dangl and Jones 2001). Avirulent strains of R. solanacearum elicit a hypersensitive response (HR) on host plants. R. solanacearum have been estimated to possess up to 60–80 TTSS effectors (Genin and Boucher 2004).
The genome sequence of R. solanacearum GMI1000 strain, which contains two replicons, 3.7-megabase chromosome and 2.1-megabase megaplasmid, revealed the presence of alternative codon-usage regions (ACURs), suggesting that these sequences were acquired by horizontal gene transfer (Salanoubat et al. 2002). Significant proportions of effector genes are located within ACURs. This is probably a reflection of its wide geographic distribution and unusually broad host range.
While the genetic diversity of R. solanacearum strains collected from diverse geographic areas has been studied, phenotypic and genotypic variation of R. solanacearum populations within localized geographical regions have been reported for several regions (Horita and Tsuchiya 2001; Jeong et al. 2007; Lewis-Ivey et al. 2007; Nouri et al. 2009; Villa et al. 2005; Wicker et al. 2007; Yu et al. 2003). Tobacco and tomato are two of the most studied hosts. In R. solanacearum AW1 strain, which belongs to phylotype II, avirulence gene avrA is responsible for eliciting HR in tobacco (Carney and Denny 1990). Sequence analysis of avrA genes in the R. solanacearum population in the southeastern United States revealed that HR-positive strains have the 792-bp wild-type avrA, which is inactivated by miniature inverted-repeat transposable elements in HR-negative strains (Robertson et al. 2004). HR-negative strains cause wilt in both tobacco and tomato. Japanese strain OE1-1 also causes wilt in both tomato and tobacco (Kanda et al. 2003). Other Japanese strains 8107 and RS1000 cause wilt in tomato, but elicit HR in tobacco (Kiba et al. 2003; Mukaihara et al. 2004). Recently we have reported that HR-eliciting Japanese strains in tobacco were divided into two types, GMI1000-type and RS1000-type, according to their avrA sequences (Liu et al. 2009). The RS1000-type avrA has 57% identity with that of GMI1000. We also reported that several Japanese strains possessing the RS1000-type avrA, such as OE1-1, did not induce HR in tobacco but wilted the plants, indicating that AvrA is not likely to be the only determinant of HR induction of Japanese strains. Although all the Japanese biovar 4 strains induce tobacco HR (Horita and Tsuchiya 2001), the scheme based on oxidative metabolism of sugars and alcohols is not sufficient to encompass the diversity of strains represented in the species R. solanacearum. The objective of this study was to investigate genetic diversity of the Japanese strains of R. solanacearum and classify them on the basis of HR induction in tobacco leaves.
Materials and methods
Bacterial strains, media and culture conditions
The R. solanacearum strains used in this study (Table 1) were streaked on BG media (Boucher et al. 1985; 1% of bacto peptone, 0.1% of yeast extract, 0.1% of casamino acids, 0.5% of glucose, and 1.5% of agar) supplemented with polymyxin B (50 μg/ml) and incubated at 28°C for 2 days. B medium (Boucher et al. 1985; 1% of bacto peptone, 0.1% of yeast extract, and 0.1% of casamino acids) was inoculated with cells of a selected single colony and incubated overnight at 28°C. Escherichia coli strain DH12S (Invitrogen Corp., Carlsbad, CA, USA) was grown in Luria–Bertani (LB) medium at 37°C. Concentrations of ampicillin, 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-Gal), and isopropyl-beta-d-thiogalactopyranoside (IPTG) were 100, 40 μg/ml, and 100 μM, respectively.
DNA preparation
Chromosomal DNA of R. solanacearum strain was prepared with AquaPure Genomic DNA Isolation Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instruction. Plasmid DNA was purified using GenElute Plasmid Miniprep Kit (Sigma–Aldrich, St. Louis, MO, USA).
Sequencing of endoglucanase gene
The partial egl fragments were amplified with primers eglA (5′-GGAGACAUATGCATGCCGCTGGTCGCCGC-3′) and eglB (5′-GGGAAAGUGCGTTGCCCGGCACGAACACC-3′). The 20-μl PCR mixture contained 10× TurboCx buffer (Stratagene, La Jolla, CA, USA), 10× PCR enhancer (Invitrogen Corp.), dNTP, primers, and PfuTurboCx DNA polymerase (Stratagene). The PCR condition was an initial denaturation at 95°C for 2 min, followed by 30 cycles of 95°C for 30 s, 65°C for 1 min, and 72°C for 1 min. The PCR products separated on 0.8% agarose gel electrophoresis were purified by E.Z.N.A. Gel Extraction Kit (Omega Bio-tek, Doraville, GA, USA) and cloned on pNEB206A with a USER Friendly cloning kit (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s protocol. Plasmids were purified from multiple numbers of white colonies on LB supplemented with ampicillin, IPTG, and X-gal. Nucleotide sequence of egl on the plasmid was determined using BigDye Terminator v3.1 Cycle Sequencing kits (Applied Biosystems, Foster City, CA, USA) with either M13-47 (5′-TGTAAAACGACGGCCAGT-3′) or RV-M (5′-CAGGAAACAGCTATGACC-3′) primer and analyzed with Applied Biosystems 3130 genetic analyzer (Applied Biosystems).
Sequencing of gyrB
The partial gyrB gene fragments were amplified with degenerate primers UP-IE (5′-CAGGAAACAGCTATGACCAYGSNGGNGGNAARTTYRA-3′) and APrU (5′-TGTAAAACGACGGCCAGTGCNGGRTCYTTYTCYTGRCA-3′) using PrimeSTAR HS DNA Polymerase (Takara Bio, Otsu, Japan). The PCR condition was one cycle at 95°C for 2 min, followed by 35 cycles at 98°C for 10 s, 58°C for 5 s and 72°C for 1 min. The PCR products were purified and directly sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) with either M13-47 or RV-M primer.
Sequencing of hrpB
The partial hrpB gene fragments were amplified with primers RShrpBf (5′-TGCCATGCTGGGAAACATCT-3′) and hrpB-SQ2 (5′-GATACTTGGCGGACAGCCG-3′) using PrimeSTAR HS DNA Polymerase (Takara Bio). The PCR condition was one cycle at 95°C for 2 min, followed by 35 cycles at 98°C for 10 s, 56°C for 5 s and 72°C for 1.5 min. The PCR products separated on 0.8% agarose gel electrophoresis were purified and cloned on pBluescript KS+ pre-digested with EcoRV. Plasmids were purified from multiple numbers of white colonies on LB supplemented with ampicillin, IPTG, and X-gal. The nucleotide sequence of hrpB on the plasmid was determined using BigDye Terminator v3.1 Cycle Sequencing kits (Applied Biosystems) with either M13-47 or RV-M primer.
Phylogenetic analysis
The determined nucleotide sequences or the translated amino acid sequences were aligned using the program CLUSTAL W (Thompson et al. 1994). A phylogenetic tree was constructed with the program CLC Sequence Viewer using the UPGMA algorithm (CLC bio, Aarhus, Denmark).
HR test
Tobacco seedlings (Nicotiana tabacum cv. Bright Yellow and N. benthamiana) were transplanted into 6-cm-diameter plastic pots containing a mixture of vermiculite/peat moss (3:1) in a growth room at 25°C under 10,000 lux for 16 h per day (Kanda et al. 2003). Eight-week-old test plants with four to five true leaves were inoculated with bacteria using leaf infiltration method. Bacterial cell suspension (108 CFU/ml) prepared in 10 mM MgSO4 were infiltrated into tobacco leaves with a syringe without needle. Each assay was repeated in three successive trials. Plants were inspected at 24, 48, and 72 h post inoculation (hpi) and daily for 14 days (Yoshimochi et al. 2009).
The sequences of partial hrpB fragments, gyrB fragments, and egl fragments have been deposited in DDBJ as accessions AB508353–AB508472, AB508473–AB508592, and AB508593–AB508712, respectively.
Results
Phylotyping of the Japanese strains of R. solanacearum
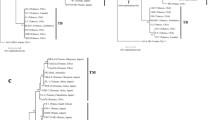
The endoglucanase gene egl has been used to classify R. solanacearum into four phylotypes (Wicker et al. 2007). The partial gene fragments of egl were amplified from 120 strains of R. solanacearum isolated across Japan from variety of host plants such as tomato, tobacco, eggplant, potato, pepper, and the zingiberaceous plants (Table 1). The nucleotide sequences of the amplified fragments were determined and compared to those of strains belonging to phylotypes I–IV. Parts of the egl sequences of MAFF 211471 (code no. 8), MAFF 211479 (code no. 9), MAFF 211266 (code no. 27), MAFF 211271 (code no. 68), MAFF 301558 (code no. 72), MAFF 301559 (code no. 73), and MAFF 211493 (code no. 87) were matched to the deposited egl sequences of these strains (AY464998, AY464997, AF295250, AY465000, AY465002, AY465001, AY465013). All but three strains were classified as phylotype I (Fig. 1). Three strains isolated from potato were classified as phylotype IV, supporting previous results (Villa et al. 2005).
Phylotype classification of the Japanese strains of R. solanacearum based on the partial egl nucleotide sequences. The 688-bp partial egl fragments were aligned with Clustal W. The phylogenetic tree was constructed by the UPGMA algorithm. Values at the branches indicate the bootstrap percentage. “RJ” is used as a prefix to indicate the Japanese strains used in this study. Numbers in parentheses indicate strain numbers having the same egl sequences. The egl sequences of S444E, R221, R230, WP20, E152, R292, Ps6-3-1, GMI1000, JT523, UW151, UW469, CFBP2958, CFBP2047, UW162, WP306, JT525, NCPPB505, NCPPB332, and CFBP3059 are from Villa et al. (2005)
HR induction in tobacco leaves by R. solanacearum
When leaves of N. tabacum were infiltrated separately with 117 strains of R. solanacearum belonging to phylotype I, 83 of these strains elicited HR in the infiltrated leaves within 24 h, while 34 strains caused wilt tobacco in 2 weeks (Table 1). Strains isolated from the zingiberaceous plants, ginger, mioga, or curcuma, elicited HR. HR induction by strains isolated from other plants was variable. No clear relationship between HR induction in tobacco leaves and the original host plants was observed. When a different Nicotiana species, N. benthamiana, was infiltrated with the strains, we observed the same phenomena.
Phylogenetic analyses
Five housekeeping genes, dispersed in the chromosome, and three virulence-related genes, located on the megaplasmid were used to classify R. solanacearum (Castillo and Greenberg 2007). Of these eight genes, we chose hrpB, egl, and gyrB for phylogenetic analysis in this study. The gyrB gene is a housekeeping gene and the egl and hrpB genes are virulence genes. The 1088-bp partial hrpB gene fragments were sequenced. The hrpB sequences of three phylotype IV strains were identical to each other, but distinct from those of other phylotype I strains. Part of the hrpB sequences of MAFF 211266 (code no. 27) was matched to the deposited hrpB sequences of this strain (AF295603). There were five alleles in phylotype I strains, represented by strain 8107 (code no. 2), strain MAFF 211479 (code no. 9), strain MAFF 301070 (code no. 32), strain OE1-1 (code no. 42), and strain SUPP1510 (code no. 205). These differed by a few nucleotides from one another and were similar to GMI1000. Almost all the allelic differences are synonymous nucleotide substitutions, and there were only two types of translated amino acid sequences (Fig. 2). HrpB sequences of OE1-1 (code no. 42), 8107 (code no. 2), MAFF 211479 (code no. 9) and MAFF 301070 (code no. 32) were identical to that of GMI1000 and differed by one amino acid from that of SUPP1510 (code no. 205).
Phylogenetic tree of the Japanese strains of R. solanacearum based on HrpB. The 362-amino acid sequences of translated partial HrpB proteins were aligned with Clustal W. The phylogenetic tree was constructed using the UPGMA algorithm. Phylotype IV strain (code no. 68) was used as the outgroup. Two letters in parentheses indicate the host plants from which each strain was initially isolated: Tm, Lycopersicon esculentum; Ep, Solanum melongena; Po, Solanum tuberosum; Zo, Zinger officinale; De, Delphinium sp.
The gyrB sequences of three phylotype IV strains were identical and distinct from those of phylotype I strains. There were 11 gyrB alleles in phylotype I strains. We constructed phylogenetic trees based on both gyrB nucleotide sequences and GyrB amino acid sequences. When two trees were compared, the resolution was higher in the amino acid-based tree than in the nucleotide-based tree. The deduced amino acid sequences of GyrB in phylotype I strains grouped into four types (Fig. 3a), represented by strain 8107 (code no. 2), strain MAFF 211479 (code no. 9), strain OE1-1 (code no. 42) and strain MAFF 301485 (code no. 66). Although the bootstrap value was not high, GyrB of GMI1000 was grouped into another type (Fig. 3a). Strains belonging to each GyrB type were isolated from a range of host species; no clear relationship between GyrB types and the original host plants was observed.
Phylogenetic tree of the Japanese phylotype I strains of R. solanacearum. The 264-amino-acid sequences of translated partial GyrB (a) and the 297-amino-acid sequences of translated partial Egl (b) were aligned with Clustal W. The phylogenetic trees were constructed with the UPGMA algorithm. The phylotype IV strain (code no. 68) was used as the outgroup. Values at the branches indicate the bootstrap percentage. The scales indicate genetic distances. Two letters in parentheses indicate host plants from which each strain was initially isolated: Tm, Lycopersicon esculentum; Tb, Nicotiana tabacum; Ep, Solanum melongena; Po, Solanum tuberosum; Pe, Capsicum annuum; Li, Limonium sp.; An, Angelia keiskei; Ca, Campanula lactiflora; Sb, Fragaria sp.; Zo, Zinger officinale; Zm, Zinger mioga; Cu, Curcuma alismatifolia; St, Strelitzia reginae; De, Delphinium sp.
The phylogenetic tree based on Egl amino acid sequences showed higher resolution than the phylogenetic tree based on egl nucleotide sequences. The deduced amino acid sequences of Egl in phylotype I grouped into five types (Fig. 3b), represented by strains MAFF 211479 (code no. 9), MAFF 211491 (code no. 26), MAFF 301070 (code no. 32), OE1-1 (code no. 42), and SUPP1510 (code no. 205). Although the bootstrap value was not high, Egl of GMI1000 was grouped into another type (Fig. 3b).
Multilocus sequence typing
We phylogenetically analyzed phylotype I strains of R. solanacearum using three independent genes. After combining the sequence data for the three genes, we applied multilocus sequence typing (MLST) to classify Japanese strains of R. solanacearum. The diversity of amino acid sequences in HrpB was very low (Fig. 2). In this study, we used amino acid sequences of GyrB and Egl for the MLST scheme and identified 10 unique sequence types within the Japanese phylotype I strains (Table 2): type A (GyrB42/Egl42), type B (GyrB2/Egl42), type C (GyrB2/Egl32), type D (GyrB2/Egl9), type E (GyrB2/Egl205), type F (GyrB66/Egl42), type G (GyrB9/Egl42), type H (GyrB9/Egl32), type I (GyrB9/Egl9), and type J (GyrB9/Egl26). We classified the phylotype I strains on the basis of MLST types and the original host plants (Table 2). Strains belonging to MLST types A and G were isolated from the family Solanaceae. Strains of MLST type F were isolated from species in the family Solanaceae and from strawberry. Strains belonging to MLST types B, C and D were isolated from a wide range of plants, including both dicots and monocots. Strains belonging to MLST types H, I, and J were isolated from species in the Zingiberaceae. MLST type E contained only one strain (code no. 205), which was isolated from Delphinium sp.
Discussion
One hundred and seventeen Japanese phylotype I strains of R. solanacearum were classified into 10 MLST types when the amino acid sequences of GyrB and Egl were combined for analysis. When HR induction in tobacco of these strains was assessed, the strains could be clearly differentiated on the basis of GyrB types (Table 3). While strains belonging to GyrB types 42 and 66, which corresponded to MLST types A and F, caused wilt of tobacco, the strains belonging to GyrB types 2 and 9 elicited HR in infiltrated tobacco leaves with two exceptions for MLST type G (Table 3). Strains GyrB42 and GyrB66 form a distinct clade from strains GyrB2 and GyrB9 (Fig. 3a). We used two proteins GyrB and Egl to conduct the MLST analysis. In comparison of two phylogenetic trees based on GyrB and Egl, the tree topologies were different (Fig. 3), suggesting that genes gyrB and egl evolved in a different manner. The housekeeping gene gyrB has been proposed to be a suitable phylogenetic marker (Yamamoto and Harayama 1998). On the other hand, the egl gene, encoding a plant cell wall-degrading enzyme, is directly involved in pathogenesis (Liu et al. 2005). Diversifying selection of the host may accelerate the accumulation of new divergent alleles (Castillo and Greenberg 2007). By combining two genes that evolved in a different manner, we could classify R. solanacearum strains at a much higher resolution. Such a case may be seen in the strains belonging to MLST type G. Type G strains cause wilt in tobacco. The GyrB type of the MLST type G strain is GyrB9. However, other GyrB9 strains elicited HR in tobacco (Table 3). When we used only GyrB for diversifying HR-inducing strains, MLST G strains became the exception. On the other hand, Egl of MLST type G is Egl42, which is the same as MLST types A and F (Table 3). Strains belonging to MLST types A and F cause wilt in tobacco. When GyrB and Egl are combined for the analysis, MLST type G strains are placed in the right group.
The strains belonging to MLST types A, F, and G, which cause wilt in tobacco, were originally isolated from the species in the Solanaceae (Table 2). The strains belonging to MLST types B, C, and D, which elicited HR in tobacco, were isolated from both Solanaceae and Zingiberaceae species. These MLST types are composed of GyrB2. When ginger plants were inoculated with strains from types B, C, and D isolated from the family Solanaceae—8107 (code no. 2), MAFF 301524 (code no. 3), MAFF 730126 (code no. 6), Kami-1 (code no. 40), MAFF 301070 (code no. 32), MAFF 301841 (code no. 38), Rst071 (code no. 224), and HAIP104 (code no. 235)—no wilting was observed (unpublished data). The type B strain isolated from ginger, MAFF 211471 (code no. 8), was fully virulent on species from the family Solanaceae (Tsuchiya et al. 2005). We can speculate that the ancestral strains of MLST types B, C, and D were avirulent on tobacco and that some strains gained the ability to cause disease on the family Zingiberaceae.
On the other hand, HR strains belonging to MLST types H, I, and J were isolated exclusively from the Zingiberaceae species. The GyrB type is GyrB9, which is a distinct clade from GyrB2 (Fig. 3a). Strains isolated from ginger and mioga have unique sequences in both 16S rRNA and egl genes (Villa et al. 2005). An AFLP analysis of R. solanacearum strains on the island of Hawaii indicated that strains from ginger have a high degree of similarity within strains and little similarity with strains from all other hosts (Yu et al. 2003). Type I strains MAFF 211479 (code no. 9), MAFF 211496 (code no. 25), MAFF 211272 (code no. 31), and MAFF 211274 (code no. 78) and type J strain MAFF 211491 (code no. 26) did not wilt plants from the family Solanaceae (Tsuchiya et al. 2005). The ancestral strains of MLST types H, I, and J might be distinct from the ginger strains of MLST types B, C, and D. A rep-PCR analysis of the ginger strains defined two fingerprint types, types I and II (Horita et al. 2004). The MLST type B strain MAFF 211471 (code no. 8) is fingerprint type II, and the MLST type I strains MAFF 211479 (code no. 9), MAFF 211272 (code no. 31), and MAFF 211274 (code no. 78), are fingerprint type I (Horita et al. 2004), which could support our speculation that the ginger strains might have two distinct origins.
In this study, while almost all strains belonged to phylotype I, three strains were classified into phylotype IV. These three strains (MAFF211271, code no. 68; MAFF301558, code no. 72; MAFF301559, code no. 73) were all race 3 and isolated from potato. They belong to phylotype IV and caused the collapse of the infiltrated area of tobacco leaves (Villa et al. 2005). They did not cause wilt in tobacco. We obtained the same results with these potato strains, indicating that tobacco plants inoculated with phylotype IV Japanese strains reacted differently from those inoculated with phylotype I strains.
The type III effectors play an important role in determining host range (Dangl and Jones 2001). The avrA gene is involved in pathogenicity on tobacco in the phylotype II population of R. solanacearum in the southeastern United States (Robertson et al. 2004). The combination of two genes, avrA and popP1, is responsible for HR induction of phylotype I strain GMI1000 (Poueymiro et al. 2009), which was collected in South America. Wild-type GMI1000 elicited HR and an avrA popP1 double mutant was virulent on tobacco. Recently, we reported that HR-eliciting Japanese strains in tobacco were divided into two types according to AvrA sequences: one type strain contained AvrA identical to that of GMI1000, and another type strain contained AvrA identical to that of OE1-1 (Liu et al. 2009). While the strains with GMI1000-type AvrA elicited HR in tobacco, both strains with OE1-1-type AvrA elicited HR or wilted tobacco. Although all the virulent strains lacked popP1, HR was elicited even by several strains without popP1. From these results, we speculate that avrA and popP1 in Japanese strains might not be responsible for HR induction in tobacco. Draft genome sequencing of strains OE1-1 and MAFF 730135 (potato strain, race 3) has been ongoing. Strain OE1-1 belonging to MLST type A caused wilt of tobacco, and strain MAFF 730135 belonging to MLST type B elicited HR in tobacco. By comparing the two genome sequences, we have found several effector genes, whose structures are quite different between OE1-1 and MAFF 730135 (unpublished data). These effector genes could be candidate virulence determinants on tobacco. As a next step, we will investigate the distribution of the candidate effector genes in the strains belonging to each of 10 MLST types.
The hrpB gene has been used to analyze the phylogenetic relationship among strains of R. solanacearum (Poussier et al. 2000; Villa et al. 2005). In this study, we also used hrpB to classify the Japanese strains. Although the hrpB sequence of phylotype IV strain was distinct from those of phylotype I strains, the sequence variation among phylotype I strains was subtle. Most of the substitution was synonymous, and only two types of amino acid sequences of partial HrpB were detected. Although South American phylotype I strain GMI1000 had different GyrB and Egl types from Japanese phylotype I strains (Fig. 3), the full length of HrpB of GMI1000 was 100% identical to OE1-1 (Liu et al. 2009). Contrary to the hrp gene cluster of other plant pathogens, the R. solanacearum hrp locus and flanking regions containing the virulence genes is not a pathogenicity island (Salanoubat et al. 2002). Instead, the hrp locus is believed to be composed of a core group of ancestral pathogenicity genes (Genin and Boucher 2004), indicating that diversity of genes in the R. solanacearum hrp locus including hrpB is low. All these data suggest that the hrpB gene might not be suitable for classifying R. solanacearum strains in the same phylotype, at least in phylotype I.
References
Alfano JR, Collmer A (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol 42:385–414
Boucher CA, Barberis PA, Trigalet AP, Demery DA (1985) Transposon mutagenesis of Pseudomonas solanacearum: isolation of Tn5-induced avirulent mutants. J Gen Microbiol 131:2449–2457
Carney BF, Denny TP (1990) A cloned avirulence gene from Pseudomonas solanacearum determines incompatibility on Nicotiana tabacum at the host species level. J Bacteriol 172:4836–4843
Castillo JA, Greenberg JT (2007) Evolutionary dynamics of Ralstonia solanacearum. Appl Environ Microbiol 73:1225–1238
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833
Fegan M, Prior P (2005) How complex is the “Ralstonia solanacearum species complex”? In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 449–461
Genin S, Boucher C (2004) Lessons learned from the genome analysis of Ralstonia solanacearum. Annu Rev Phytopathol 42:107–134
Gillings MR, Fahy P (1994) Genomic fingerprinting: towards a unified view of the Pseudomonas solanacearum species complex. In: Hayward AC, Hartman GL (eds) Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. CAB International, Wallingford, pp 95–112
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87
Horita M, Tsuchiya K (2001) Genetic diversity of Japanese strains of Ralstonia solanacearum. Phytopathology 91:399–407
Horita M, Yano K, Tsuchiya K (2004) PCR-based specific detection of Ralstonia solanacearum race 4 strains. J Gen Plant Pathol 70:278–283
Jeong Y, Kim J, Kang Y, Lee S, Hwang I (2007) Genetic diversity and distribution of Korean isolates of Ralstonia solanacearum. Plant Dis 91:1277–1287
Kanda A, Yasukohchi M, Ohnishi K, Kiba A, Okuno T, Hikichi Y (2003) Ectopic expression of Ralstonia solanacearum effector protein PopA early in invasion results in loss of virulence. Mol Plant Microbe Interact 16:447–455
Kiba A, Tomiyama H, Takahashi H, Hamada H, Ohnishi K, Okuno T, Hikichi Y (2003) Induction of resistance and expression of defense-related genes in tobacco leaves infiltrated with Ralstonia solanacearum. Plant Cell Physiol 44:287–295
Lewis-Ivey ML, McSpadden-Gardener BB, Opina N, Miller SA (2007) Diversity of Ralstonia solanacearum infecting eggplant in the Philippines. Phytopathology 97:1467–1475
Liu H, Zhang S, Schell MA, Denny TP (2005) Pyramiding unmarked deletions in Ralstonia solanacearum shows that secreted proteins in addition to plant cell-wall-degrading enzymes contribute to virulence. Mol Plant Microbe Interact 18:1296–1305
Liu Y, Kanda A, Kiba A, Hikichi Y, Ohnishi K (2009) Distribution of avirulence genes avrA and popP1 in 22 Japanese phylotype I strains of Ralstonia solanacearum. J Gen Plant Pathol. doi:10.1007/s10327-009-0189-6
Mukaihara T, Tamura N, Murata Y, Iwabuchi M (2004) Genetic screening of Hrp type III-related pathogenicity genes controlled by the HrpB transcriptional activator in Ralstonia solanacearum. Mol Microbiol 54:863–875
Nouri S, Bahar M, Fegan M (2009) Diversity of Ralstonia solanacearum causing potato bacterial wilt in Iran and the first record of phylotype II/biovar 2T strains outside South America. Plant Pathol 58:243–249
Poueymiro M, Cunnac S, Barberis P, Deslandes L, Peeters N, Cazale-Noel AC, Boucher C, Genin S (2009) Two type III secretion system effectors from Ralstonia solanacearum GMI1000 determine host-range specificity on tobacco. Mol Plant Microbe Interact 22:538–550
Poussier S, Prior P, Luisetti J, Hayward C, Fegan M (2000) Partial sequencing of the hrpB and endoglucanase genes confirms and expands the known diversity within the Ralstonia solanacearum species complex. Syst Appl Microbiol 23:479–486
Prior P, Fegan M (2005) Recent developments in the phylogeny and classification of Ralstonia solanacearum. Acta Hortic 695:127–136
Robertson AE, Wechter WP, Denny TP, Fortnum BA, Kluepfel DA (2004) Relationship between avirulence gene (avrA) diversity in Ralstonia solanacearum and bacterial wilt incidence. Mol Plant Microbe Interact 17:1376–1384
Salanoubat M, Genin S, Artiguenave F, Gouzy J, Mangenot S, Arlat M, Billault A, Brottier P, Camus JC, Cattolico L, Chandler M, Choisne N, Claudel-Renard C, Cunnac S, Demange N, Gaspin C, Lavie M, Moisan A, Robert C, Saurin W, Schiex T, Siguier P, Thébault P, Whalen M, Wincker P, Levy M, Weissenbach J, Boucher CA (2002) Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 415:497–502
Taghavi M, Hayward C, Sly LI, Fegan M (1996) Analysis of the phylogenetic relationships of strains of Burkholderia solanacearum, Pseudomonas syzygii, and the blood disease bacterium of banana based on 16S rRNA gene sequences. Int J Syst Bacteriol 46:10–15
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tsuchiya K, Yano K, Horita M, Morita Y, Kawada Y, d’Ursel CM (2005) Occurrence and epidemic adaptation of new strains of Ralstonia solanacearum associated with Zingiberaceae plants under agro-ecosystem in Japan. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 463–469
Villa JE, Tsuchiya K, Horita M, Natural M, Opina N, Hyakumachi M (2005) Phylogenetic relationships of Ralstonia solanacearum species complex strains from Asia and other continents based on 16S rDNA, endoglucanase, and hrpB gene sequences. J Gen Plant Pathol 71:39–46
Wicker E, Grassart L, Coranson-Beaudu R, Mian D, Guilbaud C, Fegan M, Prior P (2007) Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Appl Environ Microbiol 73:6790–6801
Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y (1995) Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. Nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. Nov., Ralstonia solanacearum (Smith 1896) comb. Nov. and Ralstonia eutropha (Davis 1969) comb. Nov. Microbiol Immunol 39:897–904
Yamamoto S, Harayama S (1998) Phylogenetic relationships of Pseudomonas putida strains deduced from the nucleotide sequences of gyrB, rpoD and 16S rRNA genes. Int J Syst Bacteriol 48:813–819
Yoshimochi T, Zhang Y, Kiba A, Hikichi Y, Ohnishi K (2009) Expression of hrpG and activation of response regulator HrpG are controlled by distinct signal cascades in Ralstonia solanacearum. J Gen Plant Pathol 75:196–204
Yu Q, Alvarez AM, Moore PH, Zee F, Kim MS, De Silva A, Hepperly PR, Ming R (2003) Molecular diversity of Ralstonia solanacearum isolated from ginger in Hawaii. Phytopathology 93:1124–1130
Acknowledgments
This work was supported in part by KAKENHI (Grant-in-Aid for Scientific Research) from Japan Society for the Promotion of Science (16658020 to Y.H. and 17380031 to K.O.) and on Priority Areas “Comparative Genomics” from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20017020 to K.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Kanda, A., Yano, K. et al. Molecular typing of Japanese strains of Ralstonia solanacearum in relation to the ability to induce a hypersensitive reaction in tobacco. J Gen Plant Pathol 75, 369–380 (2009). https://doi.org/10.1007/s10327-009-0188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-009-0188-7